Abstract
Effective photojournalism provokes an emotional reaction and leaves a lasting impression upon the viewer. Striking and memorable images are often said to possess ’impact’. Within cognitive neuroscience memorable emotional images evoke a greater amygdala response. Research to date has focused on arousal as a causative factor, while the contribution of appraisal dimensions relating to salience of an item, goal relevance, or impact are yet to be addressed. We explored how differences in ratings of impact influenced amygdala activity to negative emotional images matched for valence, arousal and other factors. Increased amygdala activation was found to high impact when compared to neutral images, or high impact when compared to low impact images (matched for arousal). Our findings demonstrate that the amygdala response to emotional stimuli is not a function of arousal (or valence) alone and accord more with the proposal that the amygdala responds to the significance or relevance of an event.
Keywords: Emotion, Arousal, Attention, Appraisal, fMRI
INTRODUCTION
The power of photojournalism lies in its ability to provoke an instant emotional reaction and to leave a lasting impression upon the viewer. Photographs that are particularly striking or memorable are often said to possess ‘impact’ (Hurley and McDougall, 1971). Artists and media professionals have long appreciated the attention grabbing capacity of visual images; placing particular emphasis on the impact of an image when creating and selecting pictures for use in the arts, media and advertising. In this study, we were interested in investigating the neural signature underlying the sense of impact generated when viewing images of the type that appear in news media.
Within psychological research, attempts to categorize emotional experience have largely focused on the role of arousal and valence. These two dimensions have long been considered central to emotional experience (e.g. Schlosberg, 1952); indeed, some current models claim that these dimensions alone can be used to categorize all affective experiences (Russell, 2003). Appraisal theorists have attempted to identify various criteria upon which the antecedent conditions of different emotional experiences and reactions are appraised. A large number of specific dimensions have been proposed (e.g. Scherer, 1999), and a number of these appear related to the concept of impact; emphasizing the significance or relevance of an event to the individual, such as goal relevance, novelty, unpredictability, compatibility/incongruity in relation to the self-concept or social norms (Lazarus, 1966). While there is considerable overlap between these dimensions and those that have been addressed in wider research on attention and memory, research on emotional images and concepts has focused almost exclusively on the causal role of arousal and valence. Experimental evidence provides support for the view that stimuli perceived as more arousing attract attention and are better recollected than non-arousing stimuli (Anderson, 2005; Bradley et al., 1992; Ochsner, 2000; Arnell et al., 2007). However, the contribution of various appraisal variables in these paradigms remains to be fully explored.
As discussed, there are parallels between the appraisal dimensions of significance, relevance, novelty or incongruity and the concept of impact as commonly used in visual media. Specific images may affect viewers on the basis of one or more of these attributes in ways that are not directly coupled to increased arousal. Indeed, two recent studies have found that ratings of impact proved to be a better predictor than arousal of what attracts attention to valenced emotional pictures (Murphy et al., submitted for publication) and also a better predictor than arousal of recollection using a remember/know paradigm (Croucher et al., 2006). To this extent, Croucher et al. (2006) have argued that impact may provide a convenient shorthand for the immediate significance and relevance of an item to the individual.
Within cognitive neuroscience, research addressing memory and attention for emotional material has focused on the role of the amygdala. This is thought to play an important role in directing attention towards emotional stimuli, and subsequent recollection of emotional material (Cahill et al., 1995; Phelps et al., 1997; Adolphs et al., 2001; Anderson and Phelps, 2001; Hamann, 2001); however, see Papps et al. (2003). Explanations of amygdala involvement in processing emotional images have focused on arousal as the mediating factor; however, previous studies have failed to differentiate between the influence of arousal and that of other factors that may influence the salience, or impact, of an image. Indeed, Sander et al. (2003) have proposed that the amygdala is involved in the appraisal of relevance, and codes the significance or goal-relevance of stimuli, rather than arousal per se.
Although this hypothesis has not been tested directly using stimuli equated on the dimensions of arousal and valence, recent research accords with their proposal. For example, single-cell recording in monkeys has shown that neurons in the amygdala are more sensitive to unexpected than expected presentations of both positive and negative stimuli (Belova et al., 2007). Similarly, intracranial recordings in humans have demonstrated that the N2-P300 component in the amygdala is larger to rare attended events, especially if they are in some way ‘bizarre’ (Stapleton and Halgren, 1987). The P300 scalp component is thought to emanate from the temporoparietal junction (TPJ) (Soltani and Knight, 2000), and has been shown to be particularly sensitive to novelty detection (Halgren and Marinkovic, 1995). Such findings indicate that the hypothesized dependence on arousal at a behavioural and neural level in both attention to and recollection of emotional stimuli may well have been over-emphasized at the expense of the influence of other variables and dimensions.
In the current study, we addressed whether overall ratings of impact can capture the variables that contribute to the neural signature underlying the processing of emotional images. To investigate this, we used negative emotional images taken from the IAPS image database (Lang et al., 1999) and other sources. Images were matched for ratings of several factors—arousal, valence, distinctiveness, visual complexity and approach/avoidance—but differed on subjective ratings of impact. Consistent with the role of the amygdala in the processing of highly significant and relevant stimuli, our prediction was that the amygdala response to negative high impact images would be greater than that to negative low impact images. By contrast, if the amygdala response is dependent upon perceived levels of arousal (or valence), then we would expect no difference between the high and the low impact images when matched on these dimensions.
MATERIALS AND METHODS
Participants
Twenty-four healthy volunteers (14 female, all right-handed, aged 19–41, mean age = 26.1) with normal, or corrected to normal vision participated in this study. No participants had a history of neurological disease or head injury or were currently on medication affecting the central nervous system. The data from two participants were excluded due to scanner malfunction. The study was approved by Cambridgeshire Local Research Ethics Committee. All volunteers provided written informed consent.
Task design
Thirty high and 30 low impact images were selected from a set of 213 scenes that had previously been independently rated for arousal, valence, visual distinctiveness, visual complexity, approach/avoidance and impact (Croucher et al., 2006). The ratings on most of these dimensions made use of well-established scales. Ratings of impact were obtained using a new scale in which participants were asked to consider each picture as a whole and to judge whether they felt the content of the image created an instant sense of impact on them personally. They were asked not to think in detail about the picture or its contents in terms of particular properties like the positive or negative feelings it might invoke (e.g. fear, anger, joy, etc.), how distinctive the image was or how many thoughts and ideas it led to. An intraclass correlation showed that the consistency of impact ratings across raters was highly significant (P < 0.001, Prep = 0.98). Ratings were not obtained from participants used in the scanning study as explicit processing of emotional content has been shown to reduce activation in the amygdala (Critchley et al., 2000). It was also important that participants only viewed each image once whilst in the scanner, and therefore ratings were not made before or after the scanning session as repeated viewing of the images would eliminate the novelty and immediacy that contributes to the sense of impact. Fifty-five of the 60 negative images were taken from the International Affective Picture System (IAPS) database (Lang et al., 1995), and image IDs are available from the authors on request; the remaining negative and neutral images were acquired from various sources including the world wide web.
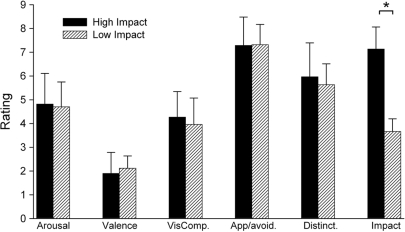
High and low impact images differed significantly on ratings of impact [t(58) = 17.71, P < 0.005, Prep = 0.96, d = 1.82], but were matched for ratings on all other dimensions (P-values > 0.25; Figure 1). Similarly, an analysis of the images from IAPS (n = 55) also showed that high and low impact images did not differ on the normative ratings of a set of arousal and valence (P-values > 0.3) (Lang et al., 1995). Thirty neutral images were also included. All sets of images were matched for the presence of people and faces. In order to examine low-level differences between image sets, we also performed an analysis on the mean luminance, global energy and colour composition of the images. This confirmed that low level features were not a confounding influence on the interpretation of the results (see Supplementary Data). An additional 30 neutral filler images were used in order to balance the number of negative and neutral upright images throughout the scan. Finally, 30 neutral inverted images were also presented. This gave a total of 150 images (30 high impact, 30 low impact, 30 neutral, 30 fillers, 30 inverted neutral).
Fig. 1.
Mean ratings for the negative emotional images used in this study. Error bars show s.d.
Subjects lay supine in the magnet bore and viewed images projected onto a screen visible via an angled mirror placed above the head of the participant. On each trial, an image appeared for a period of 1000 ms, followed by a blank screen containing a central fixation cross shown for 2500 ms. Each image subtended a visual angle of ∼12°× 9°. Images were presented in a pseudo-randomized order throughout the scan. The task of the subject was to respond, via a button press, whenever an inverted image appeared. The total scanning time was 10 min and 30 s.
Imaging parameters
MRI scanning was performed on a Siemens Tim Trio 3-Tesla MR scanner. Whole brain data were acquired with T2*-weighted echo-planar imaging (EPI) sensitive to BOLD signal contrast. Each image volume consisted of 46 descending 3 mm thick slices (gap 25%; FOV 192 × 192 mm2; voxel size 3 × 3 × 3 mm3; flip angle 78°; TE 30 ms; TR 2800 ms). Slices were acquired in a coronal orientation to optimize amygdala signal (Merbolt et al., 2001). The first three volumes were discarded to allow for the effects of magnetic saturation. T1-weighted structural images were acquired at a resolution of 1 × 1 × 1 mm3.
Image analysis
Data were analyzed using SPM 5 software (Wellcome Trust Centre for Neuroimaging, London, UK). Standard pre-processing was applied, including correction for slice-timing and head motion. Undistortion based on a field strength derived from a phase map was applied (Cusack et al., 2003). Each scan of the participants was normalized to the Montreal Neurological Institute (MNI) —ICBM avg152 T1-weighted template, using 2 mm isotropic voxels and smoothed with a Gaussian kernel of 8 mm full-width half-maximum. Individual events were modelled with a canonical hemodynamic response function. Temporal derivatives of these regressors, along with realignment parameters were also included as effects of no interest to account for motion-related variance. A high pass filter of 128 s was used to remove low-frequency noise. A random effects analysis (one-sample t-test) was performed to analyze data at a group level. All activations are reported using a threshold of P < 0.001 uncorrected. Amygdala ROIs were defined using the Automatic Anatomical Labelling (AAL) template (Tzourio-Mazoyer et al., 2002), and small volume corrections were applied.
RESULTS
Behavioural results
Behavioural data indicated that participants were successfully able to perform the inverted image detection task, with a correct response rate of 95.8% (±1.2 s.d.) and a mean response time of 731.2 ms (±24 s.d.).
Amygdala responsivity to impact
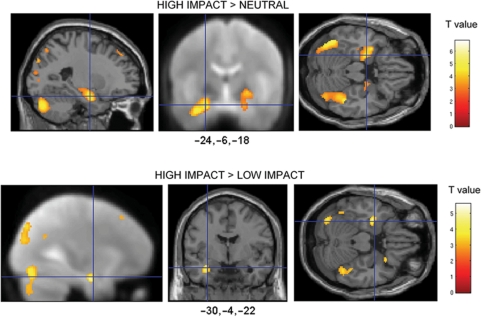
In order to determine how the amygdala response to emotional images differed according to the rated impact of the image, we performed a random effects analysis (one-sample t-test) across all participants. Firstly, we investigated the amygdala response during the presentation of negative high impact images when compared to neutrally valenced images. Consistent with our predictions, we found a significantly larger response to high impact images when compared to neutral images in the left amygdala/anterior hippocampus [–24, –6, –18, t(21) = 4.79, P < 0.005 svc; Prep > 0.96, d = 1.0], and right amygdala [28, –8, –12, t(21) = 3.62, P < 0.03 svc; Prep > 0.90, d = 0.79] (Figure 2A). We found only a borderline significant increase in left amygdala to low impact images when compared to neutral images [–26, –8, –12, t(21) = 2.68, P = 0.06 svc, Prep > 0.86, d = 0.57].
Fig. 2.
Activation maps for the contrast (A) high impact vs. neutral images and (B) high impact vs. low impact images. (Left to right show sagittal, coronal and tranverse sections of a canonical weighted T1 image or the average normalized mean EPI across all participants.) Maps are thresholded at P < 0.005 (10 contiguous voxels) for display purposes.
Finally, we compared the response of high impact images with low impact images, thus determining whether the amygdala response to negative images that were matched for arousal and valence, and other factors, would be modulated by differences in impact. The results revealed a significant increase in the left amygdala response [–30, –4, –22, t(21) = 3.34, P < 0.03 svc, Prep > 0.90, d = 0.71] (Figure 2B). Other regions showing significant activation are shown in Table 1.
Table 1.
Significant activations for high impact and low impact images
| Region | MNI coordinates |
|||
|---|---|---|---|---|
| x | y | z | t-value | |
| High impact > neutral | ||||
| Left mid-temporal gyrus | –44 | –66 | –12 | 6.89 |
| Right fusiform gyrus | 48 | –38 | –18 | 5.55 |
| Left cerebellum | –18 | –74 | –34 | 5.46 |
| Left amygdala/anterior hippocampus | –24 | –6 | –18 | 4.99* |
| Left superior frontal gyrus | –20 | 44 | 46 | 4.54 |
| Right inferior parietal lobe | 34 | –66 | 24 | 4.29 |
| Precuneus | –16 | –50 | 44 | 4.17 |
| Left inferior parietal lobe (TPJ) | –46 | –58 | 26 | 4.11 |
| Right inferior parietal lobe (TPJ) | 46 | –52 | 22 | 3.90 |
| Right amygdala | 28 | –8 | –12 | 3.62* |
| High impact > low impact | ||||
| Right inferior temporal gyrus | 44 | –72 | 4 | 4.77 |
| Left posterior parietal lobe | –38 | –78 | 38 | 4.66 |
| Posterior cingulate gyrus | 2 | –42 | 34 | 4.55 |
| Right fusiform gyrus | 44 | –52 | –14 | 4.53 |
| Left superior frontal gyrus | –36 | 16 | 54 | 4.18 |
| Left inferior temporal gyrus | –56 | –48 | –10 | 4.13 |
| Right superior frontal gyrus | 22 | 40 | 50 | 4.05 |
| Right inferior parietal lobe (TPJ) | 46 | –62 | 32 | 3.83 |
| Left amygdala | –30 | –4 | –22 | 3.34* |
| Low impact > neutral | ||||
| Right superior occipital lobe | 50 | –74 | 14 | 3.96 |
All values reported at P < 0.001 uncorrected, minimum 10 contiguous voxels.
*P < 0.05 amygdala small volume correction
Other brain regions
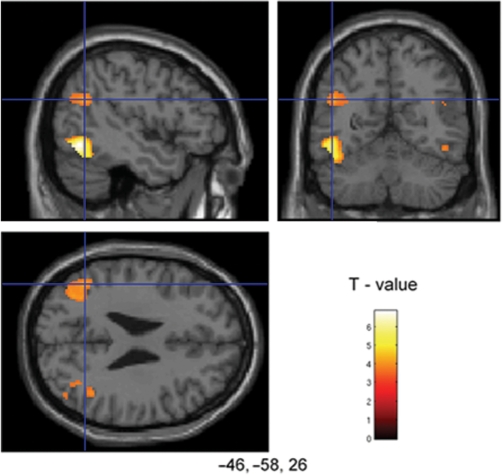
We also found increased activity in occipitotemporal cortex when participants were viewing high impact images when compared to neutral or low impact images (Table 1). This included activity in the lateral occipital cortex and posterior fusiform gyrus, regions that have been shown to be particularly sensitive to emotional and attentional modulation, thought to be mediated by projections from the amygdala (Amaral et al., 2003; Vuilleumier et al., 2004). We also found increased bilateral activation in the posterior parietal lobe, in the region of the TPJ when participants were viewing high impact images when compared to neutral images or low impact images (Figure 3 and Table 1). The TPJ responds preferentially to relevant stimuli and is thought to act as a ‘circuit breaker’; disrupting ongoing processes and orientating attention to behaviourally salient stimuli (Corbetta and Shulman, 2002).
Fig. 3.
Activation in TPJ (–46, –58, 26) for high impact when compared to low impact images shown on sagittal, transverse and coronal sections of a canonical weighted T1 image. Images are thresholded at P < 0.001 uncorrected (10 contiguous voxels).
DISCUSSION
The aim of this study was to investigate the neural signature underlying the sense of impact generated by viewing negative images. Negative high impact images produced a greater amygdala response than neutral images or low impact images. Moreover, a difference between high and low impact images was found despite the two sets being matched for ratings of arousal, pleasantness (valence) and other dimensions (approach-avoidance, distinctiveness, and visual complexity). Consistent with previous research showing an increased amygdala response to negative images, a comparison of low impact and neutral images showed a borderline increase in the amygdala. Our findings indicate that contrary to previous assumptions, the amygdala response to emotional images is not attributable primarily to their perceived arousal value alone, and that impact ratings, a generic index similar to a number of cognitive appraisals, provide a highly effective predictor of activation in this region. Indeed, as far as we are aware, our study is the first to show differential activation in the amygdala for a comparison of two sets of negative emotional images matched for arousal and valence. In addition, our results accord with behavioural evidence indicating that impact provides a better predictor than arousal for both recollection of and attention to emotional images (Croucher et al., 2006; Murphy et al., submitted for publication)
While a number of studies have tended to emphasize a role for the amygdala in coding the perceived arousal of a stimulus, our findings accord more with the Sander et al. (2003) suggestion that the amygdala may contribute to the appraisal of the significance or relevance of an event. Earlier, we discussed work showing an amygdala response to unusual, unpredictable, or bizarre stimuli. Intracranial recordings have shown that this is associated with the N2-P300 response. Given that the primary cortical source of the P300 is the TPJ, it is of particular note that high impact images also engaged this region (relative to neutral or low impact conditions). The TPJ is thought to play a general role in orientating attention to behaviourally salient stimuli (Corbetta and Shulman, 2002). In the current study, the impact (or emotional content) of the images was irrelevant to task (detection of inverted images); hence, TPJ involvement accords with additional work showing that high impact images promote attentional capture (Murphy et al., submitted for publication). Consistent with this, a recent study also indicates that the biological relevance of an image captures attention, rather than the aversive properties (Brosch et al., 2008). In the current study, high impact images also produced increased activation in extrastriate cortex, which has previously been associated with increased visual attention as a result of feedback from the amygdala (Vuilleumier et al., 2004) or parietal cortex (Ruff et al., 2007).
Additional evidence that the amygdala is responsive to significant, behaviourally relevant, or potentially ‘high impact’ stimuli comes from work showing that it is responsive to salient, infrequent novel events (Laurens et al., 2005); the same study also showed the activation of the TPJ. Wright and Liu (2006) have shown that the amygdala responds to neutral, but behaviourally relevant face stimuli, while in the gustatory and olfactory domains, amygdala activation to pleasant and unpleasant tastes and smells is predicted by their perceived intensity (Anderson et al., 2003; Small et al., 2003). Although intensity has been interpreted as being similar to arousal (Hamann, 2003), it should be noted that the two are not always equated. Rather, as suggested by Reisenzein (1994), intensity may reflect a contribution of both valence and arousal. For example, while fear is associated with increasing arousal and decreasing valence, increasing intensity of sadness is accompanied by decreasing arousal and valence. Thus, intensity may accord more with the concept of impact than arousal.
The amygdala is a heterogeneous structure, and as such it should be noted that our results are unable to speak as to the functional role of different nuclei within this region. It is possible that some regions of the amygdala may be more responsive to the arousal value or relevance of a stimulus than others. Similarly, due to limitations in the temporal resolution of fMRI, it is not possible to determine whether different properties of the stimulus, such as impact and arousal, may be coded at different time points.
Previous work has also shown increased amygdala activation to emotionally neutral ‘high-interest’ unusual photographs, such as surreal scenes (Hamann et al., 2002); however, the unusual images in this study were not compared with control images matched for arousal and valence, and so a contribution from the latter dimensions cannot be discounted. In the current study, we used the same set of negative images used in previous behavioural work. While the implications of our current findings cannot be generalized beyond unpleasant emotional images, Hamann et al. (2002) suggest that a comparable effect may be found for neutral or positive high impact images; an issue that further studies could address. Future investigations could also explore the extent to which the impact of images can be modulated by personal relevance, similar to the memory bias observed when presenting personally relevant stimuli (Conway et al., 2001). Interestingly, significance and relevance have also been emphasized as important factors in work investigating product purchases and the effects of advertising (Zaichkowsky, 1994), and further demonstrating the central role of these components in existing media strategies.
The term impact is borrowed from photojournalism where it is used to describe powerful and striking images. In the Introduction, we noted that the concept of impact has considerable overlap with a constellation of appraisal dimensions that are involved in evaluating the significance and relevance of emotional events. We do not wish to claim that these appraisal dimensions can be condensed into a unitary ‘impact’ appraisal dimension, and as with the debate among appraisal theorists (Scherer, 1999) regarding the precise definition of appraisal dimensions, the numbers and identities of specific properties that contribute to the impact of the image remain to be resolved. For example, as suggested by appraisal theory (Scherer, 1999), the significance of material may derive from some discrepancy with mental models of the self, others and the world. Similarly, the presence of ambiguity or uncertainty about how to interpret the material or how one might behave in the depicted setting may also influence the significance of an event. Images that are unusual, striking or incongruent with existing knowledge may therefore have particularly high impact, indeed, effective photographs in news media are said to inspire ‘curiosity about the causes and effects of the depicted situation’ (Zillmann et al., 2001).
A central feature of ratings of impact used in our research is that viewers are asked to provide an instant, non-deliberative judgement of the extent to which they are affected by an image. While arousal and valence may be two ways in which people are affected, those contributions are controlled for here by matching high and low impact images on both of these dimensions. It is also worth considering that the instant nature of the judgement could itself be important, perhaps reducing the influence of rational, ‘cold’ deliberations, based on conceptual content, and encouraging participants to rate impact according to the more abstract senses or feelings evoked by that content—a distinction that is made in multilevel theories concerning the relationship between the cognition and the affect (for review see Teasdale, 1999).
In the current report, we have followed conventional practice in the majority of behavioural and neuroimaging studies on attention and memory for emotional material (e.g. Anderson and Phelps, 2001; Ochsner, 2000; Anderson, 2005; Arnell et al., 2007) by relying on ratings of arousal, and in our case also of impact. Arousal ratings for IAPS material have been found to show a positive correlation with changes in skin conductance (r = 0.81) (Lang et al., 1993). Consistent with this, preliminary work in our laboratory has revealed an increased skin conductance response to high and low impact images when compared with neutral images, according to the established psychophysiological findings. However, we were unable to find any difference between the high and the low impact images matched on rated arousal, indicating that our impact effects cannot readily be attributable to a confound at this level. Rated impact, by the very way in which it is measured, emphasizes being affected by an image, but does not restrict the rater to specific dimensions (e.g. excitement or calmness) related to physiological arousal. All that matters is that they are affected in one way or another. It should be noted that our results do not suggest that ratings of impact and arousal and valence are entirely independent. Previous work on appraisal criteria has demonstrated a quadratic non-linear relationship between ratings on whether an image had an ‘effect or impact’ on the viewer, and rated arousal (Scherer et al., 2006). We propose that very high arousal images are unlikely to be dissociable from high impact images. By its very nature, an image or event that is extremely highly arousing is likely to have high relevance to the viewer.
In summary, we have shown that the media concept of impact that resembles appraisal variables other than arousal and valence can be characterized by a measurable neural response, involving brain regions involved in attentional orienting (TPJ) and emotional responsivity (amygdala). These findings accord with behavioural data demonstrating the predictive value of impact in both attention and memory studies. Our results demonstrate that the amygdala response to emotional stimuli is difficult to interpret in relation to arousal alone, and accord with the Sander et al. (2003) proposal that the amygdala may contribute to the appraisal of the significance or relevance of an event. Importantly, our results are difficult to reconcile with two-dimensional models of emotion, which predict that the affective quality of a stimulus can be fully characterized using the dimensions of arousal and pleasantness. Instead, our results suggest that future research should take a closer look at other theoretical approaches to emotion, including dimensions related to the appraisal of relevance, novelty and unpredictability of a stimulus.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Supplementary Material
ACKNOWLEDGEMENT
This research was funded by the UK Medical Research Council under project code U.1055.02.001.0001.01 (A. J. C.). We wish to thank Lauri Nummenmaa for his kind assistance in analysis of the image sets, Jill Keane and Luca Passamonti for their help in subject recruitment and data collection, and the CBU radiographers for their assistance in this project.
REFERENCES
- Adolphs R, Denburg NL, Tranel D. The amygdala's role in long-term declarative memory for gist and detail. Behavioral Neuroscience. 2001;115:983–92. doi: 10.1037//0735-7044.115.5.983. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Behniea H, Kelly JL. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience. 2003;118:1099–20. doi: 10.1016/s0306-4522(02)01001-1. [DOI] [PubMed] [Google Scholar]
- Anderson AK. Affective influences on the attentional dynamics supporting awareness. Journal of Experimental Psychology: General. 2005;134:258–81. doi: 10.1037/0096-3445.134.2.258. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, et al. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–9. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Arnell KM, Killmana KV, Fijavz D. Blinded by emotion: Target misses follow attention capture by arousing distractors in RSVP. Emotion. 2007;7:465–77. doi: 10.1037/1528-3542.7.3.465. [DOI] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–84. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Greenwald MK, Petry M, Lang PJ. Remembering pictures: Pleasure and arousal in memory. Journal of Experimental Psychology: Learning, Memory and Cognition. 1992;18:379–90. doi: 10.1037//0278-7393.18.2.379. [DOI] [PubMed] [Google Scholar]
- Brosch T, Sander D, Pourtois G, Scherer KR. Beyond fear: rapid spatial orienting toward positive emotional stimuli. Psychological Science. 2008;19:362–70. doi: 10.1111/j.1467-9280.2008.02094.x. [DOI] [PubMed] [Google Scholar]
- Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. The amygdala and emotional memory. Nature. 1995;377:295–6. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- Conway MA, Dewhurst SA, Pearson N, Sapute A. The self and recollection reconsidered: How a ‘failure to replicate’ failed and why trace strength accounts of remembering are untenable. Applied Cognitive Psychology. 2001;15:1–14. [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Critchley H, Daly E, Phillips M, et al. Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Human Brain Mapping. 2000;9:93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croucher CJ, Calder AJ, Barnard PJ. Impact, arousal and the recollection of emotional pictures. Birmingham: Paper presented at the Joint Meeting of the Experimental Psychology Society with Nederlandse Vereninging voor Psychonomie; 2006. [Google Scholar]
- Cusack R, Brett M, Osswald K. An evaluation of the use of magnetic field maps to undistort echo-planar images. NeuroImage. 2003;18:127–42. doi: 10.1006/nimg.2002.1281. [DOI] [PubMed] [Google Scholar]
- Halgren E, Marinkovic K. Neurophysiological networks integrating human emotions. In: Gazzaniga MS, editor. The Cognitive Neuroscience. Cambridge, M.A.: MIT Press; 1995. pp. 1137–51. [Google Scholar]
- Hamann S. Nosing in on the emotional brain. Nature Neuroscience. 2003;6:106–8. doi: 10.1038/nn0203-106. [DOI] [PubMed] [Google Scholar]
- Hamann SB. Cognitive and neural mechanisms of emotional memory. Trends in Cognitive Sciences. 2001;5:394–400. doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Hoffman JM, Kilts CD. Ecstasy and agony: activation of the human amygdala in positive and negative emotion. Psychological Science. 2002;13:135–41. doi: 10.1111/1467-9280.00425. [DOI] [PubMed] [Google Scholar]
- Hurley GD, McDougall A. Visual Impact in Print. Chicago: Visual Impact Inc; 1971. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): NIMH Center for the Study of Emotion and Attention. Gainsville, Florida, USA: University of Florida; 1995. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Instruction Manual and Affective Ratings. Gainesville, Florida, USA: University of Florida; 1999. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–73. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Laurens KR, Kiehl KA, Ngan ET, Liddle PF. Attention orientating dysfunction during salient novel stimulus processing in schizophrenia. Schizophrenia Research. 2005;75:159–71. doi: 10.1016/j.schres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Lazarus RS. Psychological stress and the coping process. New York: McGraw-Hill; 1966. [Google Scholar]
- Merbolt K.-D, Fransson P, Bruhn H, Frahm J. Functional MRI of the human amygdala? NeuroImage. 2001;14:253–7. doi: 10.1006/nimg.2001.0802. [DOI] [PubMed] [Google Scholar]
- Ochsner KN. Are affective events richly recollected or simply familiar? The experience and process of recognizing feelings past. Journal of Experimental Psychology: General. 2000;129:242–61. doi: 10.1037//0096-3445.129.2.242. [DOI] [PubMed] [Google Scholar]
- Papps BP, Calder AJ, Young AW, O’Carroll R. Dissociation of affective modulation of recollective and perceptual experience following amygdala damage. Journal of Neurology, Neurosurgery, and Psychiatry. 2003;74:253–4. doi: 10.1136/jnnp.74.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LaBar KS, Spencer DD. Memory for emotional words following unilateral temporal lobectomy. Brain and Cognition. 1997;35:85–109. doi: 10.1006/brcg.1997.0929. [DOI] [PubMed] [Google Scholar]
- Reisenzein R. Pleasure–arousal theory and the intensity of emotions. Journal of Personality and Social Psychology. 1994;67:525–39. [Google Scholar]
- Ruff CC, Bestman S, Blankenburg F, et al. Distinct causal influence of parietal versus frontal areas on human visual cortex: Evidence from concurrent TMS-fMRI. Cerebal Cortex. 2007;18:817–27. doi: 10.1093/cercor/bhm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA. Core affect and the psychological construction of emotion. Psychological Review. 2003;110:145–72. doi: 10.1037/0033-295x.110.1.145. [DOI] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Reviews in the Neurosciences. 2003;14:303–16. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Scherer KR. Appraisal theory. In: Dalgleish T, Power M, editors. Handbook of Cognition and Emotion. New York: Wiley; 1999. pp. 637–63. [Google Scholar]
- Scherer KR, Dan E, Flykt A. What determines a feeling's position in three-dimensional affect space? A case for appraisal. Cognition and Emotion. 2006;20:92–113. [Google Scholar]
- Schlosberg H. The description of facial expressions in terms of two dimensions. Journal of Experimental Psychology. 1952;44:229–37. doi: 10.1037/h0055778. [DOI] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam M.-M, Parrish T. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39:701–11. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Soltani M, Knight RT. Neural origins of the P300. Critical Reviews in Neurobiology. 2000;14:199–224. [PubMed] [Google Scholar]
- Stapleton JM, Halgren E. Endogenous potentials evoked in simple cognitive tasks: depth components and task correlates. Electroencephalography and Clinical Neurophysiology. 1987;67:44–52. doi: 10.1016/0013-4694(87)90162-3. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labelling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Wright P, Liu Y. Neutral faces activate the amygdala during identity matching. Neuroimage. 2006;29:628–36. doi: 10.1016/j.neuroimage.2005.07.047. [DOI] [PubMed] [Google Scholar]
- Zaichkowsky JL. The personal involvement inventory: reduction, revision and application to advertising. Journal of Advertising. 1994;23:59–70. [Google Scholar]
- Zillmann D, Knobloch S, Yu H.-S. Effects of photographs on the selective reading of news reports. Media Psychology. 2001;3:301–24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.