Abstract
Motivation for goal-directed behaviour largely depends on the expected value of the anticipated reward. The aim of the present study was to examine how different levels of reward value are coded in the brain for two common forms of human reward: money and social approval. To account for gender differences 16 male and 16 female participants performed an incentive delay task expecting to win either money or positive social feedback. fMRI recording during the anticipation phase revealed proportional activation of neural structures constituting the human reward system for increasing levels of reward, independent of incentive type. However, in men activation in the prospect of monetary rewards encompassed a wide network of mesolimbic brain regions compared to only limited activation for social rewards. In contrast, in women, anticipation of either incentive type activated identical brain regions. Our findings represent an important step towards a better understanding of motivated behaviour by taking into account individual differences in reward valuation.
Keywords: anticipation, social reward, monetary reward, gender, nucleus accumbens
INTRODUCTION
According to economical theories human behaviour is generally guided by the expectation that the benefits of one's actions outweigh the costs. Recent neuroeconomical approaches (Walter et al., 2005; Fehr and Camerer, 2007; Izuma et al., 2008) have posed the possibility that this is not only true for financial but also for social benefits. The notion takes on the social exchange theory of the late 1950s (Thibaut and Kelley, 1959) suggesting that social actions (e.g. mutual face-to-face interactions) are driven by the goal to increase one's own social standing. Accordingly, investment in socially relevant activities is charged against potential social benefits such as approval by others. In a neuroeconomical framework premises for successful social goal-directed behaviour do not differ from financial goal-directed behaviour in requiring (i) knowledge about the rewarding quality of a stimulus or event, (ii) ability to make use of learned associations to recognize when potential reward is within reach. In recent years considerable data have been accumulated corroborating the brain's sensitivity to the rewarding quality of both, money (Elliott et al., 2003; Zink et al., 2004; Pessiglione et al., 2007) and social stimuli (Aharon et al., 2001; Nitschke et al., 2004; Izuma et al., 2008). However, the neural mechanisms prompting an organism to approach the potential reward have been explored for money (Knutson et al., 2000, 2001b; Kirsch et al., 2003) and food (McClure et al., 2007) but not yet for social feedback. This is surprising given that anticipation of social feedback in the form of praise (and reprimand) has been regarded an important educative modulator of behaviour for centuries (Skinner, 1953). An amelioration of performance through anticipated positive social feedback is already seen in young children (Sorce et al., 1985; Kohls et al., in press). Also, business employees are found to rate appreciation for work done as more motivating than financial rewards (Lindahl, 1949; Graham and Unruh, 1990).
In the present fMRI study we employed an incentive delay task announcing either monetary or socially relevant feedback in the form of friendly faces. Hence, we assessed the neural mechanisms underlying the anticipation of potential social compared to monetary reward based on incentive related cues. Previous neuroimaging studies have implicated the involvement of ventral (nucleus accumbens) and dorsal (putamen and caudate nucleus) striatum, amygdala, and orbitofrontal cortex in monetary reward prediction (Breiter et al., 2001; Knutson et al., 2001a; Bjork et al., 2004). Within this circuit the ventral striatum, particularly the nucleus accumbens (NAcc), has been attributed a key function in recognizing environmental stimuli as cues for rewarding events (Kalivas and Volkow, 2005; Knutson and Cooper, 2005). Based on learned associations, dopaminergic nerve cells project into the NAcc to alert the organism that a motivationally relevant event is within reach (Schultz, 1998). FMRI studies in humans indicate that anticipation of monetary gains proportionally increases blood oxygen level dependent contrast (BOLD) in the NAcc. Stronger BOLD-responses were reported for anticipated high amounts of reward as opposed to low amounts, paired with faster reactions to the respective reward promising cues (Knutson et al., 2001b). In a previous behavioural study (K.S. et al., submitted) we offered social or monetary feedback with varying degrees of attractiveness as reward for sufficiently fast reactions. Independent of incentive type, reaction times were found to decrease with the announced level of potential reward. The same study denotes motivational differences with respect to incentive type between men and women. Incentive type modulated performance in men resulting in faster reactions for potential monetary than social reward. In contrast, women reacted equally fast to cues signalling either type of reward. Numerous studies have found women to be more sensitive to social signals than men (Rosenthal and DePaulo, 1979; Baron-Cohen and Wheelwright, 2004; Proverbio et al., 2008). For example, women perform more accurately than men when labelling others’ feelings based on external cues such as facial expressions (McClure, 2000). Personality questionnaires assessing individual motivation for appetitive behaviour primarily in social contexts [e.g. TPQ (Cloninger et al., 1991)] find women to be more reward dependent than men (Cloninger et al., 1991; Otter et al., 1995; Krebs et al., 1998). Interestingly, personality assessments which also include situations allowing personal (monetary) gain (e.g. SPSRQ, Torrubia et al., 2001), find men to be more sensitive to reward than women (Torrubia et al., 2001; Li et al., 2007). However, despite repeated findings of gender differences with respect to reward-related personality traits, most imaging studies on reward processing included only male participants. To account for gender differences in reward processing, we included 16 male and 16 age-matched female participants in our study.
The aim of the present study was to assess whether the neural mechanisms underlying the anticipation of monetary rewards also apply to the anticipation of social feedback in the form of friendly faces. Based on previous findings indicating a linear relation between reward value and striatal activation for monetary rewards (Knutson et al., 2001a), we expected to find a stronger BOLD-response in reward-related areas in response to higher levels of anticipated reward than to lower levels. Based on the findings in the preceding behavioural study (K.S. et al., submitted), we introduced gender as an additional factor in our analysis, postulating greater sensitivity to social stimuli in women than men (Baron-Cohen and Wheelwright, 2004).
MATERIALS AND METHODS
Subjects
Thirty-two right-handed, healthy non-smoking volunteers (16 men: mean age 29.0 years, range 20–48 years; 16 women: mean age 28.8 years, range 20–53 years) with normal vision and no past neurological or psychiatric history participated in the study. None of the subjects was taking psycho-pharmacologically active medication at time of study or within the last two months. The study was approved by the Ethics Committee of the Medical Faculty of the RWTH Aachen University. Subjects gave written informed consent and were paid a fee for participation in the study.
Stimuli and task
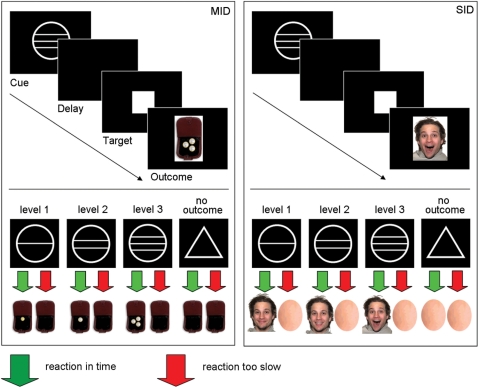
The experiment consisted of two different tasks: the classical ‘monetary incentive delay’ (MID) task as introduced by Knutson et al. (2000), and an adaptation of the former, termed ‘social incentive delay’ (SID) task which aimed to examine participants’ striving to gain positive social feedback (Figure 1).
Fig. 1.
Experimental paradigm for the Monetary (MID) and the Social Incentive Delay Task (SID). Participants were asked to hit a button as fast as possible when a white target square appeared on the screen. To generate reward anticipation, target presentation was preceded by a cue signalling the reward that would be presented if the button was hit fast enough. Circle cues presented different levels of potential reward, while the triangle announced no outcome independent of reaction time.
Each task consisted of 88 trials, yielding a total of 176 trials. In all trials potential gain depended on participants’ ability to hit a button in time whenever a cued target symbol (white square) appeared on the screen (Figure 1). Task difficulty was standardized to a hit rate of ∼66% for all participants by adjusting target duration to individual reaction times. To that end the individual mean reaction time was calculated prior to the experiment based on a simple reaction time task. In the main experiment, cues either signalled potential reward (n per task = 66; denoted by circles) or no outcome (n per task = 22; denoted by a triangle). The degree of potential reward was varied on three levels as indicated by the number of horizontal lines in a cue. The three levels of monetary reward were Euro 0.20 (n = 22, circle with one horizontal line), Euro 1.00 (n = 22, circle with two horizontal lines), and Euro 3.00 (n = 22, circle with three horizontal lines). Success was acknowledged by presenting the picture of a wallet which either contained the respective amount of money in coins or—in the case of no outcome—was empty (Figure 1). Participants were encouraged to respond as fast as possible to all cue types. For the three levels of social reward magnitude (similarly indicated by one, two or three horizontal lines inside the circle cues; see above), three types of happy face expressions with increasing intensity level (happy closed mouth; happy open mouth; happy open mouth exuberant) were used (Figure 1). For the pool of face stimuli 66 photographs displaying three different expressions of 22 different persons (11 female, 11 male) were taken from a standardized database of coloured photographs of professional actors expressing various emotions [NimStim set of Facial Expressions, available at http://www.macbrain.org; (Tottenham et al., in press)]. To generate ‘no outcome’-stimuli 22 portraits were graphically dysmorphed (Adobe PhotoDeluxe Home Edition 3.0, Adobe Systems Incorporated) eliminating all facial features but keeping size and luminance stable.
In both tasks each trial started with the presentation of one of the four cues for 240 ms, followed by a crosshair for a variable length of time (jittered between 2250 and 2750 ms) and the target (individually adjusted presentation time; between 160 and 260 ms). Feedback informing the participant about the outcome of the latest trial was presented for 1650 ms starting 300 ms after target onset. Trial types were pseudo-randomly ordered within two MID and two SID sessions of 44 trials each with inter-trial-intervals jittered between 2500 and 5000 ms. MID and SID sessions were presented interleaved with order of tasks counterbalanced across participants. At the beginning of each session, participants were informed which task (MID or SID) would follow next.
Prior to entering the scanner participants performed a practice session composed of 44 trials per task to familiarize them with the experiment.
fMRI setting
For both tasks, stimuli presentations and recording of reaction times were performed using the software Presentation (Neurobehavioral Systems, Inc., San Francisco, CA). Participants indicated their response by pressing the button of a fibreoptic custom-made response box with the index finger of their right hand.
Image acquisition
Scanning was performed on a 1.5 T whole body scanner (Phillips Medical Systems, Achieva, Best, Netherlands) using standard gradients and a standard quadrature head coil. Participants lay in a supine position, while head movement was limited by foam padding within the head coil. In order to ensure optimal visual acuity, participants were offered fMRI-compatible glasses that could be fixed to the video goggles. For each participant, a series of 840 EPI-scans, lasting approximately 28 min, was acquired. Stimuli were presented in an event-related fashion.
Functional scans covered the region constituting the reward system of the brain (Knutson et al., 2001b; Bjork et al., 2004) and were aligned parallel to the AC/PC line (see supplementary data). The fMRI recording, including five initial dummy scans, had the following parameters: number of slices (NS): 22; slice thickness (ST): 3.8 mm; interslice gap (IG): 0 mm; matrix size (MS): 64 × 64; field of view (FOV): 240 mm × 240 mm; repetition time (TR): 2000 ms; echo time (TE): 50 ms; flip angle (FA): 90°. For anatomical localization, we acquired high resolution images with a T1-weighted 3D FFE sequence (TR = 25 ms; TE = 4.59 ms; NS = 170 (sagittal); ST = 2 mm; IG = 1 mm; FOV = 256 × 256 mm2; voxel size = 1 × 1 × 2 mm3).
Image analysis
The data were preprocessed and analysed using Statistical Parametric Mapping (SPM5) (http://www.fil.ion.ucl.ac.uk) implemented in MATLAB 7.0 (Mathworks Inc., Sherborn, MA, USA). The first five volumes were discarded from further data analysis because of the non-equilibrium state of magnetization.
All remaining images were realigned to the first image to correct for head movement, coregistered with the anatomical 3D image, spatially normalized to the standard template of the Montreal Neurological Institute (MNI, Canada) using the unified segmentation approach (Ashburner and Friston, 2005). Finally the normalized data were smoothed with a resliced voxel size of 4 × 4 × 4 mm3 with a. 6 mm FWHM isotropic Gaussian kernel to allow statistical inference using Gaussian Random Field theory and temporally filtered with a high-pass filter to remove baseline drifts due to cardio-respiratory and other cyclical influences. A random-effects, event-related statistical analysis (Josephs et al., 1997) was performed with SPM5 in a two-level procedure. At the first level, a separate general linear model (GLM) was specified for each participant. Task-related changes in BOLD signal were estimated at each voxel by modelling the onsets of each trial as delta functions convolved with a hemodynamic response function (HRF). Both tasks (MID, SID) with their four levels of potential reward magnitude were modelled as separate regressors. Regression coefficients for all regressors were estimated using least squares within SPM5 (Friston et al., 1995). Condition-specific effects were tested using the appropriate linear contrasts of the parameter estimates for the HRF regressors of all conditions. The resulting set of voxel values for each contrast constitutes a statistical parametric map.
For group inferences (second level), we performed a full factorial analysis (task × reward magnitude × gender) implemented in SPM5 to test for the potential increase of activation with levels of anticipated reward.
To specifically test our hypothesis of signal changes in the NAcc correlating with increasing values of anticipated reward we extracted parameter estimates in a region of interest (ROI) (a sphere of 5 mm radius at the coordinates x = −9, y = 10, z = 0 for the left NAcc and x = 11, y = 12, z = 0 for the right NAcc) based on the results reported by Knutson et al. (2003). Parameter estimates of each side were entered into an ANOVA on repeated measures with within factors ‘task’ (monetary, social) and ‘incentive magnitude’ (low, medium, high) and between factor ‘gender’ (female, male). Effect sizes were calculated using partial eta square (ηP2).
For all group analyses, we applied a voxel-wise threshold of P < 0.001. The reported voxel coordinates of activation peaks were transformed from MNI space to Talairach & Tournoux atlas space (Talairach and Tournoux, 1988) by non-linear transformations (www.mrc-cbu.cam.ac.uk).
RESULTS
Behavioural results
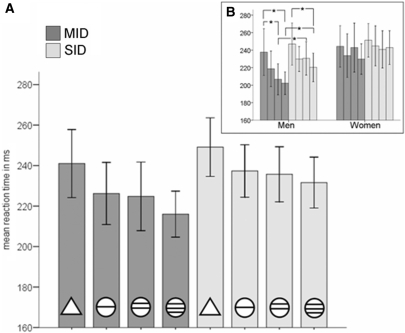
An ANOVA on repeated measures with within factors ‘task’ (monetary, social) and ‘incentive magnitude’ (low, intermediate, high) and between factor ‘gender’ (female, male) performed on reaction times revealed a significant effect of task [F(1,30) = 17.88, P < 0.001, ηP2 = 0.37] with faster mean reaction times in the MID than the SID task [227 ms (SE = 6.80) vs. 238 ms (SE = 6.02)]. A significant main effect of incentive magnitude [F(3,90) = 12.97, P < 0.001, ηP2 = 0.30] reflected a linear decrease of reaction times with increasing level of potential reward in both tasks [see Figure 2a, pairwise post-hoc comparisons revealed significantly faster reaction times for all levels of reward compared to ‘no outcome’ trials (P < 0.05 for all comparisons), linear trend calculation: F(1,30) = 30.14, P < 0.001, ηP2 = 0.5]. However, the group variable ‘gender’ was found to interact with task and incentive magnitude [interaction gender × task × incentive magnitude: F(3,90) = 3.1, P = 0.03, ηP2 = 0.09]. Male participants reacted faster to cues signalling high monetary rewards than high social rewards while reacting equally slow in both tasks to cues signalling low levels or ‘no outcome’. In contrast, female reaction times did not differ significantly with respect to incentive type or magnitude (Figure 2b).
Fig. 2.
Mean reaction times to the reward signalling cues in the MID and the SID task. (A) Mean reaction times of all participants to the cues announcing potential monetary (dark grey columns) or social reward (light grey columns). Circles with one, two, and three bars announce increasing levels of potential reward, triangles represent no outcome. (B) Mean reaction times in the MID (dark grey columns) and the SID task (light grey columns), separated for men (left) and women (right). Error bars indicate the standard error of the mean (s.e.m). *P < 0.01 (paired t-test, two-tailed).
Neuroimaging results
Linear increase of anticipated reward
Testing for a linear increase of activation in response to increasing levels of anticipated monetary or social rewards revealed significant activation of cortical structures constituting the reward system (Knutson et al., 2001a; Bjork et al., 2004), specifically the putamen, bilateral NAcc, thalamus and precuneus.
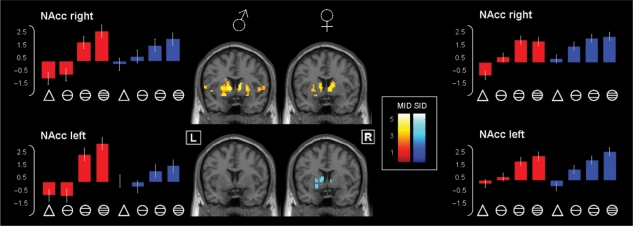
The ANOVA performed on parameter estimates extracted at the coordinates x = −9, y = 10, z = 0 (left NAcc) and x = 11, y = 12, z = 0 (right NAcc) (Knutson et al., 2003) revealed main effects of incentive magnitude in both, left (F(3,90) = 26.46, P < 0.001, η2p = 0.47) and right NAcc [F(3,90) = 29.31, P < 0.001, η2p = 0.49], reflecting a linear increase of activations [linear trend for NAcc left: F(1,30) = 49.43, P < 0.001, η2p = 0.62, and right: F(1,30) = 83.23, P < 0.001, η2p = 0.74] with higher levels of anticipated reward independent of incentive type and gender (Figure 3; box plots; see supplementary data for complete results of the ANOVA and post-hoc comparisons).
Fig. 3.
Activation foci for male and female participants during MID and SID linear anticipation. Brain areas increasingly activated by anticipated increasing amounts of money (MID task, triangle < circle with one bar < circle with two bars < circle with three bars) are displayed in red, brain areas increasingly activated by anticipated increasing amounts of social approval (SID task, triangle < circle with one bar < circle with two bars < circle with three bars) are displayed in blue separated for men (left) and women (right). The activation maps (P < 0.001, uncorrected; minimal cluster size 10 voxels) are projected onto coronal slices (y = 6) of the T1-weighted SPM-template. In the SID task, male participants did not show any activation at the chosen threshold. (It has to be noted though that if the threshold was lowered to P < 0.01, uncorrected, activation of the left striatum could also be seen in men). Bars represent parameter estimates for the MID (red bars) and the SID task (blue bars) in ROIs for the right and left NAcc, separated for men (left) and women (right). Error bars indicate the standard error of the mean (SEM).
However, the pattern of brain areas showing increased activation for increasing levels of anticipated reward was task-dependently different among men and women: at the same activation threshold (P < 0.001, uncorrected; see Figure 3) men displayed a wider network of brain areas sensitive to the increasing level of monetary reward than women, contrasted by only little activation for increasing levels of social reward. In contrast, women displayed a rather balanced cortical activation pattern with respect to increasing levels of monetary and social rewards (Figure 3 and Table 1). To test for quantitative differences between activation in men and women in the respective tasks we computed voxel-wise t-tests for all comparisons (MID: men > women and women > men; SID: men > women and women > men). This approach revealed stronger activation in response to increasing levels of anticipated monetary reward in men than women in the left putamen (−18, 18, −3; P < 0.05, SVC). In the SID task women showed stronger activation in response to increasing levels of anticipated social rewards than men in the right caudate nucleus (18, 3, 21; P < 0.05, SVC). The opposite comparisons (MID: women > men and SID: men > women) did not reveal any effects.
Table 1.
Brain regions showing proportional activation to increasing anticipated reward
| Brain regions | MNI-coordinates |
|||||
|---|---|---|---|---|---|---|
| BA | x | y | z | Peak z-score | Cluster size | |
| MID all participants (N = 32) | ||||||
| R NAcc | 9 | 15 | −1 | 7.01 | 451* | |
| R thalamus | 3 | −17 | 12 | 6.92 | ||
| L NAcc | −9 | 9 | 0 | 6.84 | ||
| R inferior middle occipital lobe | 18/19 | 24 | −89 | 18 | 7.21 | 417* |
| L inferior middle occipital lobe | 18/19 | −21 | −90 | 10 | 7.23 | 102* |
| R inferior frontal gyrus | 47 | 33 | 26 | −9 | 5.06 | 17* |
| L precuneus | 7 | −12 | −65 | 34 | 5.70 | 15* |
| SID all participants (N = 32) | ||||||
| R inferior middle occipital lobe | 18/19 | 27 | −90 | 13 | 5.15 | 52** |
| L putamen | −18 | 12 | −1 | 4.31 | 34** | |
| L NAcc | −9 | 6 | 5 | 3.78 | ||
| R putamen | 21 | 9 | 8 | 3.87 | 23** | |
| L inferior middle occipital lobe | 18/19 | −24 | −90 | 10 | 5.31 | 21** |
| R inferior frontal gyrus | 30 | 32 | −2 | 4.27 | 14** | |
| MID male participants (N = 16) | ||||||
| R inferior middle occipital lobe | 18/19 | 24 | −90 | 16 | 5.67 | 91* |
| R NAcc | 9 | 15 | −1 | 6.24 | 69* | |
| R anterior cingulum | 15 | 32 | −4 | 5.73 | ||
| R caudate | 15 | −2 | 17 | 5.44 | ||
| L NAcc | −9 | 12 | −1 | 6.23 | 58* | |
| R cuneus | 18 | 12 | −69 | 15 | 5.97 | 27* |
| R superior occipital gyrus | 39 | 39 | −77 | 26 | 5.63 | 14* |
| L amygdala | −21 | 2 | −10 | 5.64 | 13* | |
| SID male participants (N = 16) | ||||||
| R inferior middle occipital lobe | 18/19 | 24 | −90 | 10 | 2.91 | 14*** |
| L putamen | −18 | 12 | −1 | 3.01 | 11*** | |
| R putamen | 21 | 9 | 8 | 2.83 | 10*** | |
| MID female participants (N = 16) | ||||||
| L thalamus | −3 | 32 | 2 | 5.78 | 405** | |
| R thalamus | 3 | −17 | 12 | 4.73 | ||
| L putamen | −21 | 11 | −6 | 4.52 | ||
| L inferior middle occipital lobe | 18/19 | −21 | −90 | 10 | 5.56 | 112** |
| L (dorsal) anterior cingulum | −6 | 28 | 26 | 5.33 | 110** | |
| R (dorsal) anterior cingulum | 0 | 19 | 32 | 4.03 | ||
| R NAcc | 15 | 9 | 5 | 4.73 | 108** | |
| R putamen | 18 | −2 | 11 | 3.99 | ||
| R inferior middle occipital lobe | 18/19 | 21 | −90 | 10 | 4.73 | 98** |
| R cuneus | 18 | 12 | −72 | 15 | 4.07 | 89** |
| R (ventral) anterior cingulum | 3 | 49 | −13 | 4.76 | 65** | |
| R posterior cingulum | 30 | 21 | −52 | 5 | 4.04 | 22** |
| SID female participants (N = 16) | ||||||
| L putamen | −18 | 5 | −8 | 4.25 | 83** | |
| L NAcc | −9 | 6 | 5 | 4.10 | ||
| R thalamus | 0 | −17 | 9 | 3.92 | ||
| R inferior middle occipital lobe | 18/19 | 27 | −87 | 13 | 4.98 | 51** |
| R cuneus | 18 | 15 | −92 | 16 | 4.30 | |
| L inferior middle occipital lobe | 18/19 | −24 | −90 | 10 | 5.44 | 22** |
| R middle temporal gyrus | 39 | 42 | −57 | 25 | 4.41 | 19** |
| R insula | 13 | 24 | 18 | 2 | 4.04 | 15** |
| R (dorsal) anterior cingulum | 0 | 24 | 18 | 3.73 | 14** | |
| L (dorsal) anterior cingulum | −6 | 25 | 26 | 3.36 | ||
| R caudate | 6 | 0 | 3 | 4.05 | 13** | |
*Cluster defined using P < 0.05 FWE; **P < 0.001 uncorrected; ***P < 0.01, uncorrected.
DISCUSSION
In the present study we demonstrated that the prospect of earning positive social feedback in the form of an approvingly smiling face leads to activation of brain areas also active during the anticipation of monetary rewards, specifically the dorsal and ventral striatum (including NAcc). Moreover, independent of incentive type, striatal activation was found to proportionally increase in response to cues announcing increasingly higher levels of reward. In both tasks (MID and SID), striatal activation increase was accompanied by a decrease in reaction times. The result replicates previous findings based solely on monetary reward anticipation (Knutson et al., 2003). In addition, this is the first study to show that the same linear relationship applies to anticipated social feedback.
However, based on earlier data we expected that gender would differently affect performances in the two tasks. Indeed, a gender effect was found for behavioural as well as brain data. Men were more strongly affected by the magnitude of the anticipated reward than women, reacting faster to cues signalling high levels of reward than low levels. In addition male participants reacted faster to cues announcing high levels of reward in the MID than the SID task. In contrast, women's reaction times did not differ between the two tasks. The behavioural data was matched by gender-specific patterns of brain activation. Male participants displayed a wide network of reward-related brain areas sensitive to increasing levels of monetary reward, including anterior cingulum, caudate, amygdala, cuneus and bilateral NAcc, contrasted by only little activation for increasing levels of social reward. In women a wider network of brain areas was sensitive to increasing levels of social reward than in men; importantly, these cortical areas were strongly overlapping with those responsive to monetary incentives. The pattern of gender effects of the present study replicates our earlier purely behavioural findings of faster reaction times in the prospect of monetary than social reward in men but not women, while at the same time women do not exhibit a difference in performance level in the prospect of either reward (K.S. et al., submitted).
The parameter-estimates derived from ROIs in the left and right NAcc revealed a correlation between activation and level of anticipated reward for men and women in both tasks. The finding adds to data identifying the NAcci as the principle predictors of reward based on learned associations, independent of incentive type (Gottfried et al., 2002, 2003; O’Doherty et al., 2004). The value of the cued reward is hereby coded by brain activation level, reflected in the fMRI BOLD-response. Though the fMRI technique cannot provide us with a clear answer to the question what causes the activation, it can be assumed that dopamine projections from the ventral tegmental area play a crucial role (Satoh et al., 2003). It is known from animal studies that dopamine increase in the midbrain encodes the value of potential outcome (Morris et al., 2006; Wilson and Bowman, 2006) forming an important prerequisite for goal-directed behaviour (Hollerman et al., 2000). Recent fMRI studies have used pharmacological manipulations to elucidate the link between dopamine release and increased BOLD response in mesolimbic regions associated with reward (Marota et al., 2000; Dixon et al., 2005). The findings implicate that the BOLD-signal is a consequence of increased oxygen consumption caused by postsynaptic (presumably D1-receptor modulated) metabolic processes after dopamine release (Knutson and Gibbs, 2007).
What is particularly interesting about the present data is that it clearly shows the differential effect individual valuation has on the mesolimbic reaction. Though no differences in the degree of the activation between men and women was found in the ROI analysis of the NAcc, two-sample t-tests revealed stronger left putamen activation in men than women if money was to be expected. In contrast, anticipation of social approval led to stronger activation of the right caudate in women as opposed to men.
The gender-specific activation patterns for the two different incentive types, money and social approval, can be taken to reflect differences in motivational value subjectively attributed to the respective reward. A striking preponderance of brain activation as well as reaction times for monetary compared to social anticipated rewards in men is contrasted by only small differences with respect to both incentive types in women. An imbalance of striatal activation due to gender-differences in motivational value has already been reported for relatively abstract forms of reward such as humour (Azim et al., 2005) and ‘increase of one's own space’ during a computer game (Hoeft et al., 2008). Our data underlines the importance of accounting for individual preferences when studying reward processes.
In conclusion, the present study delineates the brain structures activated by the anticipation of positive social feedback. By assessing brain activity to anticipated social and anticipated monetary rewards in the same experiment, our results clearly show that anticipation of social approval shares the same neural basis as anticipation of monetary rewards, displaying proportional activation with increasing levels of anticipated reward. Turning our attention to gender effects revealed ample differences in brain activation patterns underlying anticipation of different reward types between men and women. Together with recent findings linking genetic variability in the dopaminergic system to differences in reward-related behaviour (Yacubian et al., 2007), our data underlines the importance of considering inter-individuality as modulating factor in future studies on reward processing.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Supplementary Material
Acknowledgments
This work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG), grant GR 1399/5-1 and with a scholarship of the DFG (IRTG 1328) to G.K.
REFERENCES
- Aharon I, Etcoff N, Ariely D, Chabris CF, O’Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Azim E, Mobbs D, Jo B, Menon V, Reiss AL. Sex differences in brain activation elicited by humor. Proceedings of National Academy of Sciences USA. 2005;102:16496–16501. doi: 10.1073/pnas.0408456102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. Journal of Autism and Developmental Disorders. 2004;34:163–175. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. Journal of Neuroscience. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Cloninger C, Przybeck T, Svrakic D. The tridimensional personality questionnaire: U.S. normative data. Psychological Reports. 1991;69:1047–1057. doi: 10.2466/pr0.1991.69.3.1047. [DOI] [PubMed] [Google Scholar]
- Dixon AL, Prior M, Morris PM, Shah YB, Joseph MH, Young AM. Dopamine antagonist modulation of amphetamine response as detected using pharmacological MRI. Neuropharmacology. 2005;48:236–245. doi: 10.1016/j.neuropharm.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Elliott R, Newman JL, Longe OA, Deakin JF. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. Journal of Neuroscience. 2003;23:303–307. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr E, Camerer CF. Social neuroeconomics: the neural circuitry of social preferences. Trends in Cognitive Science. 2007;11:419–427. doi: 10.1016/j.tics.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J.-P, Frith CD, Frackowiak R. SJ. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. Journal of Neuroscience. 2002;22:10829–10837. doi: 10.1523/JNEUROSCI.22-24-10829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Graham GH, Unruh J. The motivational impact of nonfinancial employee appreciation practices on medical technologists. Health Care Supervisor. 1990;8:9–17. [PubMed] [Google Scholar]
- Hoeft F, Watson CL, Kesler SR, Bettinger KE, Reiss AL. Gender differences in the mesocorticolimbic system during computer game-play. Journal of Psychiatry Research. 2008;42:253–258. doi: 10.1016/j.jpsychires.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Tremblay L, Schultz W. Involvement of basal ganglia and orbitofrontal cortex in goal-directed behavior. Progress in Brain Research. 2000;126:193–215. doi: 10.1016/S0079-6123(00)26015-9. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58:284–294. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Josephs O, Turner R, Friston KJ. Event-related fMRI. Human Brain Mapping. 1997;5:243–248. doi: 10.1002/(SICI)1097-0193(1997)5:4<243::AID-HBM7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. American Journal of Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Schienle A, Stark R, et al. Anticipation of reward in a nonaversive differential conditioning paradigm and the brain reward system: an event-related fMRI study. NeuroImage. 2003;20:1086–1095. doi: 10.1016/S1053-8119(03)00381-1. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001a;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Current Opinion of Neurology. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001b;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. NeuroImage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Knutson B, Gibbs SE. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology. 2007;191:813–822. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Kohls G, Peltzer J, Herpertz-Dahlmann B, Konrad K. Differential effects of social and non-social reward on response inhibition in children and adolescents. Developmental Science. doi: 10.1111/j.1467-7687.2009.00816.x. (in press) [DOI] [PubMed] [Google Scholar]
- Krebs H, Weyers P, Janke W. Validation of the German version of Cloninger's TPQ: replication and correlations with stress coping, mood measures and drug use. Personality and Individual Differences. 1998;24:805–814. [Google Scholar]
- Li CR, Huang C.-Y, Lin W, Sun C. WV. Gender differences in punishment and reward sensitivity in a sample of Taiwanese college students. Personality and Individual Differences. 2007;43:457–483. [Google Scholar]
- Lindahl L. What makes a good job? Personnel. 1949;Vol. 25:262–266. [Google Scholar]
- Marota JJ, Mandeville JB, Weisskoff RM, Moskowitz MA, Rosen BR, Kosofsky BE. Cocaine activation discriminates dopaminergic projections by temporal response: an fMRI study in rat. NeuroImage. 2000;11:13–23. doi: 10.1006/nimg.1999.0520. [DOI] [PubMed] [Google Scholar]
- McClure EB. A meta-analytic review of sex differences in facial expression processing and their development in infants, children, and adolescents. Psychological Bulletin. 2000;126:424–453. doi: 10.1037/0033-2909.126.3.424. [DOI] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. Journal of Neuroscience. 2007;27:5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G, Nevet A, Arkadir D, Vaadia E, Bergman H. Midbrain dopamine neurons encode decisions for future action. National Neuroscience. 2006;9:1057–1063. doi: 10.1038/nn1743. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. NeuroImage. 2004;21:583–592. doi: 10.1016/j.neuroimage.2003.10.005. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Otter C, Huber J, Bonner A. Cloninger's Tridimensional Personality Questionnaire: reliability in an English sample. Personality and Individual Differences. 1995;18:471–480. [Google Scholar]
- Pessiglione M, Schmidt L, Draganski B, et al. How the brain translates money into force: a neuroimaging study of subliminal motivation. Science. 2007;316:904–906. doi: 10.1126/science.1140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proverbio AM, Zani A, Adorni R. Neural markers of a greater female responsiveness to social stimuli. BMC Neuroscience. 2008;9:56. doi: 10.1186/1471-2202-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R, DePaulo BM. Sex differences in eavesdropping on nonverbal cues. Journal of Personality and Social Psychology. 1979;37:273–285. doi: 10.1037//0022-3514.37.10.1713. [DOI] [PubMed] [Google Scholar]
- Satoh T, Nakai S, Sato T, Kimura M. Correlated coding of motivation and outcome of decision by dopamine neurons. Journal of Neuroscience. 2003;23:9913–9923. doi: 10.1523/JNEUROSCI.23-30-09913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Skinner BF. 1953. Science and Human Behavior (New York, The Free Press)
- Sorce JF, Emde RN, Campos J, Klinnert MD. Maternal emotional signaling: its effects on the visual cliff behavior of 1-year-olds. Developmental Psychology. 1985;21:195–200. [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System – an Approach to Cerebral Imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Thibaut JW, Kelley HH. The Social Psychology of Groups. New York: Wiley; 1959. [Google Scholar]
- Torrubia R, Ávilab AC, Moltób J, Caserasa X. The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray's anxiety and impulsivity dimensions. Personality and Individual Differences. 2001;31:837–862. [Google Scholar]
- Tottenham N, Tanaka J, Leon A, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. in press. [DOI] [PMC free article] [PubMed]
- Walter H, Abler B, Ciaramidaro A, Erk S. Motivating forces of human actions. Neuroimaging reward and social interaction. Brain Research Bulletin. 2005;67:368–381. doi: 10.1016/j.brainresbull.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Wilson DI, Bowman EM. Neurons in dopamine-rich areas of the rat medial midbrain predominantly encode the outcome-related rather than behavioural switching properties of conditioned stimuli. European Journal of Neuroscience. 2006;23:205–218. doi: 10.1111/j.1460-9568.2005.04535.x. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Sommer T, Schroeder K, et al. Gene-gene interaction associated with neural reward sensitivity. Proceedings of National Academy of Science USA. 2007;104:8125–8130. doi: 10.1073/pnas.0702029104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42:509–517. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.