Abstract
Multiple lines of evidence implicate the basolateral amygdala (BLA) and the noradrenergic (norepinephrine, NE) system in responding to stressful stimuli such as fear signals, suggesting hyperfunction of both in the development of stress-related pathologies including anxiety disorders. However, no causative link between elevated NE neurotransmission and BLA hyperresponsiveness to fear signals has been established to date in humans. To determine whether or not increased noradrenergic tone enhances BLA responses to fear signals, we used functional magnetic resonance imaging (fMRI) and a strategy of pharmacologically potentiating NE neurotransmission in healthy volunteers. 18 subjects were scanned two times on a facial emotion paradigm and given either a single-dose placebo or 4 mg of the selective NE reuptake inhibitor reboxetine 2 h prior to an fMRI session. We found that reboxetine induced an amygdala response bias towards fear signals that did not exist at placebo baseline. This pharmacological effect was probabilistically mapped to the BLA. Extrapolation of our data to conditions of traumatic stress suggests that disinhibited endogenous NE signaling could serve as a crucial etiological contributor to post-traumatic stress disorder (PTSD) by eliciting exaggerated BLA responses to fear signals.
Keywords: amygdala, emotion, face, fear, fMRI, noradrenaline, reboxetine
INTRODUCTION
A capacity to perceive, assesses, control, and adequately respond to stress is of adaptive importance. However, when disproportional in intensity, chronic, or unrelated to genuine threats, stress may become maladaptive and precipitate stress-related psychiatric disorders (Millan, 2003). The amygdala is heavily implicated in stress-related pathologies such as panic disorder (Shekhar et al., 1999), chronic anxiety (Shekhar et al., 2005), and post-traumatic stress disorder (PTSD) (Rauch et al., 1996; 2006). One of the most notable findings from functional magnetic resonance imaging (fMRI) studies of anxiety disordered patients is a hyperresponsiveness of the amygdala to fear signals (Etkin and Wager, 2007).
Also heavily researched for its role in stress and stress-related pathologies is the locus coeruleus (LC) (Sved et al., 2002), a collection of 16,000 neurons (per hemisphere) located in the dorso-rostral pons (Aston-Jones and Cohen, 2005a, 2005b; Berridge and Waterhouse, 2003). The basolateral complex of the amygdala (BLA) receives a dense noradrenergic (norepinephrine, NE) innervation from the LC (Asan, 1998), and NE levels increase in the BLA with exposure to stressful stimuli (Galvez et al., 1996; Hatfield et al., 1999). Neurons in the LC are activated by stressful stimuli (Abercrombie and Jacobs, 1987) and mediate the stress-induced enhancement of NE neurotransmission in the BLA (Buffalari and Grace, 2007).
Although prefrontal cortex (PFC) subregions have been shown to exert top-down inhibitory control over the stress response, leading to dampening of response to stressful stimuli (Amat et al., 2005), the BLA and LC appear to reciprocally interact in promoting the stress response, which implicates the disinhibition of both in the development of maladaptive stress responses and anxiety disorders (Buffalari and Grace, 2007). There is indeed substantial clinical evidence for a crucial role of LC-NE overdrive as an important neurochemical substrate of – and thereby obvious therapeutic target in – PTSD (Southwick et al., 1993; 1997). β-adrenergic blockade of LC-NE signaling with propranolol attenuates symptoms of anxiety (Granville-Grossman and Turner, 1966) and appears to be effective in the secondary prevention of PTSD (Pitman et al., 2002; Vaiva et al., 2003). However, no causative link between elevated NE neurotransmission and BLA hyperresponsiveness to fear signals has been established to date in humans.
To determine whether or not such functional bias emerges under conditions of elevated BLA noradrenergic activity, we used a strategy of pharmacologically potentiating NE neurotransmission in healthy volunteers. Eighteen subjects participated in a double-blind, placebo-controlled, randomized fMRI study. Subjects underwent imaging two times (at least a week apart) and were administered either a single-dose placebo or 4 mg of the selective NE reuptake inhibitor reboxetine. We used ecologically valid dynamic facial expressions to stimulate the amygdala (van der Gaag et al., 2007), and cytoarchitectonic maximum probabilistic maps of amygdala subregions (Amunts et al., 2005; Eickhoff et al., 2005) to determine susceptibility of BLA evoked responses to increased noradrenergic tone.
MATERIALS AND METHODS
Subjects
Eighteen healthy adults (nine females, nine males; mean age, 24 years; age range, 19–33 years) volunteered after giving written, informed consent. The study had full ethical approval and was accomplished in compliance with the latest revision of the Declaration of Helsinki. All volunteers were right-handed, as assessed by the Edinburgh Handedness Inventory (Oldfield, 1971). Subjects were screened for MRI compatibility and determined to be free of current or past medical, neurological, or psychiatric illness, drug/alcohol abuse, and psychoactive medication. Psychiatric assessment included the BDI (Beck Depression Inventory) (Beck et al., 1995) and the M.I.N.I. (Mini International Neuropsychiatric Interview) (Lecrubier et al., 1997). Neuropsychological assessment included a measure of verbal IQ based on lexical decisions (MWTB, Mehrfachwahl-Wortschatz-Test) (Lehrl, 1995) and a test of facial emotion recognition (FEEST, Facial Expressions of Emotions: Stimuli and Test) (Young et al., 2002). In brief, subjects had neither neuropsychological impairments nor any psychopathology. In addition, heart rate, blood pressure, and an electrocardiogram (ECG) were obtained from each subject to exclude any cardiovascular abnormalities.
Study design
The rationale of the present study was to potentiate NE neurotransmission in healthy volunteers in order to pharmacologically model an amygdala response bias towards fear. In a within-subjects, double-blind study design, subjects received one tablet of reboxetine mesilate (4 mg) or saccharose placebo two hours prior to an fMRI session. This interval was chosen because maximum reboxetine serum concentrations in humans are usually reached two hours after oral intake (serum half-life, 13 h) (Fleishaker, 2000). The order of drug/placebo administration was counterbalanced across subjects. Reboxetine is a highly selective inhibitor of presynaptic NE reuptake and thus increases synaptic availability of NE (Kent, 2000; Scates and Doraiswamy, 2000). A 4-mg single oral dose of reboxetine was administered in analogy to previous studies investigating noradrenergic modulation of emotional and cognitive functions and their interactions (Hurlemann et al., 2005, 2007; Norbury et al., 2007; Papps et al., 2002). Heart rate and blood pressure were measured once before drug administration and once before fMRI scanning. In addition, a venous blood sample was collected after fMRI scanning. The resulting reboxetine serum levels were as follows: mean, 123.1 ng/ml; SD, 37.3 ng/ml (for a detailed synopsis of analytical procedures see Hurlemann et al., 2007).
FMRI paradigm
The fMRI paradigm consisted of a pseudorandom series of movies obtained from 10 professional actors (five females, five males) who in each clip displayed either a fearful, neutral, or happy facial expression in a standardized fashion (for a detailed description of stimuli see van der Gaag et al., 2007). In the emotionally neutral movies, actors blew up their cheeks, which served to control for facial movements in happy and fearful stimuli (Figure 1A). In previous fMRI studies, happy, fearful, and neutral movies produced equally robust amygdala responses (van der Gaag et al., 2007), making these stimuli an ideal imaging probe to study a pharmacologically induced response bias in the amygdala. Moreover, we used dynamic instead of static stimuli for higher ecological validity: in everyday life, during social interactions, dynamic facial expressions rather than static facial displays serve as the primary conveyors of social-emotional information (see also Hurlemann et al., 2008). We thereby adapted an approach also used in recent single-neuron recording studies of the primate amygdala; this approach accounts for the fact that primates never see static facial displays in their natural environment (Kuraoka and Nakamura, 2007). Each movie had duration of 3 s and was repeated two times, resulting in 30 stimulus presentations per condition. Movies occurred at a rate of one every 13.2 s (7.8–18.6 s) over a period of 20 min. A fixation cross was interspersed between each movie. During fMRI scanning, subjects were engaged in a gender judgment task requiring appropriate push-button responses. Stimulus delivery and response recording was carried out using Presentation12 (Neurobehavioral Systems, Inc., Albany, CA, USA).
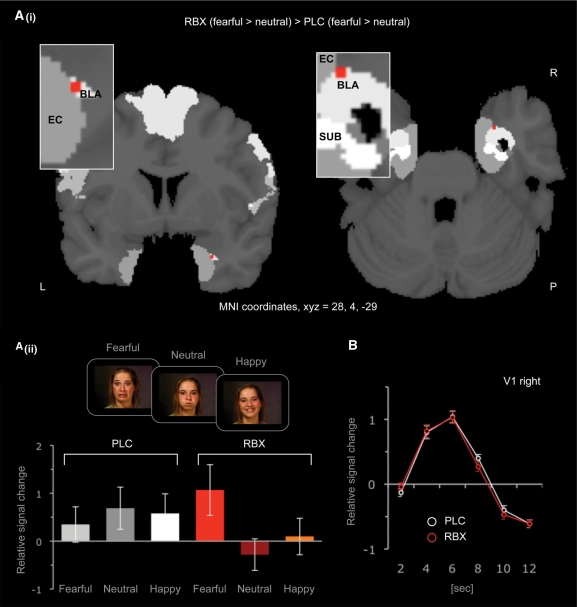
Fig 1.
(A) Activation map resulting from the fearful-minus-neutral contrast in a ROI analysis of the amygdala (i) Probabilistic mapping demonstrates increased differential activity in the right BLA (MNI-coordinates X, Y, Z = 28, 4, −29). (ii) Plotted are the relative signal changes in the BOLD response of the activated voxels for the two fMRI sessions (reboxetine, placebo) and the three experimental conditions (fearful, happy, neutral) relative to the mean of the global signal. Potentiation of NE neurotransmission with reboxetine increased BLA responses to fearful stimuli and decreased BLA responses to neutral stimuli. This effect was pharmacologically induced. BLA responses to fearful, happy or neutral stimuli did not significantly differ from each other at placebo baseline. (B) Relative signal changes in the BOLD response of activated clusters within the primary visual cortex (V1). Amplitudes and latencies of minima and maxima of the V1 hemodynamic response profile were not affected by the reboxetine challenge, which argues against a global homogeneous drug effect on the BOLD signal. Error bars indicate SEM. Abbreviations: BLA, basolateral complex of the amygdala; EC, entorhinal cortex; L, left hemisphere; P, posterior; PLC, placebo; R, right hemisphere; RBX, reboxetine; SUB, subiculum.
Data acquisition
An Avanto MRI system (Siemens, Erlangen, Germany) operating at 1.5T was used to obtain T2*-weighted echoplanar (EPI) images with blood-oxygen-level-dependent (BOLD) contrast (TR = 2.70 s, TE = 40 ms, matrix size: 64 × 64, pixel size: 3 × 3 mm2, slice thickness = 1.8 mm, distance factor = 50%, FoV = 192 mm, flip angle = 88°, 39 axial slices) with the parallel acquisition technique generalized autocalibrating partially parallel acquisitions (GRAPPA). Based on the a priori hypothesis the 39 slices were oriented centrally to the amygdala. Five hundred and fifty volumes were acquired; the first five volumes were discarded to allow for T1 equilibration effects. Stimuli were presented with liquid crystal display video goggles. In addition, high-resolution anatomical MRI images were acquired (T1 weighted 3D MPRAGE).
Data analysis
The image preprocessing was performed using Matlab7 (The MathWorks, Inc., Natick, MA, USA) and SPM5 (http://www.fil.ion.ucl.ac.uk/spm). The EPI images were corrected for head movement between scans by an affine registration (Ashburner and Friston, 2003). For realignment we used a two-pass procedure, by which images were initially realigned to the first image of the time-series and subsequently re-realigned to the mean of all images after the first step. After completing the realignment, the mean EPI image for each subject was computed and spatially normalized to the MNI template (Collins et al., 1994; Evans et al., 1992; Holmes et al., 1998) using the "unified segmentation" function in SPM5. This algorithm is based on a probabilistic framework that enables image registration, tissue classification, and bias correction to be combined within the same generative model. The resulting parameters of a discrete cosine transform, which define the deformation field necessary to move the subjects’ data into the space of the MNI tissue probability maps (Evans et al., 1994), were then combined with the deformation field transforming between the latter and the MNI single subject template. The ensuing deformation was subsequently applied to the individual EPI volumes. All images were hereby transformed into standard stereotaxic space and resampled at 2 × 2 × 2 mm3 voxel size. The normalized images were spatially smoothed using an 8 mm FWHM Gaussian kernel.
The three conditions were modeled by means of reference waveforms which correspond to stick functions placed at the onset of the stimuli convolved with a hemodynamic response function (Friston et al., 1995). A design matrix comprising contrasts of alternating intervals of the different trials, the time derivative, and movement parameters was created. Specific effects were assessed by applying appropriate linear contrasts to the parameter estimates of the experimental trials resulting in t-statistics for each voxel. These formed Statistical Parametric Maps (SPM{T}) of differences between the conditions. SPM{T}-statistics were interpreted in light of the theory of probabilistic behavior of Gaussian random fields.
Drug-specific effects on brain activation associated with the three experimental conditions were assessed by a second level analysis constituting a random effects model. For each simple effect of any of the three fMRI sessions, individual contrast images of each subject were entered into a second level analysis based on an Analysis of Variance (repeated measures ANOVA).
For a hypothesis-driven analysis, the left and right amygdala – including their basolateral, centromedial, and superficial subregions – were defined as regions-of-interests (ROIs) based on cytoarchitectonic maximum probability maps derived from histological analysis of 10 human post-mortem brains (Amunts et al., 2005; Eickhoff et al., 2005). The feasibility of this probabilistic approach has been demonstrated in previous fMRI studies (Ball et al., 2007; Hurlemann et al., 2008). The a priori focus on hypothesized areas serves to prevent false-positive findings (Stein et al., 1998). Thus, the current study used primarily a ROI approach.
RESULTS
Driven by our a priori hypothesis, we performed a ROI-analysis focused on the left and right amygdala. Reboxetine enhanced the difference between right BLA responses to fearful vs neutral stimuli [MNI-coordinates xyz = 28, 4, –29, P < 0.001, uncorrected; P = 0.07, family-wise error (FWE)-corrected]. This enhancement was driven by an increased activation to fearful stimuli and by a decreased activation to neutral stimuli (Figure 1A). The opposite contrast did not reveal any significant effect of reboxetine. The observation that enhancement of right BLA responses to fearful vs neutral stimuli was restricted to the reboxetine fMRI session and absent in the placebo fMRI session, indicates that this effect is pharmacologically induced (Figure 1A).
One important consideration in this context is the differentiation between reboxetine effects on neural tissue or on the BOLD response. To exclude any effect of reboxetine on global neural activation based on changes in baseline cerebral blood flow or neural coupling, we analyzed the relative signal change profile in the primary visual cortex (area V1). Specifically, we determined the relative signal change profile from the onset of the stimuli to the end of the hemodynamic response function (for a similar approach see Miskowiak et al., 2007; Paulus et al., 2005). This analysis revealed that reboxetine left the amplitudes as well as the latencies of minima and maxima of the V1 hemodynamic response function unchanged. Comparison of standard deviations did not reveal any significant differences either (Figure 1B). These results indicate that the effects of reboxetine reflect regionally specific pharmacological modulation of neural responses.
Extending our analysis of reboxetine effects to the whole brain (P < 0.001, uncorrected; voxel extent threshold >5), we identified increased activations of the right amygdala, right hippocampus, right Heschl's gyrus, inferior frontal gyrus (bilateral), fusiform gyrus (bilateral), and higher-order visual cortical areas when contrasting the fearful with the neutral condition. Reboxetine decreased responses in the left hippocampus, left middle occipital gyrus, and cerebellum. The contrast between the happy and the neutral condition identified increased activations of the left caudate nucleus, left insula, left superior medial frontal gyrus, cingulate gyrus, cerebellum, and higher-order visual cortical areas, whereas responses in the left superior orbital frontal gyrus, right inferior temporal gyrus, and right calcarine gyrus were decreased by reboxetine. Regional activations are listed in Tables 1 and 2.
Table 1.
List of brain regions showing a significant interaction effect of condition (fearful/happy vs neutral facial expressions) and drug (reboxetine vs placebo) (P < 0.001, uncorrected; voxel extent threshold >5)
| Contrast | Region | Side | k | MNI-coordinates |
T | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| (Fear > Neutral) Reboxetine > (Fear > Neutral) Placebo | |||||||
| Heschl's gyrus | R | 33 | 50 | −16 | 8 | 4.16 | |
| Inferior frontal gyrus | R | 33 | 42 | 42 | −10 | 3.97 | |
| Fusiform gyrus | R | 26 | 32 | −60 | −14 | 3.87 | |
| Precuneus | R | 26 | 20 | −54 | 44 | 3.85 | |
| Lingual gyrus | R | 22 | 18 | −86 | −4 | 3.89 | |
| Fusiform gyrus | L | 15 | −36 | −56 | −8 | 3.91 | |
| Inferior frontal gyrus | L | 13 | –22 | 30 | −12 | 3.71 | |
| Angular gyrus | R | 12 | 54 | −50 | 30 | 3.65 | |
| Supramarginal gyrus | L | 11 | −48 | −44 | 32 | 3.63 | |
| Hippocampus/Amygdala | R | 8 | 26 | 6 | −32 | 3.27 | |
| Supramarginal gyrus | R | 7 | 60 | −24 | 22 | 3.56 | |
| (Happy > Neutral) Reboxetine > (Happy > Neutral) Placebo | |||||||
| Supramarginal gyrus | L | 42 | −46 | −40 | 30 | 3.66 | |
| Caudate nucleus | L | 38 | −18 | −4 | 26 | 4.11 | |
| Cerebellum | R | 33 | 16 | −64 | −34 | 3.96 | |
| Rectal gyrus | R | 27 | 14 | 28 | −20 | 4.60 | |
| Superior medial frontal gyrus | L | 23 | −12 | 38 | 28 | 3.76 | |
| Superior medial frontal gyrus | L | 20 | −8 | 48 | 6 | 3.70 | |
| Middle cingulum | R | 12 | 12 | −16 | 34 | 3.94 | |
| Middle occipital | L | 10 | −32 | −74 | 14 | 3.47 | |
| Precuneus | R | 8 | 12 | −56 | 42 | 3.40 | |
| Insula | L | 6 | −28 | 14 | −10 | 3.34 | |
| Middle occipital | L | 6 | −28 | −86 | 42 | 3.33 | |
| Cerebellar vermis | R | 6 | 4 | −68 | −20 | 3.32 | |
Table 2.
List of brain regions showing a significant interaction effect of condition (fearful/happy vs neutral facial expressions) and drug (placebo vs reboxetine) (P < 0.001, uncorrected; voxel extent threshold >5)
| Contrast | Region | Side | k | MNI-coordinates |
T | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| (Fear > Neutral) Placebo > (Fear > Neutral) Reboxetine | |||||||
| Middle occipital gyrus | L | 29 | −40 | −74 | 24 | 3.86 | |
| Whitte matter | L | 9 | −26 | −14 | 16 | 3.55 | |
| Hippocampus | L | 7 | −12 | −28 | −6 | 3.55 | |
| Cerebellum | R | 6 | 56 | −58 | −34 | 3.54 | |
| (Happy > Neutral) Placebo > (Happy > Neutral) Reboxetine | |||||||
| Inferior temporal gyrus | R | 32 | 48 | −56 | −16 | 4.29 | |
| Superior orbital frontal gyrus | L | 20 | −18 | 54 | −10 | 3.91 | |
| Calcarine gyrus | R | 7 | 18 | −44 | 6 | 3.29 | |
DISCUSSION
We used a strategy of pharmacologically potentiating NE neurotransmission in healthy volunteers to demonstrate an enhanced difference between right BLA responses to fearful vs neutral stimuli under conditions of increased noradrenergic tone. Specifically, a hyperresponsiveness to fearful stimuli was coupled with a hyporesponsiveness to neutral stimuli. This shift in BLA response characteristics was pharmacologically induced.
Notably, the pharmacological challenge had no effect on neural responses in the primary visual cortex, arguing against a global homogeneous drug effect on the BOLD signal. Moreover, this study is the first to probabilistically map a single-dose effect of reboxetine to the BLA. This result is in keeping with studies in rodents that demonstrated susceptibility of BLA function to pharmacological manipulations of NE signaling (McGaugh, 2000).
Our finding that pharmacological enhancement of BLA noradrenergic activity is required to induce a response bias towards fear conflicts with concepts of the amygdala as a neural module with an intrinsic fear selectivity (Phan et al., 2002). However, fMRI studies that used movies of facial expressions instead of static photographs to stimulate the amygdala under more ecological conditions found no differences between amygdala responses to fearful, neutral, or happy facial expressions (van der Gaag et al., 2007). Similar results were obtained within the chemosensory (Anderson et al., 2003; Small et al., 2003) and cognitive domains (Hamann et al., 1999; Hurlemann et al., 2005) when appetitive and aversive stimuli were matched for intensity (arousal). Thus, from our findings it appears that stress-induced increases in NE signaling act to convert a subset of BLA neurons into a fear module, perhaps by selectively augmenting the signal-to-noise ratio for fearful information at the cost of neutral information. One scenario would be that those neurons that are potently activated by NE project to downstream BLA targets mediating fear-related behavioral responses (Buffalari and Grace, 2007).
Extrapolation of our results to conditions of traumatic stress suggests that disinhibited LC-NE signaling could serve as a crucial etiological contributor to the onset and maintenance of PTSD by eliciting exaggerated BLA responses to the traumatic stressor. Several lines of evidence suggest that the degree of top-down inhibitory control orchestrated by the medial PFC (mPFC) is the variable that ultimately determines the magnitude of the endogenous stress response (Arnsten and Goldman-Rakic, 1984; Aston-Jones and Cohen, 2005a, 2005b; Berridge and Waterhouse, 2003; Jodo et al., 1998; Kim and Diamond, 2002; Robbins, 2005). Seminal findings in rodents indicate that, when a stressor is controllable, activation of autonomic nuclei is inhibited by descending connections from mPFC, and the behavioral sequelae of uncontrollable stress are blocked; in turn, experimental inactivation of mPFC eliminates stressor controllability and evokes maladaptive stress responses (Amat et al., 2005). Given this empirical background, we suggest that disinhibited LC-NE bottom-up signaling is responsible for exaggerated amygdala responses to fear signals, leading to excessive acquisition of conditioned fear responses (Rauch et al., 2006) as well as overlearning and overconsolidation of traumatic memories (Hurlemann, 2008). We thus speculate that the efficacy of propranolol in the secondary prevention of PTSD (Pitman et al., 2002; Vaiva et al., 2003) relies to a considerable extent on a normalization of BLA hyperfunction by attenuating the detrimental effects of disinhibited LC-NE input.
Our results contrast with fMRI studies where a daily 4-mg dose of reboxetine was administered to healthy volunteers over a one-week period to establish a positive response bias in the amygdala, evident in decreased amygdala responses to fearful faces and increased amygdala responses to happy faces (Norbury et al., 2007). These changes in the amygdala response profile would be consistent with an antidepressive effect of reboxetine in patient populations undergoing (sub)chronic treatment with this agent (Burrows et al., 1998). However, experiments in rodents demonstrate that the response to reboxetine varies substantially as a function of treatment duration. According to these experiments, single-dose reboxetine increased anxiety-related behaviors, whereas subchronic administration of reboxetine over a one-week period decreased anxiety-related behaviors (Inoue et al., 2006; see also Miyata et al., 2007). These results may reconcile discrepant observations derived from single-dose and (sub)chronic administrations of reboxetine. In this context, we note that reboxetine treatment has been reported to provoke symptom exacerbation in patients with borderline personality disorder (Anghelescu et al., 2005), underscoring the potential risks associated with acute elevations of NE signaling in patient populations, a finding that definitely merits further investigation.
One limitation of the present study is the relatively low field strength of the MRI system (1.5T), which complicates the probabilistic assignment of activation sites to micro-anatomically defined amygdala subregions. To account for the short distances between these subregions, we used a rather narrow slice thickness of 1.8 mm (pixel size, 3 × 3 mm2). Nevertheless, the accuracy of probabilistic mapping could be further improved by using MRI systems with field strengths of 3T or higher and a slice thickness of 1 mm or less.
Conclusions: We used pharmacological fMRI to model a BLA response bias towards fear signals. This model might help to advance our understanding of how stress-evoked escalations in LC-NE signaling could distort BLA function and thus predispose to stress-related pathologies including anxiety disorders.
Acknowledgments
The authors wish to thank M. Diessel, J. Kukolja, C. Santoro, and K. Schnell for assistance with data collection, C. Frahnert and H. Kölsch for analyzing blood samples, and A. Patin for proofreading. This work was supported by a German Federal Ministry of Education and Research (BMBF) grant (01GW0671) (to R.H.), a German Research Foundation (DFG) grant (HU1302/2-1) (to R.H.), an N.W.O Vidi grant (to C.K.), and a European Commission Marie Curie Excellence grant (to C.K.).
REFERENCES
- Abercrombie ED, Jacobs BL. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. I. Acutely presented stressful and nonstressful stimuli. Journal of Neuroscience. 1987;7:2837–43. doi: 10.1523/JNEUROSCI.07-09-02837.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nature Neuroscience. 2005;8:365–71. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and Embryology. 2005;210:343–52. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, et al. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Anghelescu I, Janen B, Schindler F, Lammers CH. Worsening of borderline symptoms under reboxetine treatment. The Journal of Neuropsychiatry and Clinical Neuroscience. 2005;17:559–60. doi: 10.1176/jnp.17.4.559. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Selective prefrontal cortical projections to the region of the locus coeruleus and raphe nuclei in the rhesus monkey. Brain Research. 1984;306:9–18. doi: 10.1016/0006-8993(84)90351-2. [DOI] [PubMed] [Google Scholar]
- Asan E. The catecholaminergic innervation of the rat amygdala. Advances in Anatomy, Embryology and Cell Biology. 1998;142:L1–118. doi: 10.1007/978-3-642-72085-7. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Rigid Body Registration. In: Frackowiak RS, Friston KJ, Frith CD, et al., editors. Human Brain Function. 2nd. London, UK: Academic Press; 2003. pp. 635–55. [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual Reviews – Neuroscience. 2005a;28:403–50. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. The Journal of Comparative Neurology. 2005b;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Ball T, Rahm B, Eickhoff SB, Schulze-Bonhage A, Speck O, Mutschler I. Response properties of human amygdala subregions: evidence based on functional MRI combined with probabilistic anatomical maps. PLoS ONE. 2007;2:e307. doi: 10.1371/journal.pone.0000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Hautzinger M, Bailer M, Worall H, Keller F. Beck-Depressions-Inventar (BDI) 2nd. Göttingen: Hogrefe; 1995. [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Research and Brain Research Review. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Grace AA. Noradrenergic modulation of basolateral amygdala neuronal activity: opposing influences of alpha-2 and beta receptor activation. Journal of Neuroscience. 2007;27:12358–66. doi: 10.1523/JNEUROSCI.2007-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows GD, Maguire KP, Norman TR. Antidepressant efficacy and tolerability of the selective norepinephrine reuptake inhibitor reboxetine: a review. Journal of Clinical Psychiatry. 1998;14:4–7. [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. Journal of Computer Assisted Tomography. 1994;18:192–205. [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AC, Marrett S, Neelin P, et al. Anatomical mapping of functional activation in stereotactic coordinate space. Neuroimage. 1992;1:43–53. doi: 10.1016/1053-8119(92)90006-9. [DOI] [PubMed] [Google Scholar]
- Evans AC, Kamber M, Collins DL, MacDonald D. An MRI based probabilistic atlas of neuroanatomy. In: Shorvon S, Fish D, Andermann F, Bydder GM, editors. Magnetic Resonance Scanning and Epilepsy. 1994. pp. 263–74. [Google Scholar]
- Fleishaker JC. Clinical pharmacokinetics of reboxetine, a selective norepinephrine reuptake inhibitor for the treatment of patients with depression. Clinical Pharmacokinetics. 2000;39:413–27. doi: 10.2165/00003088-200039060-00003. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Galvez R, Mesches MH, McGaugh JL. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiology of Learning and Memory. 1996;66:253–7. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- Granville-Grossman KL, Turner P. The effect of propranolol on anxiety. Lancet. 1966;1:788–90. doi: 10.1016/s0140-6736(66)91863-0. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nature Neuroscience. 1999;2:289–93. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Spanis C, McGaugh JL. Response of amygdalar norepinephrine to footshock and GABAergic drugs using in vivo microdialysis and HPLC. Brain Research. 1999;835:340–5. doi: 10.1016/s0006-8993(99)01566-8. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography. 1998;22:324–33. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Hurlemann R. Noradrenergic-glucococorticoid mechanisms in emotion-induced amnesia: from adaptation to disease. Psychopharmacology. 2008;197:13–23. doi: 10.1007/s00213-007-1002-x. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Hawellek B, Matusch A, et al. Noradrenergic modulation of emotion-induced forgetting and remembering. Journal of Neuroscience. 2005;25:6343–9. doi: 10.1523/JNEUROSCI.0228-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Matusch A, Hawellek B, et al. Emotion-induced retrograde amnesia varies as a function of noradrenergic-glucocorticoid activity. Psychopharmacology. 2007;194:261–9. doi: 10.1007/s00213-007-0836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Rehme AK, Diessel M, et al. Segregating intra-amygdalar responses to dynamic facial emotion with cytoarchitectonic maximum probability maps. Journal of Neuroscience Methods. 2008;172:13–20. doi: 10.1016/j.jneumeth.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Inoue T, Nakagawa S, Izumi T, Kitaichi Y, Koyama T. Effect of combined treatment with noradrenaline and serotonin reuptake inhibitors on conditioned freezing. European Journal of Pharmacology. 2006;540:91–5. doi: 10.1016/j.ejphar.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Jodo E, Chiang C, Aston-Jones G. Potent excitatory influence of prefrontal cortex activity on noradrenergic locus coeruleus neurons. Neuroscience. 1998;83:63–79. doi: 10.1016/s0306-4522(97)00372-2. [DOI] [PubMed] [Google Scholar]
- Kuraoka K, Nakamura K. Responses of single neurons in monkey amygdala to facial and vocal emotions. Journal of Neurophysiology. 2007;97:1379–87. doi: 10.1152/jn.00464.2006. [DOI] [PubMed] [Google Scholar]
- Kent JM. SNaRIs, NaSSAs, and NaRIs: new agents for the treatment of depression. Lancet. 2000;355:911–8. doi: 10.1016/S0140-6736(99)11381-3. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nature Reviews Neuroscience. 2002;3:453–62. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y, Sheehan D, Weiler E, et al. The MINI International Neuropsychiatric Interview (M.I.N.I.) A Short Diagnostic Structured Interview: Reliability and Validity According to the CIDI. European Psychiatry. 1997;12:224–31. [Google Scholar]
- Lehrl S. Mehrfachwahl-Wortschatz-Test (MWT-B) Erlangen: Straube; 1995. [Google Scholar]
- McGaugh JL. Memory – a century of consolidation. Science. 2000;287:248–51. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The neurobiology and control of anxious states. Progessive Neurobiology. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Miskowiak K, Papadatou-Pastou M, Cowen PJ, Goodwin GM, Norbury R, Harmer CJ. Single dose antidepressant administration modulates the neural processing of self-referent personality trait words. Neuroimage. 2007;37:904–11. doi: 10.1016/j.neuroimage.2007.05.036. [DOI] [PubMed] [Google Scholar]
- Miyata S, Shimoi T, Hirano S, Yamada N, Hata Y, Yoshikawa N, et al. Effects of serotonergic anxiolytics on the freezing behavior in the elevated open-platform test in mice. Journal of Pharmacological Sciences. 2007;105:272–8. doi: 10.1254/jphs.fp0070314. [DOI] [PubMed] [Google Scholar]
- Norbury R, Mackay CE, Cowen PJ, Goodwin GM, Harmer CJ. Short-term antidepressant treatment and facial processing. Functional magnetic resonance imaging study. British Journal of Psychiatry. 2007;190:531–2. doi: 10.1192/bjp.bp.106.031393. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Papps BP, Shajahan PM, Ebmeier KP, O’Carroll RE. The effects of noradrenergic re-uptake inhibition on memory encoding in man. Psychopharmacology. 2002;159:311–8. doi: 10.1007/s00213-001-0924-y. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB. Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Archives of General Psychiatry. 2005;62:282–8. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–448. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Sanders KM, Zusman RM, et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biological Psychiatry. 2002;51:189–92. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- Rauch SL, van der Kolk BA, Fisler RE, et al. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Archives of General Psychiatry. 1996;53:380–7. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research–past, present, and future. Biological Psychiatry. 2006;60:376–82. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Chemistry of the mind: neurochemical modulation of prefrontal cortical function. Journal of Comparative Neurology. 2005;493:140–6. doi: 10.1002/cne.20717. [DOI] [PubMed] [Google Scholar]
- Scates AC, Doraiswamy PM. Reboxetine: a selective norepinephrine reuptake inhibitor for the treatment of depression. Annals of Pharmacotherapy. 2000;34:1302–12. doi: 10.1345/aph.19335. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Sajdyk T, Keim SR, Yoder KK, Sanders SK. Role of the basolateral amygdala in panic disorder. Annals of New York Academy of Sciences. 1999;877:747–50. doi: 10.1111/j.1749-6632.1999.tb09315.x. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Truitt W, Rainnie D, Sajdyk T. Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress. 2005;8:209–19. doi: 10.1080/10253890500504557. [DOI] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39:701–11. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Morgan CA, et al. Abnormal noradrenergic function in posttraumatic stress disorder. Archives of General Psychiatry. 1993;50:266–74. doi: 10.1001/archpsyc.1993.01820160036003. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Bremner JD, et al. Noradrenergic and serotonergic function in posttraumatic stress disorder. Archives of General Psychiatry. 1997;54:749–58. doi: 10.1001/archpsyc.1997.01830200083012. [DOI] [PubMed] [Google Scholar]
- Stein EA, Pankiewicz J, Harsch HH, et al. Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. American Journal of Psychiatry. 1998;155:1009–15. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- Sved AF, Cano G, Passerin AM, Rabin B. The locus coeruleus, Barrington's nucleus, and neural circuits of stress. Physiology & Behavior. 2002;77:737–42. doi: 10.1016/s0031-9384(02)00927-7. [DOI] [PubMed] [Google Scholar]
- Vaiva G, Ducrocq F, Jezequel K, et al. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biological Psychiatry. 2003;54:947–49. doi: 10.1016/s0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]
- van der Gaag C, Minderaa RB, Keysers C. The BOLD signal in the amygdala does not differentiate between dynamic facial expressions. Social Cognitive and Affective Neuroscience. 2007;2:93–103. doi: 10.1093/scan/nsm002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AW, Perret DI, Calder A, Sprengelmeyer R, Ekman P. Facial Expressions of Emotion: Stimuli and Test. San Antonio, Texas: Harcourt Assessment; 2002. [Google Scholar]