Abstract
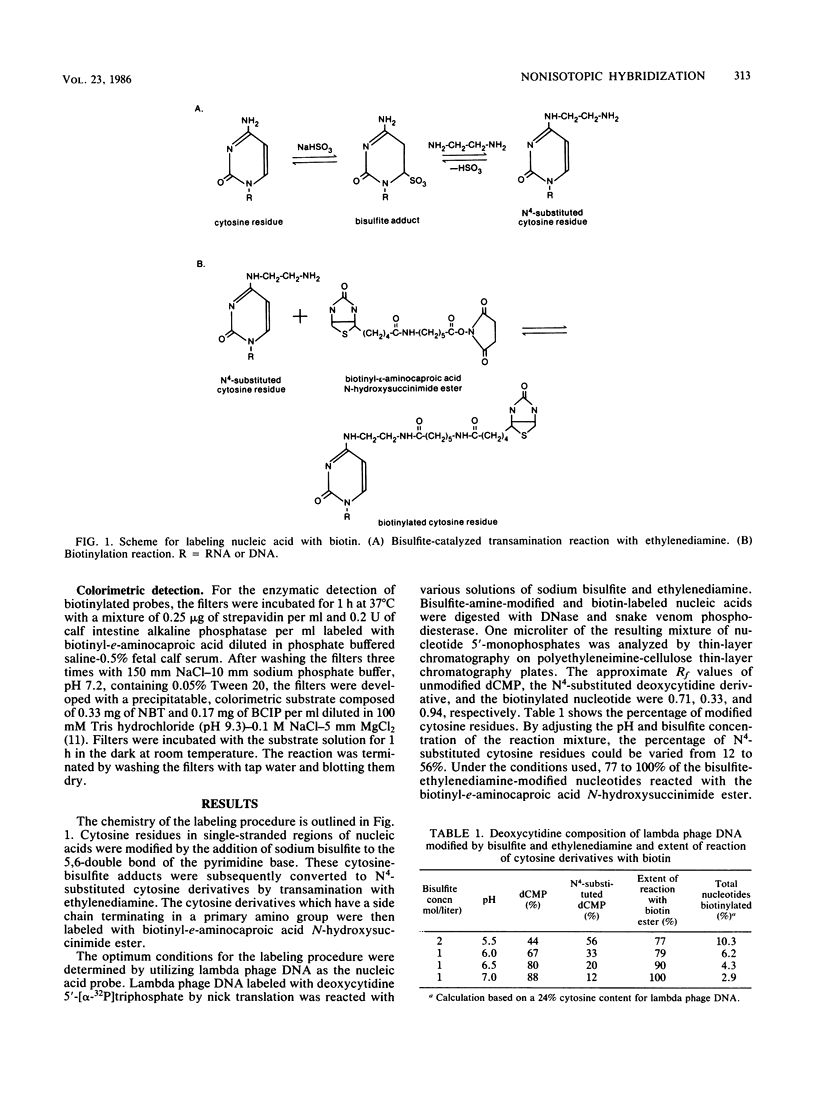
A novel chemical method was used to prepare biotin-labeled nucleic acids for nonisotopic hybridization. The method involves the transamination of unpaired cytosine residues in polynucleotides with sodium bisulfite and ethylenediamine. Primary amino groups on the cytosine derivatives are then reacted with biotinyl-e-aminocaproic acid N-hydroxysuccinimide ester. Biotinylated probes hybridized with 1 to 2 pg of nitrocellulose filter-bound DNA and were visualized with a colorimetric detection technique. This method is simpler and less expensive than other methods for the preparation of nonisotopic probes. In addition, it is more versatile since the chemically modified bases can potentially react with other "indicator" molecules or proteins such as an enzyme. The specificity for unpaired cytosine residues is another advantage which could allow for the selective labeling of a specific region of a double-stranded nucleic acid. This improved labeling method should lead to the wider application of hybridization techniques in diagnostic microbiology and basic research in infectious diseases.
Full text
PDF


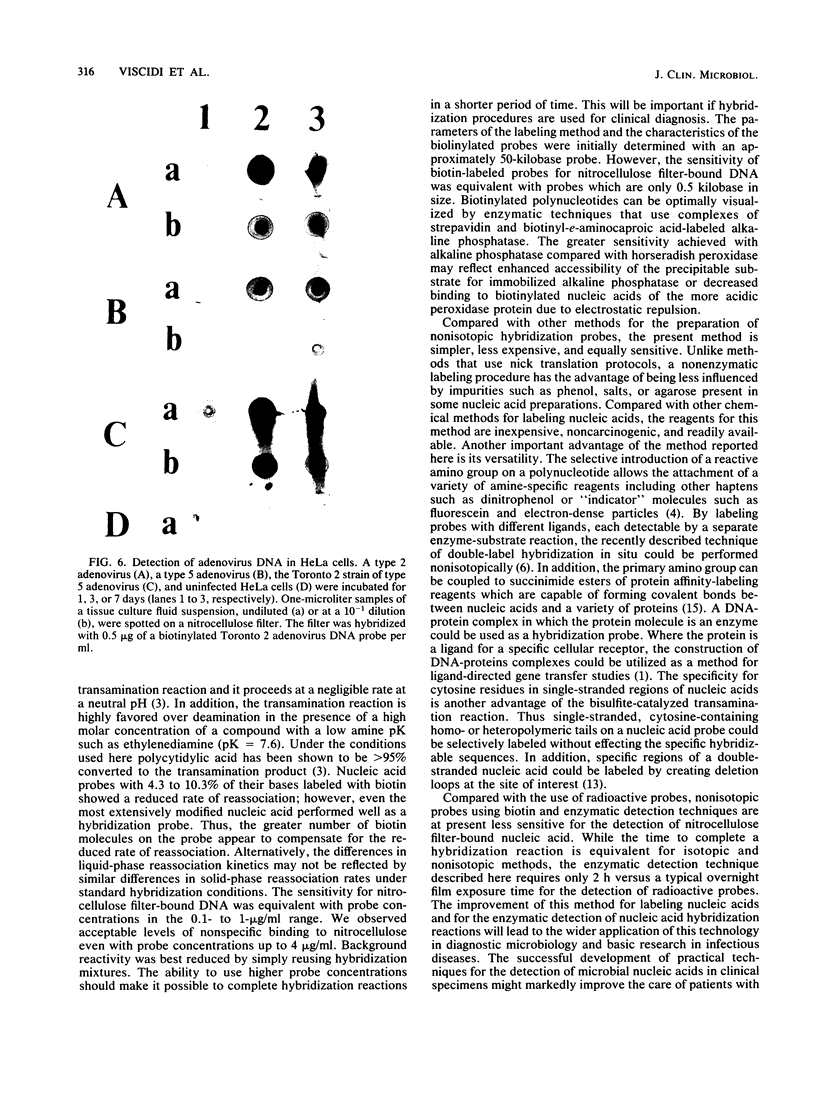

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng S., Merlino G. T., Pastan I. H. A versatile method for the coupling of protein to DNA: synthesis of alpha 2-macroglobulin-DNA conjugates. Nucleic Acids Res. 1983 Feb 11;11(3):659–669. doi: 10.1093/nar/11.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet A., Kawashima E. H. Biotin-labeled synthetic oligodeoxyribonucleotides: chemical synthesis and uses as hybridization probes. Nucleic Acids Res. 1985 Mar 11;13(5):1529–1541. doi: 10.1093/nar/13.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper D. E. Attachment of reporter groups to specific, selected cytidine residues in RNA using a bisulfite-catalyzed transamination reaction. Nucleic Acids Res. 1984 Jan 25;12(2):989–1002. doi: 10.1093/nar/12.2.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper D. E., Gold L. A method for linking fluorescent labels to polynucleotides: application to studies of ribosome-ribonucleic acid interactions. Biochemistry. 1980 Apr 29;19(9):1774–1781. doi: 10.1021/bi00550a008. [DOI] [PubMed] [Google Scholar]
- Forster A. C., McInnes J. L., Skingle D. C., Symons R. H. Non-radioactive hybridization probes prepared by the chemical labelling of DNA and RNA with a novel reagent, photobiotin. Nucleic Acids Res. 1985 Feb 11;13(3):745–761. doi: 10.1093/nar/13.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A. T., Walker D., Stowring L., Ventura P., Geballe A., Blum H., Brahic M., Goldberg R., O'Brien K. Detection of two viral genomes in single cells by double-label hybridization in situ and color microradioautography. Science. 1985 Jan 11;227(4683):189–192. doi: 10.1126/science.2981430. [DOI] [PubMed] [Google Scholar]
- Hayatsu H., Wataya Y., Kazushige K. The addition of sodium bisulfite to uracil and to cytosine. J Am Chem Soc. 1970 Feb 11;92(3):724–726. doi: 10.1021/ja00706a062. [DOI] [PubMed] [Google Scholar]
- Hyypiä T. Detection of adenovirus in nasopharyngeal specimens by radioactive and nonradioactive DNA probes. J Clin Microbiol. 1985 May;21(5):730–733. doi: 10.1128/jcm.21.5.730-733.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempe T., Sundquist W. I., Chow F., Hu S. L. Chemical and enzymatic biotin-labeling of oligodeoxyribonucleotides. Nucleic Acids Res. 1985 Jan 11;13(1):45–57. doi: 10.1093/nar/13.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P. R., Waldrop A. A., Ward D. C. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden K. W., Nathans D. Local mutagenesis within deletion loops of DNA heteroduplexes. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7214–7217. doi: 10.1073/pnas.79.23.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A. K., Schulman L. H. Attachment of cross-linking reagents to tRNA for protein affinity labeling studies. Methods Enzymol. 1979;59:156–166. doi: 10.1016/0076-6879(79)59077-6. [DOI] [PubMed] [Google Scholar]
- Schulman L. H., Pelka H., Reines S. A. Attachment of protein affinity-labeling reagents of variable length and amino acid specificity to E. coli tRNAfMet. Nucleic Acids Res. 1981 Mar 11;9(5):1203–1217. doi: 10.1093/nar/9.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R., Weisgras J. M. Bisulfite-catalyzed transamination of cytosine and cytidine. Biochem Biophys Res Commun. 1970 Aug 24;40(4):839–843. doi: 10.1016/0006-291x(70)90979-4. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Tchen P., Fuchs R. P., Sage E., Leng M. Chemically modified nucleic acids as immunodetectable probes in hybridization experiments. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3466–3470. doi: 10.1073/pnas.81.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H. Enzyme immunoassays for the detection of infectious antigens in body fluids: current limitations and future prospects. Rev Infect Dis. 1982 Jan-Feb;4(1):35–68. doi: 10.1093/clinids/4.1.35. [DOI] [PubMed] [Google Scholar]