Abstract
In the era of communication technology, new options are now available for following-up patients implanted with pacemakers (PMs) and defibrillators (ICDs). Most major companies offer devices with wireless capabilities that communicate automatically with home transmitters, which then relay data to the physician, thereby allowing remote patient follow-up and monitoring. These systems are being widely used in the USA for remote follow-up, and have been more recently introduced in Europe, where their adoption is increasing. In this article, we describe the currently existing systems, review the available evidence in the literature regarding remote follow-up and monitoring of PMs and ICDs, and finally discuss some unresolved issues.
Keywords: Pacemaker, Implantable cardioverter defibrillator, Telemedicine, Remote monitoring, Remote follow-up
Introduction
With increasing awareness of indications for pacemakers (PMs) and especially implantable cardioverter defibrillators (ICDs), the number of patients with implantable devices has been growing steadily. According to the recent ACC/AHA/HRS guidelines,1 patients with a PM should be followed-up every 3–12 months, and those with an ICD every 3–6 months (with more frequent follow-ups as the battery approaches elective replacement, Table 1). Altogether, this implies frequent visits for the patient, and for the outpatient clinic a significant increase in workload. Transtelephonic monitoring has been available for many years, but provides only basic information on battery status and capture thresholds, and is limited to follow-up of PMs. More recently, home transmitters are available from most major device companies that are able to interrogate the device, either manually by the patient using a telemetry wand or automatically using wireless technology. The data downloaded from the device by the transmitter is then sent to the physician, using either the landline phone or the GSM network. Many current PMs and ICDs are able to automatically execute the tests that are performed manually at the outpatient clinic, such as battery status, lead impedances, or sensing and capture thresholds. Data acquired automatically on a pre-defined periodic basis by the device can then be sent from the patient's home to the physician using the transmitter (thus avoiding an unnecessary in-clinic visit), hence the term remote follow-up. Another aspect is remote monitoring, which concerns data acquired automatically on a daily basis by the device, with unscheduled transmission of any pre-defined alerts to the physician. These alerts may involve device integrity (e.g. battery status, lead impedance), programming issues (e.g. disabling of ventricular fibrillation therapy, insufficient safety margins for sensing or capture), or medical data (e.g. arrhythmias, indication of lung fluid accumulation). Therefore, remote monitoring has the potential to offer improved patient safety and quality of care. A comprehensive review on remote monitoring and follow-up of implantable devices has been published last year.2 Our aim is to provide an update on this rapidly evolving topic by presenting new data and describing the currently available technology.
Table 1.
Minimum frequency of pacemakers/implantable cardioverter defibrillator/cardiac resynchronization therapy monitoring and possibilities of remote follow-up
| Direct follow-up | Remote follow-up | |
|---|---|---|
| Within 72 h of implantation | X | — |
| 2–12 weeks post-implantation | X | — |
| Every 3–12 months (PM/CRT-P) | X | X |
| Every 3–6 months (ICD/CRT-D) | X | X |
| Annually until battery depletion | X | — |
| Every 1–3 months at signs of battery depletion | X | X |
Adapted from Wilkoff et al.1
Existing remote monitoring systems
Most major PM/ICD manufacturers have introduced their version of remote monitoring system (Figure 1 and Table 2):
Home Monitoring™ (Biotronik, Berlin, Germany)
CareLink Network™ (Medtronic, Inc., MN, USA)
Latitude Patient Management system™ (Boston Scientific, St Paul, USA)
Merlin.net™ (St Jude Medical, Sylmar, USA).
Figure 1.
Currently available transmitters from different device manufacturers.
Table 2.
Comparison of different remote monitoring systems
| Biotronik Home Monitoring™ | Medtronic CareLink™ | Boston Scientific Latitude™ | St Jude Merlin.net™ | |
|---|---|---|---|---|
| Wireless communication with implanted device | Radiofrequency | Radiofrequency | Radiofrequency | Radiofrequency |
| Data transmission | GSM network | Analogue phoneline | Analogue phoneline | Analogue phoneline |
| Transmitter | Mobile | Stationary | Stationary | Stationary |
| Frequency of transmissions | Daily FU; Alert events | Scheduled FU; Alert events | Scheduled FU; Alert events | Scheduled FU; Alert events |
| Remote follow-up | Yes | Yes | Yes | Yes |
| Remote monitoring | Yes | Yes | Yes | Yes |
| Physician notification | SMS, e-mail, fax | SMS, e-mail | Fax, phone | Fax, e-mail, SMS |
| Feedback to patient via transmitter | LED indicating normal status or call to clinic | LED indicating normal status or call to clinic | Automatic text and audio messages | LED indicating call to clinic, automated phone calls |
| IEGM (real-time at remote follow-up) | 30 s (monthly periodic EGMs) | 10 s | 10 s | 30 s |
| IEGM (arrhythmic episodes) | All memorized episodes | All memorized episodes | All memorized episodes | All memorized episodes |
| Special features | Alerts fully configurable online | Automatic RA, RV and LV (only Consulta) pacing thresholds | Optional wireless weight scales and BP cuffs | Alerts fully configurable online |
| Automatic RV and LV thresholds (only Lumax 500/540) | Optivol® lung fluid status alert | Configurable data transmission to associated caregivers | Possibility of sending automated phone calls to patients | |
| Wireless PMs | Configurable red and yellow alerts | Configurable red and yellow alerts | Automatic RA, RV, and LV pacing thresholds (next generation of ICDs) | |
| Electronic health record data export capability |
RA, Right atrial; RV, right ventricular; LV, left ventricular; IEGM, intracardiac electrogram; BP, blood pressure.
All current models of PMs and ICDs from these manufacturers are able to be manually interrogated by the patient using a telemetry wand incorporated in the home transmitter and are thus able to perform remote follow-ups. However, automatic wireless interrogation of the device is preferable, as it depends less on patient compliance and allows frequent transmissions, which is mandatory for effective remote monitoring. Automatic wireless interrogation requires that the implanted device is equipped with a micro-antenna for communication with the transmitter located close to the patient. Data are then sent to a central database using either the analogue landline phone system and a toll-free number (Boston Scientific, Medtronic and St-Jude Medical systems) or via the GSM network (Biotronik). The data are processed and made accessible to the physician on a secured webpage. The physician is informed by e-mail, SMS, fax, or phone messages whenever critical data are available for consultation. The types of events which trigger an alert can be customized for each patient. None of the systems currently allows remote device programming (although this is technically feasible), essentially for safety issues.
Biotronik (Home Monitoring®)
This company is the pioneer in the field of remote PM/ICD follow-up and monitoring, with FDA approval of their first system in 2001. The transmitter (CardioMessenger®) is a little bigger than a cell phone and communicates wirelessly with the implanted device within a radius of 2 m. It sends the retrieved data to a centre in Germany using the GSM network, which is the main advantage of the system. This is an important issue today, as the patient may not have a landline phone connection (due to increased use of cell phones) or may have a digital landline connection (currently incompatible with all device systems) or a DSL/VOIP connection that requires special filters and converters. It also means that the patient can be monitored continuously around the clock, as the CardioMessenger has a rechargeable battery that allows it to be carried around by the patient. Furthermore, the patient can continue to be monitored while travelling abroad with the CardioMessenger, as the system is compatible with most available GSM networks throughout the world. Parameters for the alerts can be fully configured on the secured webpage, without having to bring the patient into the clinic for a manual transmission (Figure 2). EGMs of 30 s duration are sent periodically that may assist with data interpretation (Figure 3). Biotronik is the only company today that offers PMs with wireless technology for remote monitoring (other manufacturers are also going to offer wireless PMs in the future).
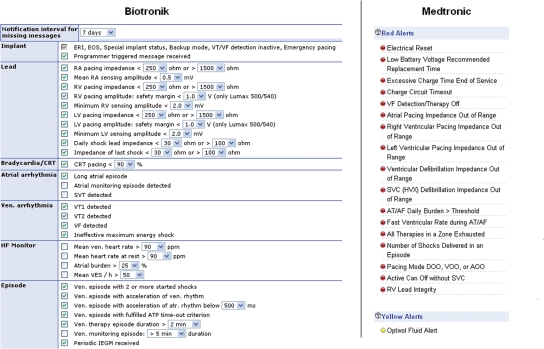
Figure 2.
Remote monitoring parameters (print screens) for CRT-D devices available at the University Hospital of Geneva at the time this manuscript was submitted. The Biotronik Lumax-540 CRT-D (left) can be fully configured online. Parameters of a Medtronic Consulta CRT-D (right) can be defined as red or yellow alerts (or website alert only) that trigger different urgencies of notification.
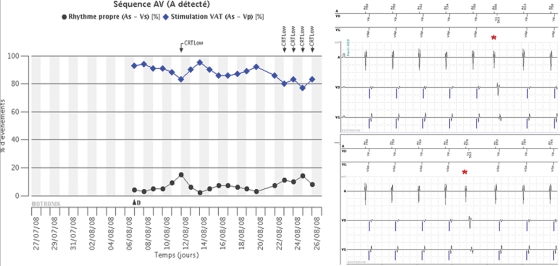
Figure 3.
(A) Biotronik Home Monitor showing trends of AS–VS events (black dots) and As–Vp events (blue diamonds). Alerts of reduced (< 85%) CRT delivery are indicated (‘CRT low’ arrows) and coincide with increased percentage of AS–VS events (black dots). This would suggest that the AV interval is programmed too long. (B) The real-time EGM strip available by remote monitoring however shows that the AS–VS events are due to premature atrial beats (red asterisks) with a short intrinsic AV interval (most probably due to proximity of the ectopic focus with the AV node). Doses of beta-blockers were increased, with improvement in CRT delivery.
Medtronic (Carelink Network™)
The system has been introduced in Europe in a pilot study in 2005.3 The ICD communicates with a wireless transmitter (Home Monitor) within a radius of 3 m, which is usually placed in proximity to the patient's bed. Data are sent via an analogue phone landline, which can only be used in the patient's country of residence (a carrier-independent, international solution is being developed). Pre-scheduled remote follow-up transmissions (that take place at 03:00) may be programmed remotely, but can be executed only after 21 days. In addition, for remote monitoring purposes, detection of an event (e.g. abnormal lead impedance etc.) will immediately trigger the ICD to attempt communication with the Home Monitor (with repeated attempts every 3 h during 3 days in case of inability to establish communication, and then by audible alerts). The parameters for remote monitoring can be configured individually in each patient, with pre-defined degrees of urgency (‘red’ and ‘yellow’ alerts, Figure 2), which helps with data triage. However, the threshold settings for each alert require in-clinic programming of the device. An advantage with the latest generation of Medtronic implantable devices is the ability to automatically perform a wide range of tests, including automatic atrial, right ventricular, and left ventricular capture thresholds, which facilitates full remote follow-up. A unique feature with Medtronic dual-chamber and biventricular ICDs is the Optivol® algorithm that monitors transthoracic impedance for detecting lung fluid accumulation, which may be potentially useful for remote monitoring of patients with heart failure.
Boston Scientific (Latitude Patient Management system™)
This system is to be introduced in Europe in 2009. The transmitter also uses an analogue landline for data transmission, which may be configured for use in various countries (but may require plug adapters). A unique feature of the system is the possibility to connect wireless weight scales and blood pressure cuffs for remote monitoring of heart failure status (Figure 1). Also, the patient can self-report heart failure symptoms into the system on a weekly basis (such as fatigue, ankle swelling, orthopnea etc.). Event notifications can be configured individually in each patient based upon ‘red’ and ‘yellow’ alerts. Furthermore, the system allows customizable data transmission to different physicians (for example to the general practitioner or general cardiologist in addition to the heart rhythm specialist), which improves networking of heart failure management.
St Jude Medical (Merlin.net™)
The wireless transmitter (Merlin@home) has been introduced in Europe in 2008 at pilot centres. The system communicates automatically by radiofrequency with the implantable device and sends data to the physician using the analogue landline system (a cellular adapter card is planned for 2009). As with the other available systems, remote monitoring is possible with alerts being sent by e-mail, fax, or SMS to notify the physician of events. A useful feature is the ability for the physician to indicate alerts or reminders of scheduled in-office visits on the patient's transmitter, and to send automated phone calls to patients indicating the results of the remote follow-up (e.g. that everything is normal). The next generation of ICDs will also have capture thresholds for all leads, which will facilitate full remote follow-up.
Potential benefits and existing evidence of utility
Reduction of in-clinic visits
In a study by Brugada,4 271 patients with a Biotronik ICD and Home Monitoring® system were followed for 12 months with routine follow-up every 3 months. Retrospective analysis of the Biotronik Home Monitoring® data showed that as many as half of the regular scheduled visits may have been skipped, without impairing patient safety.
More recently Heidbuchel et al.5 retrospectively analysed data from 1739 in-clinic ICD visits in 169 patients. The authors found that only 6% of scheduled in-clinic visits resulted in device reprogramming or patient hospitalization. Thus, in 94% of all scheduled visits, remote follow-up would have sufficed. Furthermore, they estimated that ICD remote monitoring could potentially diagnose > 99% of arrhythmia- or device-related problems, if combined with clinical follow-up by the local general practitioner and/or the referring cardiologist.
Preliminary results of the TRUST (Lumax-T/Lumos-T safely RedUceS rouTine office device follow-up) study were recently presented.6 Data on 1312 patients with a Biotronik VR/DR ICD were randomized to standard (3 months) in-office visits vs. remote monitoring (with in-office visits at 3 and 15 months). Remote monitoring and follow-up resulted in a 43% relative reduction in visits (from 3.0 to 2.5 visits/patient-year, P < 0.001) with a better adherence to the scheduled follow-up timetable. The trial demonstrated also similar safety (death, stroke, or event-related surgery) by remote monitoring compared with in-clinic control group.
In addition to reducing scheduled in-clinic visits, remote follow-up may avoid unscheduled visits following an ICD shock.7,8 After such an event, the patient may perform manual interrogation to upload data to the physician for determining if the shock was appropriate or not, and it may then be decided whether the patient should be seen for device reprogramming or modification of drug therapy.
Patients with cardiac resynchronization therapy (CRT) may require special consideration, as they are usually sicker and have more complex devices with specific issues (e.g. requirement for atrio-ventricular interval optimization, greater variations in left ventricular thresholds, phrenic nerve capture etc.). Thus frequency of follow-up and requirement for in-office device reprogramming may be greater than in patients with standard PMs or ICDs. In a report from the Insync ICD Italian Registry,9 there was a marked reduction in number of interrogations requiring reprogramming between the first 6 months of follow-up and subsequent periods, as pacing and CRT delivery parameters were usually optimized relatively quickly after implantation and maintained unmodified thereafter. This means that remote follow-up is an acceptable alternative in these patients, especially with the advent of algorithms that automatically adjust device settings such as left ventricular pacing output based upon daily threshold measurements.10
Even though remote device monitoring and interrogation has the potential to reduce numbers of in-clinic visits, it does not entirely replace direct contact (that is valued by many patients). According to current guidelines, in-office visits need to take place at least yearly (Table 1), but this may change in the future for subsets of patients (e.g. primary prevention ICD patients without clinical events or elderly/disabled patients etc.).
Improved patient safety
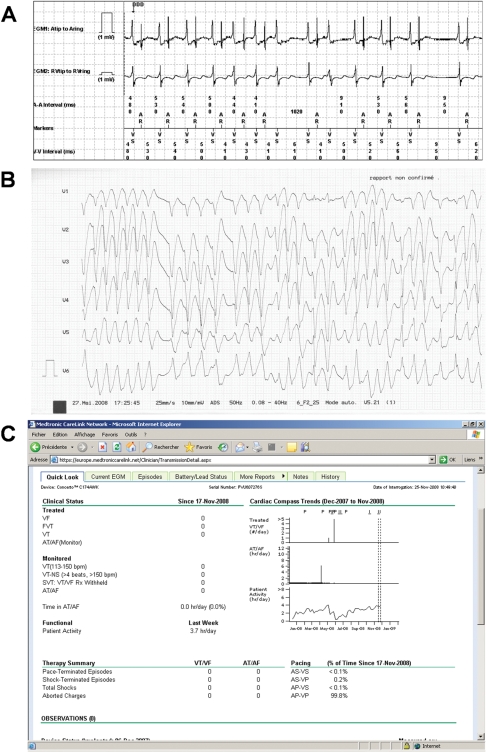
Figure 4 shows an example of a patient in whom remote follow-up proved to be life-saving.
Figure 4.
A 66-year-old patient with a Medtronic Concerto CRT-D for primary prevention of sudden death phoned the clinic complaining of fatigue since 2 days, without any malaise or ICD shocks. (A) Remote interrogation of the device with the real-time 10 s rhythm strip (partly displayed) showed slow irregular VT (a ventricular far-field is seen in the atrial EGM). No episodes of SVT or ventricular arrythmias (faster than 140 b.p.m.) were recorded by the device. On the basis of the EGM findings, the patient was asked to come directly to the clinic. (B) Immediately upon arrival in the clinic 15 min later, the patient collapsed due to rapid incessant ventricular tachycardia (VT) that led to 22 ICD shocks. Injection of amiodarone resulted in slowing and finally disappearance of the VT. The patient developed multiorgan failure, but recovered subsequently and was seen in-office 1 month later, with no events. (C) Print screen of the CareLink website of remote interrogation of the device 6 months after the arrhythmic storm (asterisk) showing no arrhythmia (faster than 113 b.p.m.), with improvement of the patients’ physical activity (arrow).
In a recent study by Nielsen et al.,11 patients with a Biotronik ICD and Home Monitoring™ system were prospectively enrolled in a registry for a mean follow-up of 10 months. At least one Home Monitoring event occurred in 41% of patients, consisting mostly of clinical events (such as arrhythmias). Technical events (inappropriate shock, lead impedance issues etc.) occurred in ∼3% of patients. The temporal distribution of home monitoring events is interesting, as over 60% occurred during the first month following the last visit. In another report using the Biotronik Home Monitoring system with over 3 million transmissions in > 11 000 patients,12 the mean interval between last follow-up and occurrence of events notified by home monitoring was 26 days. This represented a temporal gain of 154 days for event notification in patients usually followed at 6 months intervals and of 64 days in patients usually followed at 3 months intervals. This has implications for diagnosing technical issues such as lead fracture, device malfunction,13 or clinical events such as onset of atrial fibrillation (AF). Continuous monitoring for AF is particularly interesting, as it offers new treatment strategies for anti-arrhythmic drug therapy and anticoagulation. In a report by Ricci et al.14 of 166 patients implanted with a Biotronik PM or ICD using the Home Monitor system during a mean follow-up of 16 months, remote monitoring alerts for AF were triggered in 25% of patients, with an unscheduled follow-up (in-office or by phone) resulting in interventions involving anti-arrhythmic drug therapy, anticoagulation, or antiplatelet drug initiation, external cardioversion, or device reprogramming, in 17% of all patients.
Remote monitoring may be particularly useful in patients with CRT, as they are most likely to have transmissions of medically related events.12 A number of different randomized trials are currently underway to assess the utility of monitoring lung fluid overload in patients with heart failure (Table 3).
Table 3.
Ongoing multicentre randomized studies on remote device monitoring and follow-up registered on www.clinicaltrials.gov
| Study | Sponsor | Device | Projected size | Primary outcome measures | Status |
|---|---|---|---|---|---|
| CONNECT28,29 | Medtronic | CRT-D, ICD-DR | 2000 | Reduction in time to clinical decision for arrhythmias, cardiovascular disease progression, and system issues | Active, not recruiting |
| effecT30 | Biotronik | CRT-D | 300 | Mortality and morbidity in patients with AF | Recruiting |
| EuroEco31 | Biotronik | ICD-VR, ICD-DR | 312 | Costs | Recruiting |
| EVATEL32 | French Ministry of Health | ICD-VR, ICD-DR | 1600 | Safety and cost-effectiveness | Recruiting |
| HomeCARE II33 | Biotronik | CRT-D, ICD-VR, ICD-DR | 300 | Performance of intrathoracic impedance for monitoring heart failure | Recruiting |
| IMPACT34 | Biotronik | CRT-D, ICD-DR | 2718 | Thromboembolic events and bleeding in patients with AF managed by pre-defined anticoagulation plan. | Recruiting |
| IN-TIME35 | Biotronik | CRT-D, ICD-VR, ICD-DR | 620 | Mortality, hospitalization for heart failure, clinical status | Recruiting |
| OptiLink-HF36 | Medtronic | CRT-D, ICD-VR, ICD-DR | 1000 | Intrathoracic impedance monitoring for reducing death or cardiovascular hospitalization | Recruiting |
| QUANTUM37 | Biotronik | ICD-VR, ICD-DR | 150 | Psychosomatic evaluation | Recruiting |
| TRUST6,38 | Biotronik | ICD-VR, ICD-DR | 1516 | Number of ICD follow-ups and safety | Active, not recruiting |
Data were retrieved on 22 February 2009.
Failure of ICD leads is a major issue and has been highlighted by the Fidelis lead recall. The Medtronic lead integrity alert algorithm has been shown to give a warning of impending inappropriate shocks by at least 3 days advance in 76% of patients,15 and triggers an audible alert that may prompt the patient to seek attention. However, these audible alerts may not be heard by the patient in time, resulting in inappropriate shocks.16,17 Detection of lead failure may therefore be enhanced by remote monitoring. In a report of 54 patients with an ICD lead failure, remote monitoring by the Biotronik Home Monitoring system resulted in a significant reduction in the incidence of inappropriate shocks and symptomatic pacing inhibition compared with those without remote monitoring (27.3 vs. 53.4%, P = 0.04).18 Inappropriate shocks occur for a variety of reasons other than lead dysfunction. In a recent report on 35 patients with Brugada syndrome implanted with a Biotronik ICD followed by Home Monitoring,19 identification of problems by remote monitoring such as T-wave oversensing, external electromagnetic interference and sinus tachycardia led to device reprogramming that may have prevented shocks.
Remote follow-up and monitoring can also be used for tracking product performance in a large number of patients, and may allow earlier identification of issues with specific models. The large amount of data gathered in a consistent manner also has the potential to facilitate medical research.
Increased patient satisfaction
The first studies evaluating patient satisfaction by remote follow-up of ICDs were published in 2004 using the Medtronic Carelink system20 and the St Jude Medical HouseCall II™.21 Patient satisfaction with the systems was high in both studies. In an Italian study, 67 patients implanted with a Medtronic CRT-D were followed remotely using the CareLink™ system.8,22 Remote follow-ups were preferred to in-clinic visits by 78% of the patients. Satisfaction by the physicians was also very favourable. Likewise, in another study using the Medtronic CareLink™ conducted in Finland,23 the patients’ and physicians’ level of satisfaction with the system was high. Our own experience is that most patients readily accept remote monitoring, and feel secured by the use of this technology to improve their healthcare.
Potential cost savings
There are a few studies that assess the potential cost/benefit of remote monitoring-assisted care compared with conventional follow-up, and all are based on the fact that remote data access may decrease the rate of inhospital patient visits. Using a French database of 502 ICD-patients followed in tertiary care hospitals, Fauchier et al.24 estimated a decrease in costs for follow-up visits of as much as $2149 over a 5 years device lifespan. However, savings were closely related to the distance between home and medical facilities. It is noteworthy that this study did not include reimbursement for the time spent reviewing remote monitoring data.
Recently, an interesting Finnish study23 replaced standard ICD follow-up at 3 and 6 months after implantation, by remote data transmission using the Medtronic CareLink™ system. This was safe, reduced time burden for patient and inhospital staff, and was also cost-effective. However, one should note that the economic impact of remote follow-up may not be the same in all countries, due to the fact that indirect costs (such as travel expenses and sickness allowance) played a major role.
Specific issues
Optimal workflow
Even though reports suggest that the physician is unlikely to be submerged by incoming data from his patients (for example, the mean numbers of events per patient per month reported in the largest study to date12 was only 0.6), this will probably depend to a large extent on programming of the alerts and data triage. Ricci et al.25 have described a system whereby a specialized nurse connects to the home monitoring website for checking data from all their 117 patients at least every 15 days and whenever an event report was received. Only 6% of events were then relayed to the physician for further evaluation. This workflow resulted in a mean of 59 min/week for the nurse and 12 min/week for the physician to analyse home monitoring data. A well-organized clinic will also ensure that alerts are responded to in a timely manner.
Several companies are working on compatibility of their systems with electronic medical records for exporting remote follow-up data. This will considerably alleviate the clinic's workload and help streamline workflow.
Legal aspects
The patient needs to be informed of the purpose and limitations of remote monitoring, such as the fact that it does not replace an emergency service or absence of dealing with alert events outside office hours. Before initiating remote monitoring and follow-up, the patient may be requested to sign a written informed consent stating these points and authorizing transmission of personal data to third parties, respect of privacy, and confidentiality of patient data by device companies should be subjected to strict rules, described in contracts.
Patient privacy and security
In order to test the vulnerability of security breaches by hackers accessing devices with wireless capability, Halperin et al.26 performed laboratory tests on a Medtronic Maximo DR ICD. After having partially reversed the ICD's communications protocol with an oscilloscope and a software radio, they performed several software radio-based attacks that were able to retrieve uncrypted personal patient data, as well as change device settings (including commanded shocks). This report triggered considerable media coverage, although it is believed that the risk of unauthorized access to an ICD is unlikely, given the considerable technical expertise required.27 There have been no reports to date of hacking of implantable devices. Another consideration, however, is hacking of the internet server database.
Reimbursement
In the USA, Medicare and Medicaid have expanded reimbursement for remote device monitoring for all states since 2006. Reimbursement rates vary from state to state, and in some instances are the same as an in-office visit without device programming. In the UK, Germany, and Portugal, reimbursement for remote monitoring is similar to that offered for standard follow-up visits.
Conclusions
Remote monitoring and follow-up are likely to become the standard of care for patients with PMs and ICDs, as they have the potential to improve patient safety and satisfaction, to support efficient use of resources, and to reduce costs. Existing data show that current technology for remote monitoring is reliable and that it is readily accepted by patients and their physicians. Large randomized trials are underway that will hopefully prove that remote monitoring improves patient outcome. Specific issues such as reimbursement need to be dealt with by the authorities, in order to ensure that this solution is a viable one.
Conflicts of interest: D.S. is a member of the Boston Scientific fellowship program. H.B. receives research grants from Medtronic, Boston Scientific, and St Jude Medical, has been on the speakers’ bureau for Medtronic and Boston Scientific, and is a member of the Latitude advisory board.
Funding
H.B. was supported in part by a research grant from the Foundation for Cardiovascular Research of the Hôpital de la Tour, Meyrin, Switzerland. Funding to pay the Open Access publication charges for this article was provided equally by Biotronik, Boston Scientific, Medtronic and St. Jude Medical.
Acknowledgements
The authors would like to thank Mr Tim Montgomery (Biotronik), Mr Matthias Wollenstein (Boston Scientific), Mr Bertjo Frick (Medtronic), Mrs Severine Pradere (Sorin), and Mr Blaise Sahli (St Jude Medical) for their kind assistance and technical review of the manuscript.
References
- 1.Wilkoff BL, Auricchio A, Brugada J, Cowie M, Ellenbogen KA, Gillis AM, et al. HRS/EHRA Expert Consensus on the Monitoring of Cardiovascular Implantable Electronic Devices (CIEDs): Description of Techniques, Indications, Personnel, Frequency Ethical Considerations: Developed in partnership with the Heart Rhythm Society (HRS), the European Heart Rhythm Association (EHRA); and in collaboration with the American College of Cardiology (ACC), the American Heart Association (AHA), the European Society of Cardiology (ESC), the Heart Failure Association of ESC (HFA), and the Heart Failure Society of America (HFSA) Endorsed by the Heart Rhythm Society, the European Heart Rhythm Association (a registered branch of the ESC), the American College of Cardiology, the American Heart Association. Europace. 2008;10:707–25. doi: 10.1093/europace/eun122. [DOI] [PubMed] [Google Scholar]
- 2.Jung W, Rillig A, Birkemeyer R, Miljak T, Meyerfeldt U. Advances in remote monitoring of implantable pacemakers, cardioverter defibrillators and cardiac resynchronization therapy systems. J Interv Card Electrophysiol. 2008;23:73–85. doi: 10.1007/s10840-008-9311-5. [DOI] [PubMed] [Google Scholar]
- 3.Raatikainen MJP, Uusimaa P, van Ginneken MME, Janssen JPG, Linnaluoto M. Remote monitoring of implantable cardioverter defibrillator patients: a safe, time-saving, and cost-effective means for follow-up. Europace. 2008;10:1145–51. doi: 10.1093/europace/eun203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brugada P. What evidence do we have to replace in-hospital implantable cardioverter defibrillator follow-up? Clin Res Cardiol. 2006;95:III3–9. doi: 10.1007/s00392-006-1302-x. [DOI] [PubMed] [Google Scholar]
- 5.Heidbuchel H, Lioen P, Foulon S, Huybrechts W, Ector J, Willems R, et al. Potential role of remote monitoring for scheduled and unscheduled evaluations of patients with an implantable defibrillator. Europace. 2008;10:351–7. doi: 10.1093/europace/eun010. [DOI] [PubMed] [Google Scholar]
- 6.Varma N. Lumax-T/Lumos-T safely RedUceS rouTine office device follow-up. American Heart Associaton Annual Scientific Sessions. 2008 [Google Scholar]
- 7.Santini M, Ricci RP, Lunati M, Landolina M, Perego GB, Marzegalli M, et al. Remote monitoring of patients with biventricular defibrillators through the CareLink system improves clinical management of arrhythmias and heart failure episodes. J Interv Card Electrophysiol. 2009;24:53–61. doi: 10.1007/s10840-008-9321-3. [DOI] [PubMed] [Google Scholar]
- 8.Masella C, Zanaboni P, Di Stasi F, Gilardi S, Ponzi P, Valsecchi S. Assessment of a remote monitoring system for implantable cardioverter defibrillators. J Telemed Telecare. 2008;14:290–4. doi: 10.1258/jtt.2008.080202. [DOI] [PubMed] [Google Scholar]
- 9.Lunati M, Gasparini M, Santini M, Landolina M, Perego GB, Pappone C, et al. Follow-up of CRT–ICD: implications for the use of remote follow-up systems. Data from the InSync ICD Italian Registry. Pacing Clin Electrophysiol. 2008;31:38–46. doi: 10.1111/j.1540-8159.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 10.Crossley GH, Mead H, Kleckner K, Sheldon T, Davenport L, Harsch MR, et al. Automated left ventricular capture management. Pacing Clin Electrophysiol. 2007;30:1190–200. doi: 10.1111/j.1540-8159.2007.00840.x. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen JC, Kottkamp H, Zabel M, Aliot E, Kreutzer U, Bauer A, et al. Automatic home monitoring of implantable cardioverter defibrillators. Europace. 2008;10:729–35. doi: 10.1093/europace/eun099. [DOI] [PubMed] [Google Scholar]
- 12.Lazarus A. Remote, wireless, ambulatory monitoring of implantable pacemakers, cardioverter defibrillators, and cardiac resynchronization therapy systems: analysis of a worldwide database. Pacing Clin Electrophysiol. 2007;30:S2–12. doi: 10.1111/j.1540-8159.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- 13.Neuzil P, Taborsky M, Holy F, Wallbrueck K. Early automatic remote detection of combined lead insulation defect and ICD damage. Europace. 2008;10:556–7. doi: 10.1093/europace/eun009. [DOI] [PubMed] [Google Scholar]
- 14.Ricci RP, Morichelli L, Santini M. Remote control of implanted devices through Home Monitoring technology improves detection and clinical management of atrial fibrillation. Europace. 2009;11:54–61. doi: 10.1093/europace/eun303. [DOI] [PubMed] [Google Scholar]
- 15.Swerdlow CD, Gunderson BD, Ousdigian KT, Abeyratne A, Stadler RW, Gillberg JM, et al. Downloadable algorithm to reduce inappropriate shocks caused by fractures of implantable cardioverter-defibrillator leads. Circulation. 2008;118:2122–9. doi: 10.1161/CIRCULATIONAHA.108.796136. [DOI] [PubMed] [Google Scholar]
- 16.Kallinen LM, Hauser RG, Lee KW, Almquist AK, Katsiyiannis WT, Tang CY, et al. Failure of impedance monitoring to prevent adverse clinical events caused by fracture of a recalled high-voltage implantable cardioverter-defibrillator lead. Heart Rhythm. 2008;5:775–9. doi: 10.1016/j.hrthm.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 17.Vollmann D, Erdogan A, Himmrich E, Neuzner J, Becker D, Unterberg-Buchwald C, et al. Patient alert to detect ICD lead failure: efficacy, limitations, and implications for future algorithms. Europace. 2006;8:371–6. doi: 10.1093/europace/eul023. [DOI] [PubMed] [Google Scholar]
- 18.Spencker S, Coban N, Koch L, Schirdewan A, Muller D. Potential role of home monitoring to reduce inappropriate shocks in implantable cardioverter-defibrillator patients due to lead failure. Europace. 2009;11:483–8. doi: 10.1093/europace/eun350. [DOI] [PubMed] [Google Scholar]
- 19.Sacher F, Probst V, Bessouet M, Wright M, Maluski A, Abbey S, et al. Remote implantable cardioverter defibrillator monitoring in a Brugada syndrome population. Europace. 2009;11:489–94. doi: 10.1093/europace/eup034. [DOI] [PubMed] [Google Scholar]
- 20.Schoenfeld MH, Compton SJ, Mead RH, Weiss DN, Sherfesee L, Englund J, et al. Remote monitoring of implantable cardioverter defibrillators: a prospective analysis. Pacing Clin Electrophysiol. 2004;27:757–63. doi: 10.1111/j.1540-8159.2004.00524.x. [DOI] [PubMed] [Google Scholar]
- 21.Joseph GK, Wilkoff BL, Dresing T, Burkhardt J, Khaykin Y. Remote interrogation and monitoring of implantable cardioverter defibrillators. J Interv Card Electrophysiol. 2004;11:161–6. doi: 10.1023/B:JICE.0000042356.52369.89. [DOI] [PubMed] [Google Scholar]
- 22.Marzegalli M, Lunati M, Landolina M, Perego GB, Ricci RP, Guenzati G, et al. Remote monitoring of CRT–ICD: the multicenter Italian CareLink evaluation—ease of use, acceptance, and organizational implications. Pacing Clin Electrophysiol. 2008;31:1259–64. doi: 10.1111/j.1540-8159.2008.01175.x. [DOI] [PubMed] [Google Scholar]
- 23.Raatikainen MJ, Uusimaa P, van Ginneken MM, Janssen JP, Linnaluoto M. Remote monitoring of implantable cardioverter defibrillator patients: a safe, time-saving, and cost-effective means for follow-up. Europace. 2008;10:1145–51. doi: 10.1093/europace/eun203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fauchier L, Sadoul N, Kouakam C, Briand F, Chauvin M, Babuty D, et al. Potential cost savings by telemedicine-assisted long-term care of implantable cardioverter defibrillator recipients. Pacing Clin Electrophysiol. 2005;28:S255–9. doi: 10.1111/j.1540-8159.2005.00071.x. [DOI] [PubMed] [Google Scholar]
- 25.Ricci RP, Morichelli L, Santini M. Home monitoring remote control of pacemaker and implantable cardioverter defibrillator patients in clinical practice: impact on medical management and health-care resource utilization. Europace. 2008;10:164–70. doi: 10.1093/europace/eum289. [DOI] [PubMed] [Google Scholar]
- 26.Halperin D, Heydt-Benjamin TS, Ransford B, Clark SS, Defend B, Morgan W, et al. Pacemakers and implantable cardiac defibrillators: software radio attacks and zero-power defenses. Proceedings of the 2008 IEEE Symposium on Security and Privacy. 2008:129–42. [Google Scholar]
- 27.Wireless ICD programming vulnerable to hackers, report claims. http://www.theheart.org/article/847781.do. (13 March 2008, date last accessed) [Google Scholar]
- 28.Crossley G, Boyle A, Vitense H, Sherfesee L, Mead RH. Trial design of the clinical evaluation of remote notification to reduce time to clinical decision: the Clinical evaluation Of remote NotificatioN to rEduCe Time to clinical decision (CONNECT) study. Am Heart J. 2008;156:840–6. doi: 10.1016/j.ahj.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 29.Clinical Evaluation Of Remote NotificatioN to rEduCe Time to Clinical Decision (CONNECT) http://www.clinicaltrials.gov/ct2/show/NCT00402246. (22 February 2009, date last accessed) [Google Scholar]
- 30.Clinical effecT of Heart Failure Management Via Home Monitoring With a Focus on Atrial Fibrillation (effecT) http://www.clinicaltrials.gov/ct2/show/NCT00811382. (22 February 2009, date last accessed) [Google Scholar]
- 31.European Health Economic Trial on Home Monitoring in ICD Therapy (EuroEco) 2009 Accessed February 22 http://www.clinicaltrials.gov/ct2/show/NCT00776087 . [Google Scholar]
- 32.Evaluation of the ‘Tele-Follow-up ‘for the Follow-up of Implantable Defibrillators (EVATEL) http://www.clinicaltrials.gov/ct2/show/NCT00598026. (22 February 2009, date last accessed) [Google Scholar]
- 33.Monitoring of Fluid Status in Heart Failure Patients by Intrathoracic Impedance Measurement (HomeCARE II) http://www.clinicaltrials.gov/ct2/show/NCT00711360. (22 February 2009, date last accessed) [Google Scholar]
- 34.The IMPACT of BIOTRONIK Home Monitoring Guided Anticoagulation on Stroke Risk in Patients With Implanted ICD and CRT-D Devices (IMPACT) http://www.clinicaltrials.gov/ct2/show/NCT00559988. (22 February 2009, date last accessed) [Google Scholar]
- 35.Influence of Home Monitoring on the Clinical Status of Heart Failure Patients With an Impaired Left Ventricular Function (IN-TIME) http://www.clinicaltrials.gov/ct2/show/NCT00538356. (22 February 2009, date last accessed) [Google Scholar]
- 36.OptiLink HF Study:. Optimization of Heart Failure Management Using Medtronic OptiVol Fluid Status Monitoring and Medtronic CareLink Network (OptiLink-HF) doi: 10.1093/eurjhf/hfr045. http://www.clinicaltrials.gov/ct2/show/NCT00769457. (22 February 2009, date last accessed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Psychosomatic Effects of Implantable Cardioverter Defibrillator With Home Monitoring Function (QUANTUM) http://www.clinicaltrials.gov/ct2/show/NCT00325221. (22 February 2009, date last accessed) [Google Scholar]
- 38.Varma N. Rationale and design of a prospective study of the efficacy of a remote monitoring system used in implantable cardioverter defibrillator follow-up: the Lumos-T Reduces Routine Office Device Follow-Up Study (TRUST) study. Am Heart J. 2007;154:1029–34. doi: 10.1016/j.ahj.2007.07.051. [DOI] [PubMed] [Google Scholar]