Abstract
Objective
To assess prescriber adherence to standard anti-hypertensive treatment guidelines in South Africa, determine if supply data are useful indicators of drug use, and assess the cost implications of not complying with the guidelines.
Methods
We undertook two studies: an analysis of records of the anti-hypertensive drugs supplied to all 54 public-sector hospitals with a hypertension clinic in KwaZulu-Natal, and a direct-observation survey of anti-hypertensive drug prescriptions presented to pharmacies in a subset of 16 of the 54 hospitals. We calculated the relative use of each anti-hypertensive drug group as a proportion of all anti-hypertensive drugs supplied or prescribed. We ranked drug groups in order of use for comparison with recommended South African standard treatment guidelines, and we compared the proportions derived from supply data with those derived from the prescription survey.
Findings
Supply data showed that, in line with treatment guidelines, diuretics and angiotensin-converting enzyme inhibitors were the most frequently supplied medicines (42% and 27%, respectively). However, methyldopa – not included in the treatment guidelines – represented 10% of all anti-hypertensives supplied, but the proportion varied widely between hospitals (0–37%). Reserpine, second choice in the treatment guidelines, was used in high amounts by only two hospitals. Calcium channel blockers and beta blockers represented a small proportion of the anti-hypertensive drugs supplied: 6% each. Results from the prescription survey were in concordance with supply data for the most frequently prescribed drugs but gave slightly different estimates of the use of others.
Conclusion
Supply data, the most available source of information about drug use in developing countries, are (with some provisos) a reliable data source for the evaluation of adherence to treatment guidelines. Our results showed substantial non-adherence to standard treatment guidelines.
Résumé
Objectif
Evaluer l’observation par les prescripteurs des directives thérapeutiques standard dans l’utilisation des antihypertenseurs en Afrique du Sud, déterminer si les données de délivrance sont des indicateurs utiles de l’usage de ces médicaments et estimer les conséquences en termes de coût du non-respect des directives standard.
Méthodes
Nous avons entrepris deux études : une analyse des enregistrements de médicaments antihypertenseurs délivrés aux 54 hôpitaux publics de KwaZulu-Natal disposant d’une consultation d’hypertension et une enquête par observation directe des prescriptions d’antihypertenseurs présentées en pharmacie, effectuée sur un sous-groupe d’hôpitaux comprenant 16 des 54 hôpitaux susmentionnés. Nous avons calculé l’utilisation de chaque groupe d’antihypertenseurs en proportion de l’ensemble des antihypertenseurs délivrés ou prescrits. Nous avons classé les groupes de médicaments en vue d’établir des comparaisons avec les directives thérapeutiques standard sud-africaines et nous avons confronté les proportions obtenues à partir des données de délivrance et celles déterminées à partir de l’enquête sur les prescriptions.
Résultats
Les données de délivrance ont montré que, conformément aux directives thérapeutiques, les diurétiques et les inhibiteurs de l’enzyme de conversion de l’angiotensine étaient les médicaments les plus fréquemment prescrits (42 et 27 % respectivement). Néanmoins, la méthyldopa - non préconisée par les directives thérapeutiques - représentait 10 % de l’ensemble des antihypertenseurs délivrés, avec de grandes variations entre les hôpitaux (0 à 37 %). La réserpine, médicament de deuxième choix selon les directives thérapeutiques, était employée en grandes quantités par deux hôpitaux seulement. Les agents bloquants des canaux calciques et les bêta-bloquants représentaient une faible proportion des antihypertenseurs délivrés : 6 % pour chacune de ces catégories. Les résultats de l’enquête sur les prescriptions étaient en accord avec les données de délivrance à propos des médicaments les plus prescrits, mais donnaient des estimations légèrement différentes pour l’utilisation des autres groupes.
Conclusion
Les données de délivrance, source la plus facilement disponible d’informations sur l’utilisation des médicaments dans les pays en développement, constituent (avec quelques réserves) une source de données fiable pour l’évaluation de l’observation des directives thérapeutiques. Nos résultats mettent en évidence un non-respect substantiel de ces directives.
Resumen
Objetivo
Determinar la observancia de las directrices sudafricanas sobre el tratamiento antihipertensor por parte de los prescriptores, determinar si los datos sobre el suministro son indicadores útiles del uso de los fármacos, y evaluar las repercusiones de la no observancia de las directrices en el costo del tratamiento.
Métodos
Realizamos dos estudios: un análisis de los registros de los antihipertensores suministrados a 54 hospitales del sector público de KwaZulu-Natal que disponían de consultas de hipertensión, y una observación directa de las prescripciones de antihipertensores presentadas a las farmacias en 16 de esos 54 hospitales. El uso relativo de cada antihipertensor se calculó como la proporción con respecto a todos los antihipertensores suministrados o prescritos. Los grupos de fármacos fueron ordenados por frecuencia de uso para compararlos con las directrices terapéuticas sudafricanas. También se compararon los resultados proporcionados por los estudios del suministro y de las prescripciones.
Resultados
Los datos sobre el suministro revelaron que, de acuerdo con las directrices terapéuticas, los fármacos suministrados con más frecuencia fueron los diuréticos y los inhibidores de la enzima de conversión de la angiotensina (42% y 27%, respectivamente). Sin embargo, la metildopa, que no está incluida en las directrices terapéuticas, representó un 10% de las totalidad de los antihipertensores suministrados, aunque hubo una gran variabilidad entre hospitales (0 a 37%). La reserpina, un tratamiento de segunda elección según las directrices, sólo era muy utilizada en dos hospitales. Los bloqueantes de los canales del calcio y los betabloqueantes representaron una pequeña proporción de la totalidad de los antihipertensores suministrados (6% cada uno). Los resultados del estudio sobre las prescripciones fueron concordantes con los del suministro con respecto a los antihipertensores prescritos con más frecuencia, pero presentaron ligeras diferencias con respecto al uso de otros fármacos.
Conclusión
Los datos sobre el suministro, que son los más fáciles de obtener para determinar el uso de los fármacos en los países en desarrollo, constituyen (con algunos condicionamientos) una fuente de información fiables para evaluar la observancia de las directrices terapéuticas. Nuestros resultados muestran una inobservancia considerable de las directrices terapéuticas.
ملخص
الهدف
تقييم امتثال من يصف الأدوية الخافضة للضغط بالدلائل الإرشادية المعيارية للمعالجة بتلك الأدوية في جنوب أفريقيا، وللتعرف على ما إذا كانت المعطيات حول الإمداد بتلك الأدوية تعد مؤشراً مفيداً لاستخدامها، ولتقييم تأثير التكاليف عند عدم الامتثال لتلك الدلائل الإرشادية.
الطريقة
أجرينا دراستين؛ تحليلاً لسجلات الأدوية الخافضة للضغط التي زود بها 54 مستشفى من القطاع العام مع عيادة لمعالجة ارتفاع ضغط الدم في كوازولو ناتال؛ ومسحاً للمراقبة المباشرة لوصفات الأدوية الخافضة للضغط التي ترد إلى الصيادلة في مجموعة فرعية تتألف من 16 مستشفى من بين المستشفيات الـ 54. وقد حسبنا الاستخدام النسبي لكل مجموعة من مجموعات الأدوية الخافضة للضغط كنسبة مئوية إلى جميع الأدوية الخافضة للضغط التي ترد أو توصف. وأجرينا ترتيباً لمجموعات الأدوية وفقاً لمدى استخدامها بغرض مقارنتها بالدلائل الإرشادية المعيارية للمعالجة بتلك الأدوية في جنوب أفريقيا، كما قارنا النسب المئوية المشتقة من معطيات الإمداد مع النسب المئوية المشتقة من المسح الذي أجريناه حول وصف تلك الأدوية.
الموجودات
أظهرت معطيات الإمداد بالأدوية أنها تتماشى مع الدلائل الإرشادية للمعالجة، فقد كانت المدرات ومثبطات الإنزيم القالب للأنجيوتنسين هي أكثر الأدوية إمداداً (42% و27 على التوالي)؛ إلا أن المثيل دوبا لم يدرج في الدلائل الإرشادية للمعالجة، وقد مثّل 10% من مجمل إمداد الأدوية الخافضة للضغط، إلا أن نسبته المئوية تختلف اختلافاً واسعاً بين مستشفى وآخر (0%-37%). أما الرزربين الذي يمثل الخط الثاني من الاختيارات فقد استخدم بكميات كبيرة في مستشفيين اثنين فقط. فيما مثّلت حاصرات قنيات الكالسيوم وحاصرات المستقبلات البنيانية نسبة مئوية ضئيلة من الإمداد بالأدوية الخافضة للضغط، فلم تتجاوز 6% لكل منها. وقد كانت النتائج التي حصلنا عليها من مسح الوصفات متماشية مع معطيات الإمداد في معظم الأدوية الموصوفة ولكنها أعطت تقديرات مختلفة اختلافاً طفيفاً حول استخدام الأدوية الأخرى.
الاستنتاج
تعد معطيات الإمداد بالأدوية أكثر مصادر المعلومات إتاحة حول استخدام الأدوية في البلدان النامية، وهي (مع اتخاذ بعض الاحتياطات) مصادر موثوقة للمعلومات حول تقييم الامتثال للدلائل الإرشادية للمعالجة. وقد أظهرت النتائج التي توصلنا إليها قدراً هاماً من عدم الامتثال للدلائل الإرشادية المعيارية للمعالجة.
Introduction
In 1994, the new South African Government of National Unity formulated a national drug policy based on standards for national medicines polices advocated by WHO.1 Implementation strategies included the development of a list of essential medicines and the compilation of evidence-based standard treatment guidelines for common disorders, including hypertension, to be adopted throughout South Africa’s public sector.
According to the results of the first Demographic Health Survey in South Africa in 1998, hypertension is the most prevalent chronic health condition reported by men and women across all provinces. With more than 80% of the South African population receiving care through the public health system, these guidelines can have a significant impact on the nation’s health care. However, follow-up evaluation of hypertension guidelines 4 years and 5 years after their introduction by means of hospital prescription surveys suggested that the recommendations were not being followed.2,3 In an unpublished study in outpatients attending a hospital hypertension clinic in the province of KwaZulu-Natal in 1999, one-third of the 1288 prescriptions surveyed were for methyldopa, even though this drug was not recommended in the guidelines except during pregnancy. This finding mirrored those of Edwards et al.,4 who noted that 28% of patients with hypertension at a community health centre were prescribed methyldopa.
We wanted to examine the extent to which hospital prescribers followed the guidelines in their everyday work. We therefore undertook further studies of drug prescription patterns in South African public hospitals.
Quantitative drug-use studies can take several forms.5,6 Rarely is it possible to measure the actual consumption of medicines by patients. More commonly, the number of prescriptions issued is assumed to reflect actual use.
In settings where prescriptions are recorded on an electronic database that also includes the indication for the drug, prescription data may be readily obtained and matched to the indication. However, in most of South Africa, and in developing countries in general, such electronic records are not available, and drug-use data must be obtained by direct survey, a laborious and time-intensive process.
More sophisticated methods for linking prescription to indication make connections between large databases (e.g. those of Health Maintenance or other administrative organizations in the United States of America).7 What such methods lack in precision they make up in quantity, but they are currently beyond the means of most developing countries.
As a preliminary assessment of prescribers’ adherence to South African standard treatment guidelines for hypertension, we compared data from records of medicine supply to hospitals (supply data) with data from direct observation of prescriptions presented to hospital pharmacies. Even though supply data from a central depot to hospitals are acknowledged to be only an approximation of prescription data, they are readily obtainable worldwide and provide an indication of trends in drug use. Few studies have compared these two sources.
Specifically, in this study we pursued several objectives: (i) to identify the relative use of anti-hypertensive drugs using data from records of drugs supplied to hospitals and a survey of prescriptions presented to hospital pharmacies; (ii) to determine whether supply data are useful indicators of drug use by comparing them with data derived from direct observation (i.e. the prescription survey); (iii) to compare prescribing patterns for anti-hypertensive drugs with standard treatment guidelines; and (iv) to determine the cost implications of non-compliance with the guidelines.
Methods
Drug supply data
All 63 public-sector hospitals in KwaZulu-Natal purchase pharmaceuticals from a central store, the provincial medical supply centre (PMSC). The PMSC is responsible for purchasing, storing, pre-packing and delivering drugs to all public hospitals in the province. To minimize work at the hospitals, PMSC supplies most drugs in patient-ready packs (PRPs) that contain a pre-packaged volume of tablets, capsules or liquid sufficient for 28 days of therapy.
All drugs available from the PMSC are listed on an official catalogue which has been approved by the provincial Pharmacy and Therapeutics Committee. The volume and value of drugs supplied to each hospital are recorded on the PMSC database, along with information about the dosage, pack size and cost of each drug supplied. Nine of the 63 hospitals in KwaZulu-Natal were excluded from the study because they did not offer an outpatient clinic service to patients with hypertension.
The PMSC catalogue was used to identify all anti-hypertensive drugs supplied in the province. The catalogue numbers for these drugs were used to generate a report of the volume of drugs supplied to hospitals over a 6-month period. Most anti-hypertensive drugs are supplied in 28-day PRPs, except for a few bulk packs that are supplied to hospitals with facilities for repacking the drugs. Pharmacy managers at hospitals that ordered bulk packs were contacted and asked about the size of the packs being dispensed to patients at their institution, and they confirmed that they issued standard 28-day packs.
The total number of PRPs was calculated in three steps. First, the total number of PRPs of each anti-hypertensive agent supplied to each hospital over a 6-month period was taken to reflect the total number of units of each anti-hypertensive prescribed. For example, if a hospital was supplied 10 000 PRPs of hydrochlorothiazide tablets during 6 months, we assumed this represented 10 000 prescriptions for hydrochlorothiazide tablets.
Second, the PRPs of the anti-hypertensive drugs were divided into pharmacological groups: diuretics (hydrochlorothiazide, indapamide), beta blockers (atenolol, propranolol), calcium channel blockers (isradipine, nifedipine, amlodipine, verapamil, diltiazem), angiotensin-converting enzyme (ACE) inhibitors (captopril, perindopril), methyldopa, reserpine, hydralazine and prazosin. Finally, the sum of the PRPs of all individual drug groups was calculated to represent the total number of PRPs for all anti-hypertensive drugs supplied over the 6-month period.
Methyldopa as an indicator
Methyldopa use was selected as an initial indicator of non-adherence to the hypertension treatment guidelines. We calculated the amount of methyldopa supplied to each hospital as a proportion of all the anti-hypertensive drugs supplied over a 6-month period (total number of PRPs of methyldopa divided by total PRPs of all anti-hypertensive agents). We calculated the overall proportion of methyldopa from the mean proportion used in each hospital rather than by pooling all the data.
To assess whether our 6-month supply data was representative of longer-term methyldopa use, we compared the number of PRPs supplied each year from 1997 to 2001 with the number supplied in 1997. Overall, methyldopa supply had decreased from 1997 but had stabilized during the 2 years before the prescription survey.
Prescription survey
The second part of the study was a survey of anti-hypertensive prescriptions at 16 hospitals. To provide an approximate indicator of compliance with treatment guidelines, we used supply data to categorize hospitals into usage groups: low (< 5% of all anti-hypertensives supplied), intermediate (5–12%) and high (> 12%).
We selected five institutions from each category of methyldopa use and included urban and rural hospitals from across the province. Permission to conduct the study was requested from hospital superintendents in the selected institutions, and hospitals that did not respond were followed up with telephone reminders.
We visited superintendents to explain the survey procedure. One of the hospitals that failed to respond initially requested participation at the end of the recruitment period. Therefore, 16 hospitals were included in the prescription survey – 8 urban and 8 rural. This was a pragmatic, rather than a random, sample of the larger group of 54 hospitals included in the supply data study.
Hospitals were visited on the day allocated for treatment of outpatients with hypertension, and the prescription survey took place in the hospital pharmacy department. Consecutive, original prescriptions (new and repeats) presented to the outpatient pharmacy department for the management of uncomplicated essential hypertension were used to record the names and doses of all anti-hypertensive drugs prescribed. We did not record any prescriber information or patient names. Prescriptions were attached to the clinical notes, so we were able to confirm the diagnosis of uncomplicated essential hypertension in all cases. Prescriptions for patients with co-morbid conditions were not included in the survey.
Statistical analysis
Regional hospital pharmacies fill approximately 3500 prescriptions for anti-hypertensives per month, and smaller district hospitals fill around 1500.
In one hospital, the prescription rate for methyldopa was approximately 30%, according to a survey.4 The number of prescriptions needed to detect a 30% methyldopa prescription rate was calculated using StatsDirect (StatsDirect, Altrincham, England), and the maximum acceptable deviation of the sample rate from the true population rate was set at 10%, with a confidence level of 95%. The sample size required was 79; therefore, we included 100 prescriptions at each institution.
To determine whether prescribing practices varied at different times of the day, prescriptions from one urban and one rural hospital were collected every 3 hours during a hypertension clinic day. No differences were found in the prescription pattern between hospitals or time periods.
For each hospital we calculated the proportion of prescriptions for every drug type, as well as for monotherapy and combination therapy, and we recorded the doses prescribed. We then compared the results obtained with the two methods for measuring the use of anti-hypertensive drugs. To allow for comparisons, results are expressed as percentages of all prescribed anti-hypertensives.
Ethics approval
The study was approved by the University of Newcastle Human Research Ethics Committee (New South Wales, Australia) and the Nelson Mandela Medical School Ethics Committee (KwaZulu-Natal, South Africa).
Results
Supply data
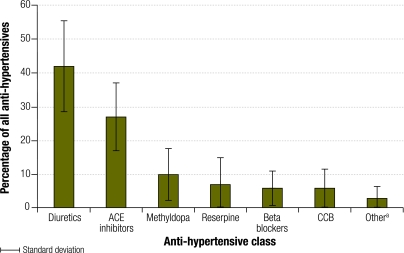
Fig. 1 shows the anti-hypertensives supplied over a 6-month period. Hydralazine and prazosin represented less than 2% of all the anti-hypertensive medications supplied, and data for these drugs are not shown separately in the figure. Diuretics were the most commonly supplied anti-hypertensive agents at 40 hospitals (74%) in the province. The average relative supply of diuretics in the province was 42%, with hydrochlorothiazide being the most commonly supplied. Diuretics are also used to treat conditions other than hypertension.
Fig. 1.
Relative proportions of anti-hypertensive drugs supplied to 54 hospitals in KwaZulu-Natal, South Africa, over a 6-month period
ACE, angiotensin-converting enzyme inhibitor; CCB, calcium channel blocker.
a Hydralazine and prazosin.
ACE inhibitors were the second most commonly supplied drugs in the province. At 14 hospitals (26%), ACE inhibitors were supplied more often than diuretics. The average relative supply of ACE inhibitors was 27%; However, ACE inhibitors may also be prescribed for conditions other than hypertension.
The average relative supply of methyldopa was 10%. However, there was significant variation between hospitals, from 0% to 37%. Reserpine represented 7% of all the anti-hypertensive drugs supplied in all the hospitals. Its supply was high only in two hospitals, where it represented 20% and 40% of all the anti-hypertensive medications supplied. Because reserpine is used only to treat hypertension, these supply data accurately reflect the number of patients with hypertension who received the drug. Calcium channel blockers and beta blockers each represented 6% of all anti-hypertensives supplied. The most commonly supplied beta blocker was atenolol. However, there appeared to be no preferred agent among the calcium channel blockers; amlodipine, isradipine and nifedipine were supplied in roughly the same proportions. Hydralazine represented less than 5% of all the anti-hypertensive drugs supplied at all hospitals except one, where it represented 11%.
Prescription survey
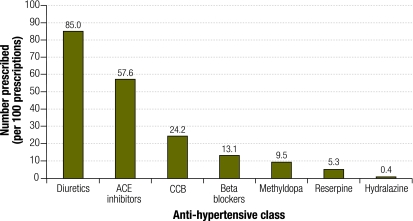
Fig. 2 shows the average number of anti-hypertensive drugs prescribed by class per hundred prescriptions. We noted substantial differences in methyldopa use between hospitals: the percentage of prescriptions for methyldopa ranged from 0% to 37%. Of the 1600 prescriptions surveyed, 152 included methyldopa (9.5%).
Fig. 2.
Mean number of anti-hypertensive drugs in each group, per 100 prescriptions, in 16 hospitals in KwaZulu-Natal, South Africa
ACE, angiotensin-converting enzyme; CCB, calcium channel blocker.
Although reserpine is the preferred second-line treatment in the guidelines, we noted this drug was rarely prescribed, with only 84 prescriptions in total (5.3% of all prescriptions). Three hospitals with the highest rates of reserpine prescription accounted for 56% of reserpine use overall. In 6 hospitals reserpine was not prescribed at all.
Monotherapy and combination therapy
Most patients (51–91%) were prescribed combination therapy for uncomplicated essential hypertension. The mean number of items per prescription was 1.95. Approximately 63% of the patients on monotherapy were prescribed a diuretic; 19%, an ACE inhibitor; 8% a calcium channel blocker, and 7%, a beta blocker. Overall, only 1% of patients were prescribed methyldopa or reserpine as monotherapy.
Comparison of supply data and prescription survey
Supply data were available for the 54 hospitals in the supply study and for the 16 hospitals that also took part in the prescription survey. Table 1 shows drug groups ranked in order of actual relative use (calculated using supply data as well as data from the prescription survey) and suggested relative use, as recommended by the national guidelines.
Table 1. Compared results of using supply data and a prescription survey for measuring anti-hypertensive drug use in hospitals in KwaZulu-Natal, South Africa.
| Actual or suggested use | Treatment guidelines | Supply data | Prescription survey |
|---|---|---|---|
| (%)a | (%)a | ||
| 1st line | Diuretic (HCT) | Diuretic (42) | Diuretic (43.5) |
| 2nd line | Reserpine | ACE inhibitor (27) | ACE inhibitor (29.5) |
| 3rd line | Beta blocker | Methyldopa (10) | CCB (12.4) |
| 4th line | ACE inhibitor | Reserpine (7) | Beta blocker (6.7) |
| 5th line | CCB | Beta blocker (6) | Methyldopa (4.9) |
| 6th line | Alpha blocker | CCB (6) | Reserpine (2.7) |
| 7th line | – | Hydralazine (1) | Hydralazine (0.2) |
ACE, angiotensin-converting enzyme; CCB, calcium channel blocker; HCT, hydrochlorothiazide. a The percentages in parentheses represent the proportion of all anti-hypertensive drugs supplied or prescribed.
Costs of actual practice versus guideline recommendations
We used results of the prescription survey and information from the PMSC price list to calculate the cost of a 1-month supply of anti-hypertensive drugs for 100 patients. We also calculated the total expenditure had treatment been given in accordance with standard treatment guidelines. To perform this calculation we assumed that the proportions of second-, third-, fourth- and fifth-line anti-hypertensive drugs prescribed, as determined from the prescription survey, would be identical irrespective of the agent that had been used. Actual costs were 19% greater than would have been the case if standard treatment guidelines had been followed (Table 2).
Table 2. Total cost of anti-hypertensive drugs in 16 hospitals in KwaZulu-Natal, South Africa, based on hospital prescription practices, versus total cost had hypertension treatment guidelines been followed.
| Anti-hypertensive drug | Cost | ||
|---|---|---|---|
| (in US$a, per 100 prescriptions)b | |||
| Under current practice | Under treatment guidelines | ||
| Diuretic | 27.20 | 27.20 (1st line) | |
| ACE inhibitor | 67.25 | 15.07 (4th line) | |
| CCB | 86.33 | 57.55 (5th line) | |
| Beta blocker | 6.05 | 11.16 (3rd line) | |
| Methyldopa | 20.61 | – | |
| Reserpine | 5.15 | 59.68 (2nd line) | |
| Hydralazine | 0.724 | – | |
| Total | 213.30 | 170.67 | |
ACE, angiotensin-converting enzyme; CCB, calcium channel blocker. a 1 United States dollar (US$) = 10 South African rands. b A prescription for a single patient may contain one or more drugs.
Discussion
Evaluating the success of a national drug policy always calls for assessing prescription practices through appropriate measurements, but poor data availability may be a limiting factor in resource-poor countries. Therefore, it is important to establish whether surrogate measures, such as drug supply data, can be used where routinely measuring prescription data is impossible. Our results confirm that supply data are relatively easy to obtain and yield a broad pattern of use that closely resembles the pattern revealed by direct observation. However, discrepancies were noted in the estimated use of some groups of medicines, and several factors could account for this.
First, “losses” occurred in the supply data study because some of the medicines either expired, were stolen or were used for conditions other than hypertension. Such losses would not have been captured by the prescription survey. Second, our prescription survey was conducted in only a subset of hospitals, since it was not feasible to perform prescription studies in the 54 hospitals included in the supply data study. Third, supply data were obtained for a 6-month period, whereas the hospital prescription surveys took place on a single day. Variation over these unequal periods possibly accounts for some differences, even though one indicator – the proportional use of methyldopa – had been stable over almost 2 years before the study started.
Our main conclusions based on supply data study and the results of the prescription survey are the same. Diuretics and ACE inhibitors are the two most frequently supplied or prescribed drugs for essential hypertension. All other anti-hypertensive agents are used much less frequently, although the two measurement methods do not yield the same relative order of use. As methyldopa is not listed in the hypertension guidelines for any indication other than gestational hypertension, any use outside of pregnancy is inappropriate. Both measurement methods identify high rates of methyldopa prescription as a problem. Likewise, reserpine is recommended in the guidelines as second-line therapy but is clearly not being used as such, and the recommended third-line drugs, the beta blockers, are also used less than expected.
To determine whether the guidelines for the treatment of hypertension are being followed, initial supply data are as useful as more time-consuming and labour-intensive prescription surveys. They might serve to indicate, at least, whether more precise and focused drug utilization studies are required to identify lack of adherence to specific recommendations.
Our findings reinforce those of earlier studies2–4 that hypertension guidelines were not being followed in some health settings. Reasons for this non-adherence can only be ascertained through a qualitative study. Finally, it is of interest that the costs of drug treatment were calculated to be higher by 19% than would have been the case if national guidelines had been followed. ■
Footnotes
Funding: Thamizhanban Pillay was supported by a Commonwealth of Australia postgraduate research scholarship awarded through AusAID. There were no other sources of funds.
Competing interests: None declared.
References
- 1.National Essential Drugs List Committee. South African Standard Treatment Guidelines and Essential Drugs List for Primary Health Care, 1998 edition Pretoria: National Department of Health; 1998. Available from: http://www.doh.gov.za/docs/factsheets/pharma/primary/edlphc1-35.pdf [accessed on 1 February 2009].
- 2.Lunt DW, Edwards PR, Steyn K, Lombard CJ. Fehrsen. Hypertension care at a Cape Town community health centre. S Afr Med J. 1998;88:544–8. [PubMed] [Google Scholar]
- 3.Naidoo DP, Randeree IGH, Reddy P, Singh A, Moss R. Blood pressure control at a hospital day clinic – a medical audit. Cardiovasc J S Afr. 1999;89(Suppl 1):C15–8. [Google Scholar]
- 4.Edwards PR, Lunt DW, Fehrsen GS, Lombard CJ, Steyn K. Improving cost-effectiveness of hypertension management at a community health centre. S Afr Med J. 1998;88:549–54. [PubMed] [Google Scholar]
- 5.Ross-Degnan D. Investigating drug use [Chapter 29]. In. Managing drug supply 2nd ed. West Hartford: Kumarian Press; 1997. pp. 431-449. [Google Scholar]
- 6.How to investigate drug use in health facilities: selected drug use indicators [EDM Research Series No. 7]. Geneva: World Health Organization; 1993. [Google Scholar]
- 7.Soumerai SB, Lipton HL. Computer-based drug utilization review – risk benefit or boondoggle? N Engl J Med. 1995;332:1641–5. doi: 10.1056/NEJM199506153322411. [DOI] [PubMed] [Google Scholar]