Abstract
Identifying another's mistakes requires a basic representation of other's action patterns as well as recognition and understanding of their failed goal-attainment. In previous work, we identified several regions, including inferior parietal cortex and rostral/ventral anterior cinguli (r/vACC), that show unique sensitivity to the observation of another's errors. Here we utilize the same sample to show that participants’ level of self-reported perspective-taking (but not empathic concern) correlated with hemodynamic response in IPC, while participants’ level of self-reported empathic concern (but not perspective taking) correlated with hemodynamic response in r/vACC. This functional dissociation provides strong evidence for separate roles for IPC and r/vACC in the processing of observed errors. IPC may foster a sense of agency by distinguishing self- from other-performed actions; r/vACC may, in turn, promote a more contextually-mediated understanding of the other's failed goal-attainment.
Keywords: empathy, perspective-taking, error-monitoring, cingulate, inferior parietal cortex, fMRI
Recent electrophysiological and neuroimaging work has been dedicated to identifying the neural circuitry underlying the observation and representation of other's actions. While much of this work has focused on demonstrating parallel neural responses during the performance and observation of actions (Grafton et al., 1996; Rizzolatti et al., 1996), more recent work has begun identifying those neural regions that show unique sensitivity during the observation, but not the performance, of given actions (Decety and Grezes, 1999; Shane et al., 2008). Indeed, a growing body of work has identified a distributed network, including cortical and limbic regions, that appears to show specific sensitivity during the observation of a wide variety of simple hand and body movements (Grafton et al., 1996; Rizzolatti et al., 1996; Iacoboni et al., 1999), as well as during more complex observations including movie clips displaying human actors (Iacoboni et al., 2004), joint attention experience (Williams et al., 2005) and judgments of strangers vs judgments about one's self (Ochsner et al., 2005; see also Devinsky et al., 1995; Craig, 2005). The prevailing logic has been that these regions of unique observer-related activity may underlie processes involved in basic and/or more contextual representations of another's actions.
Recent work in our laboratory has been tackling this problem by investigating those regions that show sensitivity when one commits, or observes another person committing, successful and failed attempts at goal-attainment (Shane et al., 2008). In this work, participants were asked to perform and/or observe a video of another person performing, a speeded Go/Nogo task designed to elicit errors. A wide body of electrophysiological and neuroimaging research has identified the dorsal anterior cingulate (dACC) as central to the processing of goal-directed errors (Falkenstein et al., 1991; Gehring et al., 1993; Carter et al., 1998; Holroyd and Coles, 2002; van Veen and Carter, 2002; Luu et al., 2003) and our work has converged with several recent ERP studies (Miltner, 2004; van Schie et al., 2004; Bates et al., 2005) in demonstrating similar dACC activity after watching another person make a mistake.
In addition, we identified several regions that showed specific sensitivity to the observation, but not to the performance, of goal-directed failures. The first area incorporated inferior parietal cortex (IPC) bilaterally; the second area incorporated a constellation of frontal regions comprising anterorostral and ventral cinguli (r/vACC) and adjacent medial-frontal cortex (Shane et al., 2008). That these regions showed sensitivity unique to the observation condition suggests that they do not underlie core error monitoring processes. Rather, consistent with a number of previous reports, we believe these regions may be fundamental to processes underlying basic and/or more contextually mediated representations of another's actions [(IPC: Ruby and Decety, 2001; Chaminade and Decety, 2002; Decety and Jackson, 2004; Lou et al., 2004) (r/vACC: (Moriguchi et al., 2006; Somerville et al., 2006)].
Importantly, the IPC and r/vACC activity identified in our previous study manifested under slightly different contexts. Specifically, bilateral IPC activity was witnessed when hemodynamic response unique to observed errors was compared to hemodynamic response unique to performed errors. This ‘Observed error – Performed error’ (Oe–Pe) contrast holds constant the processing of errors, and may thus be construed as interrogating regions that distinguish between first- and third-person performance. Consistent with other recent reports, we have hypothesized this IPC response to support generalized action representation, potentially in a manner that fosters a sense of agency (Ruby and Decety, 2001; Chaminade and Decety, 2002; Decety and Jackson, 2004; Lou et al., 2004). Indeed, we find similarly increased IPC activity when hemodynamic response to observed correct responses are compared to hemodynamic response to performed correct responses, as would be expected of such a generalized response (Shane, unpublished results).
In contrast, increased r/vACC activity was observed when hemodynamic response to observed errors was directly compared to hemodynamic response to observed correct responses. Unlike the previous contrast, this ‘Observed error – Observed correct (Oe–Oc) contrast holds constant the third-person perspective, and thus specifically interrogates regions that distinguish observed successful and unsuccessful action patterns. This distinction necessitates a more contextually mediated representation of the observed action (Somerville et al., 2006), and thus suggests a role for r/vACC in such contextually mediated processing. That r/vACC was particularly active during the observation of the actor's errors suggests that r/vACC may play an important role in the contextually mediated understanding of another's failed goal-attainment.
To formally evaluate these hypotheses, we collected self-report measures of perspective-taking and empathic-concern [as evaluated with the Interpersonal Reactivity Index (IRI) (Davis et al., 1999)] from the 20 participants who participated in our original study, described above. Here we report on the extent to which individual differences in these self-report measures moderated observer-related hemodynamic response within the IPC and r/vACC regions during error-observation. Our rationale was as follows: if IPC plays an important role in differentiating first- and third-person perspectives, then it may be that the magnitude of IPC response to observed errors would correlate with individual differences in perspective-taking ability. In a similar vein, if r/vACC plays an important role in more contextually-mediated processing of observed actions, with particular sensitivity to other's failures, then the magnitude of r/vACC response to observed errors may correlate with individual differences in empathic concern. Our hypotheses were thus 2-fold: First, we predicted that IPC activity in the Oe–Pe contrast would correlate with self-reported perspective-taking scores from the IRI. Second, we predicted that r/vACC activity in the Oe–Oc contrast would correlate with self-reported empathic-concern scores from the IRI.
METHOD
Participants
Participants were 20 healthy right-handed volunteers (14 men) ranging in age from 18–50 (M = 28.48; s.d. = 9.07) who had participated in a previous functional magnetic resonance imaging (fMRI) study at the Olin Neuropsychiatric Research Center. These participants were recontacted and asked to complete several additional self-report measures including the IRI (Davis et al., 1999). Recruitment was undertaken via advertisements and word of mouth at the Olin Neuropsychiatry Research Center (Hartford, CT). Participants provided written informed consent in protocols approved by Hartford Hospital's Institutional Review Board. All research procedures were conducted in adherence to ethical standards required for human subject protection.
Experimental design
In the performance condition, participants were required to perform two runs of a challenging Go/Nogo task designed to elicit errors. This task was identical to a paradigm described in previous reports (Kiehl et al., 2000; Bates et al., 2005; Shane et al., 2008) and involved the speeded, pseudo-random presentation of X's [the ‘Go’ stimulus, presented 412 times (∼80% of trials)] and K's [the ‘No-go’ stimulus, presented 78 times, (∼20% of trials)]. Stimuli were presented on-screen for 1000 ms, with an inter-stimulus interval jittered between 750 ms, 1000 ms and 1250 ms. Participants were instructed to go as quickly and as accurately as they could, and were reminded of this instruction between runs. All participants performed the Go-Nogo task before observing the actor performing the task.
In the observation condition, participants watched two 7 min videos portraying an actor (the first author) seated at a computer, performing the same Go-Nogo task described above. As in the performance condition, the actor was required to press the button on the response box with his index finger every time an X was presented, and to withhold his button-press every time a K was presented. Using a predetermined order, the actor successfully withheld his button-press for half of the K presentations (39 trials), and committed a button-press error for the other half of the K presentations (39 trials). While watching the video, the observer received the following instructions:
I’m now going to ask you to watch a video of another person performing the same task that you just performed. So in this video, another participant is going to be trying to press the button every time that an ‘X’ appears, and to stop that button press every time a ‘K’ appears. What I want you to do, while you’re watching this video, is to press one of two buttons every time a ‘K’ appears, to indicate whether the person in the video is able to withhold their button press response or not. If the person is successful, and is able to stop from pressing the button, then I want you to press the button under your index finger, because this button indicates that the person in the video was successful. If, however, the person makes a mistake, and is unable to stop their button press, then I want you to press the button under your middle finger, because this button indicates that the person failed.
Participant were then required to repeat the finger mappings back to the experimenter, and to indicate why they would press that button (i.e. ‘If the person is successful, and is able to stop from pressing the button, then I will press the button with my index finger. If, however, the person makes a mistake, and is unable to stop their button press, then I will press the button with my middle finger.’). This was required to maximize the extent to which participants would process the actor's responses in terms of ‘successes’ and/or ‘failures’.
MATERIALS
The videos were filmed with a Canon 2R40 digital video camera set up on a tripod behind the actor's right shoulder, and thus displayed an ‘over-the-shoulder’ view of the computer, and of task performance. The actor was facing the computer monitor, providing the participant with a view of the back of the actor's head, his right shoulder and arm, and his right hand. The actor's right hand rested on a four-button response box with which all responses were made (see Supplementary Figure 1 for a still-shot of the video). Effort was taken to ensure that the primary stimuli in the video—the presented Go/Nogo stimuli, and the actor's button press responses—were centered as closely as possible within the video frame. To this end, feedback from piloting, and from participants' performance, indicated that participants were fully capable of attending to both stimuli without decreasing identification of the actor's responses.
Measurement of perspective-taking and empathic-concern
Perspective-taking and empathic-concern were evaluated with the perspective-taking and empathic-concern subscales of the IRI (Davis et al., 1999). This scale was chosen because of its strong psychometric properties (Alterman et al., 2004), and to promote easy synthesis with relevant work that has also utilized this measure (e.g. Singer et al., 2004).
Imaging parameters
A Siemens Allegra 3T system located at the Olin Neuropsychiatry Research Center was utilized for the scanning protocol. Each participant's head was firmly secured using a custom head holder, and head motion was restricted using a custom-built cushion inside the head coil. Localizer images were acquired to determine functional image volumes. The echo planar image (EPI) gradient-echo pulse sequence (TR/TE = 1500/28 ms; flip angle = 65°; FOV = 24 × 24 cm2; 64 × 64 matrix; 3.4 × 3.4 mm2 in plane resolution; 5 mm effective slice thickness; 30 total slices) effectively covered the entire brain (150 mm) in 1.5 s. Each video lasted just over 7 min, or 281 scans. A 9 s rest period was included prior to the start of each run to allow for T1 effects to stabilize. The six initial images from these 9 s were not included in the reported analyses.
Image processing
Functional images were reconstructed offline and reoriented to approximately the anterior commissure/posterior commissure (AC/PC) plane. Functional image runs were motion corrected using an algorithm unbiased by local signal changes (INRIAlign; Freire and Mangin, 2001) as implemented in Statistical Parametric Mapping II (SPM2). No participants showed head movements in excess of 5 mm and were thus all retained for the analyses reported below.
A mean functional image volume was constructed for each run from the realigned image volumes. The mean EPI image was normalized to the EPI template. The spatial transformation into standard MNI space was determined using a tailored algorithm with both linear and nonlinear components (Friston et al., 1995). The normalization parameters determined for the mean functional volume were then applied to the corresponding functional image volumes for each participant. The normalized functional images were smoothed with a 9 mm full width at half-maximum (FWHM) Gaussian filter. Event-related responses were modeled using a synthetic hemodynamic response function composed of two gamma functions. The first gamma function modeled the hemodynamic response using a peak latency of 6 s. A term proportional to the derivative of this gamma function was included to allow for small variations in peak latency. The second gamma function and associated derivative was used to model the small ‘overshoot’ of the hemodynamic response on recovery. A low-pass (cutoff period 116 Hz) filter was applied to remove any high-frequency confounds. A latency variation amplitude-correction method was used to provide a more accurate estimate of hemodynamic response for each condition (Calhoun et al. 2004).
Data analytic strategies
Following preprocessing of the functional images, group analyses utilized a random effects model to determine voxel-wise t-statistics. At the first level, mean images corresponding to correct inhibitions and incorrect responses to ‘Nogo’ stimuli were computed, and condition-specific images were contrasted using the general linear model. Our a priori hypotheses were constrained to specific regions of interest (ROIs) within IPC and r/vACC. Central coordinates for these ROIs were based on previous work in our lab, which identified these regions as uniquely sensitive to the observation of error (p = 0.001, uncorrected; Shane et al., 2008; see Supplementary Table 1). Beta-weights representing peak activation within these ROIs were extracted from SPM2 and were correlated with participant's empathic concern and perspective taking scores within SPSS (Table 1 and Figures 1 and 2). Correlational analyses were used to evaluate peak activity within these ROIs against participants’ self-reported levels of perspective-taking and empathic-concern.
Table 1.
Whole-brain search for regions showing activity within the Oe–Pe contrast that correlated with empathic concern and/or perspective taking
| Region | L/R | Peak voxel coordinates | t-scores |
|---|---|---|---|
| Positive correlation with empathic concern | No regions reached threshold | ||
| Negative correlation with empathic concern | |||
| Hippocampus | L | −3, −3, −15 | 3.25 |
| Positive correlation with perspective taking | |||
| Parahippocampal Gyrus | L | −9, 0, 18 | 2.46 |
| PostCentral Cortex | R | 18, −45, 63 | 2.43 |
| Negative correlation with perspective taking | |||
| Superior Frontal Cortex | R | 18, 27, 42 | 2.62 |
| Middle Occipital Cortex | L | −45, −78, 36 | 2.57 |
| Inferior Parietal Cortex | L | −51, −39, 57 | 1.81 |
| Inferior Parietal Cortex | R | 54, −42, 57 | 1.79 |
Bolded regions small volume corrected, p < 0.05, for 10 mm sphere around the following coordinates: IPC coordinates: x = 45, y = −45, z = 60 and x = −45, y = −39, z = 60. All other activations p < 0.001, uncorrected for whole-brain volume.
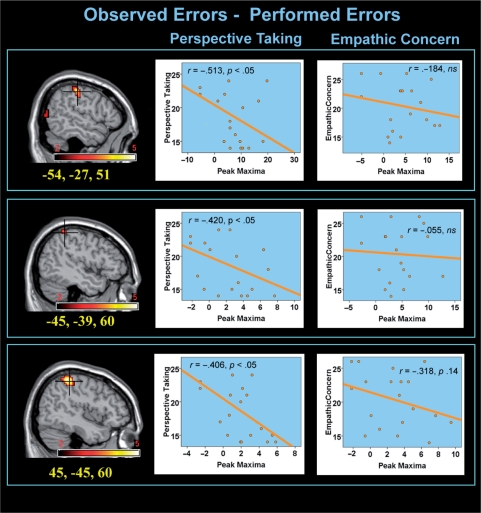
Fig. 1.
Significant relationships were found between relative IPC response to observed errors (compared to performed errors) and self-reported levels of perspective taking. Note that this ‘observed–performed’ contrast controls for error processing, and thus specifically interrogates differences between reported self- and other-perspective.
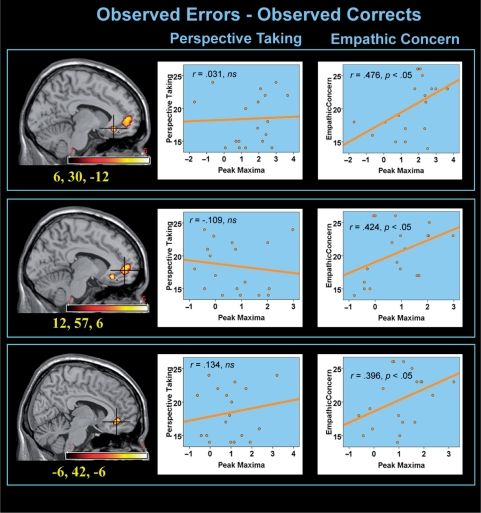
Fig. 2.
Significant relationships were found between relative r/vACC response to observed errors (compared to observed correct responses) and self-reported levels of empathic concern. Note that this ‘observed error–observed correct’ contrast controls for action observation, and thus specifically interrogates differences between the observation of another's successes and failures.
RESULTS
As displayed in Figure 1, participant's levels of self-reported perspective-taking, but not empathic concern, showed substantive correlations with peak IPC activity in the ‘Oe–Pe’ contrast. Specifically, individuals who rated themselves as more skilled at representing other's perspectives manifested decreased IPC activity in response to other's failures compared to their own failures (r = 0.41–0.46). Of particular import, this relationship between peak IPC activity and perspective-taking was not similarly identified when activity within the ‘Oe–Oc’ contrast was evaluated (during which the self/other perspective was held constant). Moreover, at no time did activity within these IPC regions show any correlation with level of empathic concern. Additional regions demonstrating correlations with IPC activity via a whole-brain search included parahippocampal gyrus and postcentral cortex, and are displayed in Table 1.
In contrast, and as displayed in Figure 2, participant's levels of self-reported empathic-concern, but not perspective taking, was related to peak r/vACC activity (and surrounding medial-frontal cortex) in the ‘Oe–Oc’ contrast (r = 0.41–0.51). Specifically, individuals who rated themselves higher in empathic concern showed increased r/vACC activation when witnessing the actor's mistakes. Importantly, this relationship was specific to the Oe–Oc contrast, and did not hold for r/vACC activity in the ‘Oe–Pe’ contrast, when the processing of errors was held constant (r = 0.01–0.11). Moreover, a similar relationship was not identified when performed errors were contrasted against performed correct responses (Shane et al., 2008). Thus, the correlation between r/vACC activity and empathic concern was quite specific to the observation of another's errors. Additional regions demonstrating correlations with r/vACC activity via a whole-brain search included middle temporal cortex and dorsal anterior cingulate, and are displayed in Table 2.
Table 2.
Whole-brain search for regions showing activity within the Oe–Oc contrast that correlated with empathic concern and/or perspective taking
| Region | L/R | Peak voxel coordinates | t-scores |
|---|---|---|---|
| Positive correlation with empathic concern | |||
| Middle temporal pole | R | 33, 3, −39 | 2.98 |
| Dorsal anterior cingulate | L | −9, 12, 21 | 2.84 |
| Middle temporal cortex | L | −45, −21, −18 | 2.72 |
| Middle temporal cortex | L | −48, −3, −30 | 2.66 |
| Ventral anterior cingulate | L | −12, 30, −9 | 2.16 |
| Ventral anterior cingulate/medial frontal cortex | R | −3, 33, −12 | 1.93 |
| Negative correlation with empathic concern | |||
| Supplementary motor area | L | −9, −12, 72 | 3.25 |
| Superior frontal cortex | L | −15, 21, 66 | 2.78 |
| Orbitofrontal cortex | L | −21, 57, 6 | 2.70 |
| Calcarine | R | 21, −102, 6 | 2.64 |
| Parahippocampal gyrus | L | −3, −6, −21 | 2.60 |
| Positive correlation with perspective taking | No regions reached threshold | ||
| Negative correlation with perspective taking | |||
| Parahippocampal gyrus | R | 15, −15, −30 | 3.99 |
| Parahippocampal gyrus | L | −18, 0, −27 | 3.63 |
| Cerebellum | R | 6, −21, −48 | 3.42 |
| Caudate | L | 0, 3, −15 | 3.42 |
| Vermis | L | −6, −45, −30 | 3.31 |
| Fusiform | R | 27, −33, −18 | 3.22 |
| Parahippocampal gyrus | R | 36, −42, −6 | 3.19 |
| Fusiform | L | −27, −48, −12 | 3.18 |
Bolded regions small volume corrected, p < 0.05, for 10 mm sphere around the following coordinates: Ventral ACC coordinates: x = 3, y = 23, z = −8. All other activations p < 0.001, uncorrected for whole-brain volume.
DISCUSSION
In previous research, we reported that IPC and r/vACC activity were associated with unique aspects of error-observation. IPC activity showed associations with error-observation in the ‘Oe–Pe’ contrast, which interrogated regions with specific sensitivity to differences between self- and other-performed actions; r/vACC, in contrast, showed associations with error-observation in the ‘Oe–Oc’ contrast, which interrogated regions with specific sensitivity to differences between the observation of successful and failed actions. This led us to postulate that IPC may be involved in the basic representation of observed actions, while r/vACC may be involved in more contextually-mediated representations of the actor's goal-attainment (or lack thereof). The present study was designed to elaborate on these postulations, and to further clarify the specific roles for IPC and r/vACC in action observation.
Role of IPC in action observation
Our first hypothesis was that if IPC was involved in differentiating first- and third-person perspectives, then individual differences in perspective-taking should correlate with IPC response to observed errors. Indeed, individuals who self-reported higher levels of perspective-taking tended to evidence greater reductions in IPC activity during the observation, compared to the performance, of committed errors. Importantly, participant's self-reported levels of empathic concern did not show similar relationships with IPC activity. Moreover, this IPC effect was not found when observed errors were contrasted with observed correct responses, a contrast that held third-person perspective constant. Together, these findings suggest a role for IPC in differentiating between one's own and another's performed actions, and support recent research suggesting that IPC may serve a fundamental role in fostering a sense of agency (Ruby and Decety, 2001; Chaminade and Decety, 2002; Decety and Jackson, 2004; Lou et al., 2004).
It may seem curious that the relationship between IPC activity and perspective taking was reciprocal. That is, those individuals who self-reported higher perspective-taking skills manifested decreased IPC activity to observed errors. At this point we can only speculate on the nature of this reciprocal relationship, but determining its underlying cause may be important for fully understanding the role of IPC in self/other distinctions. One possibility is that this is an example of neural efficiency, wherein those more skilled at perspective-taking are capable of undertaking the necessary action representation with a minimum of resource output (see Haier et al., 1992; Poldrack et al., 1998; Rypma et al., 2005 for theories regarding neural efficiency). A second possibility is that optimal perspective-taking may actually be represented neurally by similar IPC response to both first- and third-person representation. In this case, an increase in IPC activity during action observation may actually represent a deviation from this symmetry, and thus lead to inferior perspective-taking ability. Finally, it may be important to think carefully about other potential differences that exist between the performance and observation of action. For instance, it is possible that the observer may have experienced a conflict when they observed a behavior that was different from the behavior they were required to perform (i.e. they were asked to press a button to indicate when the actor in the video withheld their button press). While no evidence currently points to this IPC region in the experiencing or resolution of such conflict, this remains a possibility that future research would be apt to clarify.
Role of r/vACC in action observation
Our second hypothesis was that if r/vACC activity occurred in the course of developing a contextually mediated representation of the actor's successful or failed goal-attainment, then individual differences in empathic concern may correlate with r/vACC response to observed errors within the Oe–Oc contrast. Indeed, individuals who self-reported higher levels of empathic-concern tended to evidence increased r/vACC activity during the observation of goal-directed failures than to comparable goal-directed successes. Importantly, IPC activity was not similarly associated with empathic concern within this contrast. Moreover, empathic concern did not show a similar relationship with r/vACC activity in the Oe–Pe contrast. Together, this suggests that r/vACC activity was specifically attuned to differences in the outcome of the actor's actions.
One question concerns the extent to which r/vACC represents either a cognitive representation of the outcome of the actor's actions, or an emotional reaction to the actor's failed goal-attainment. The present data cannot speak conclusively to this issue, however, consideration of the items that comprise the empathic concern subscale of the IRI, which includes items focused heavily on the individual's emotional consideration of another's situation, make plausible an emotionally based interpretation. This interpretation coincides with the understanding that r/vACC is a cingulate region with close ties to limbic regions (Vogt et al., 1992), and with specific involvement in mood-related phenomena including recovery from major depressive states (Mayberg et al., 1997). Thus, while the current data can only go as far as to support a role for r/vACC in the contextually-mediated representation of observed action, it is worth postulating a more specific role for r/vACC in the manifestation of empathic concern.
IPC and r/vACC activity within the context of a broader neural system
It is worth noting that dACC also showed a positive relationship with the empathic concern subscale of the IRI. Rather than imposing a specific role for dACC in empathic processing, we suggest that this dACC response may indicate that individuals with greater empathic concern showed heightened monitoring of the actor's incorrect responses. With this in mind, it is interesting to consider the composition of a distributed neural network underlying error-monitoring. Dorsal ACC, sensitive to both self- and other-committed errors, appears to serve as a generalized error detector (Ruby and Decety, 2001; Chaminade and Decety, 2002; Decety and Jackson, 2004; Lou et al., 2004). When the error is one's own, dACC error-detection may be quite direct. When the error is committed by another person, however, additional information may often be necessary before identification of success or failure can be completed. Within this context, we suggest that dorsal ACC may exist as a high-level component within a distributed network, and may utilize inputs from regions including IPC and r/vACC toward determination of success and failure. Alternately, r/vACC activity may represent processing subsequent to dorsal ACC, and may be related to the extent to which recognition of the other's errors initiates empathic concern in the observer. Future research utilizing techniques with temporal specificity greater than that of fMRI, such as EEG, may prove capable of evaluating these competing hypotheses.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Supplementary Material
REFERENCES
- Alterman A.I., McDermott P.A., Cacciola J.S., Rutherford M.J. Latent structure of the Davis Interpersonal Reactivity Index in methadone maintenance patients. Journal of Psychopathology and Behavioral Assessment. 2004;25:257–5. [Google Scholar]
- Bates AT, Patel TP, Liddle PF. External behavior monitoring mirrors internal behavior monitoring error-related negativity for observed errors. Journal of Psychophysiology. 2005;19:281–8. [Google Scholar]
- Carter C.S., Braver T.S., Barch D.M., Botvinick M.M., Noll D., Cohen J.D. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–9. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Chaminade T., Decety J. Leader or follower? Involvement of the inferior parietal lobule in agency. Neuroreport. 2002;13:1975–8. doi: 10.1097/00001756-200210280-00029. [DOI] [PubMed] [Google Scholar]
- Craig A.D. Forebrain emotional asymmetry: a neuroanatomical basis? Trends in Cognitive Sciences. 2005;9:566–71. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Davis M.H., Mitchell K.V., Hall J.A., Lothert J., Snapp T., Meyer M. Empathy, expectations, and situational preferences: personality influences on the decision to participate in volunteer helping behaviors. Journal of Personality. 1999;67:469–503. doi: 10.1111/1467-6494.00062. [DOI] [PubMed] [Google Scholar]
- Decety J., Grezes J. Neural mechanisms subserving the perception of human actions. Trends in Cognitive Sciences. 1999;3:172–8. doi: 10.1016/s1364-6613(99)01312-1. [DOI] [PubMed] [Google Scholar]
- Decety J., Jackson P.L. The functional architecture of human empathy. Behavioral and Cognitive Neuroscience Reviews. 2004;3:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Devinsky O., Morrell M.J., Vogt B.A. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Falkenstein M., Hohnsbein J., Hoormann J., Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78:447–55. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Freire L., Mangin J.F. Motion correction algorithms may create spurious brain activations in the absence of subject motion. Neuroimage. 2001;14:709–22. doi: 10.1006/nimg.2001.0869. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Frith C.D., Frackowiak R.S., Turner R. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage. 1995;2:166–72. doi: 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–90. [Google Scholar]
- Grafton S.T., Arbib M.A., Fadiga L., Rizzolatti G. Localization of grasp representations in humans by positron emission tomography. 2. Observation compared with imagination. Experimental Brain Research. 1996;112:103–11. doi: 10.1007/BF00227183. [DOI] [PubMed] [Google Scholar]
- Haier R.J., Siegel B.V., Jr, MacLachlan A., Soderling E., Lottenberg S., Buchsbaum M.S. Regional glucose metabolic changes after learning a complex visuospatial/motor task: A positron emission tomographic study. Brain Research. 1992;570:134–43. doi: 10.1016/0006-8993(92)90573-r. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Coles M.G. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Iacoboni M., Lieberman M.D., Knowlton B.J., Molnar-Szakacs I., Moritz M., Throop C.J., et al. Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. Neuroimage. 2004;21:1167–73. doi: 10.1016/j.neuroimage.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Iacoboni M., Woods R.P., Brass M., Bekkering H., Mazziotta J.C., Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–8. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Kiehl K.A., Liddle P.F., Hopfinger J.B. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology. 2000;37:216–23. [PubMed] [Google Scholar]
- Lou H.C., Luber B., Crupain M., Keenan J.P., Nowak M., Kjaer T.W., et al. Parietal cortex and representation of the mental self. Proceedings of the National Academy of Sciences. 2004;101:6827–32. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu P., Tucker D.M., Derryberry D., Reed M., Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychological Science. 2003;14:47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Mayberg H.S., Brannan S.K., Mahurin R.K., Jerabek P.A., Brickman J.S., Tekell J.L., et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–61. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Miltner W., Brauer J., Hecht H., Trippe R., Coles M.G.H. Parallel brain activity for self-generated and observed errors. In: Falkenstein M.U.M., editor. Error, Conflict and the Brain. MPI of Cognitive Neuroscience: Leipzig; 2004. pp. 124–28. [Google Scholar]
- Ochsner K.N., Beer J.S., Robertson E.R., Cooper J.C., Gabrieli J.D.E., Kihsltrom J.F., et al. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Poldrack R.A., Desmond J.E., Glover G.H., Gabrieli J.D. The neural basis of visual skill learning: an fMRI study of mirror reading. Cereb Cortex. 1998;8:1–10. doi: 10.1093/cercor/8.1.1. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Fadiga L., Matelli M., Bettinardi V., Paulesu E., Perani D., et al. Localization of grasp representations in humans by PET: 1. Observation versus execution. Experimental Brain Research. 1996;111:246–52. doi: 10.1007/BF00227301. [DOI] [PubMed] [Google Scholar]
- Ruby P., Decety J. Effect of subjective perspective taking during simulation of action: a PET investigation of agency. Nature Neuroscience. 2001;4:546–50. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- Rypma B., Berger J.S., Genova H.M., Rebbechi D., D’Esposito M. Dissociating age-related changes in cognitive strategy and neural efficiency using event-related fMRI. Cortex. 2005;41:582–94. doi: 10.1016/s0010-9452(08)70198-9. [DOI] [PubMed] [Google Scholar]
- Shane M.S., Stevens M., Harenski C.L., Kiehl K.A. Neural correlates of the processing of another's mistakes: A possible underpinning for social and observational learning. NeuroImage. 2008;42:450–59. doi: 10.1016/j.neuroimage.2007.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T., Seymour B., O’Doherty J., Kaube H., Dolan R.J., Frith C.D. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience. 2006;9:1007–8. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- van Schie H.T., Mars R.B., Coles M.G., Bekkering H. Modulation of activity in medial frontal and motor cortices during error observation. Nature Neuroscience. 2004;7:549–54. doi: 10.1038/nn1239. [DOI] [PubMed] [Google Scholar]
- van Veen V., Carter C.S. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Vogt B.A., Finch D.M., Olson C.R. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–43. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Williams J.H.G., Waiter G.D., Perra O., Perrett D.I., Whiten A. An fMRI study of joint attention experience. Neuroimage. 2005;25:133–40. doi: 10.1016/j.neuroimage.2004.10.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.