Abstract
The Gram-negative opportunistic pathogen Legionella pneumophila replicates in phagocytes within a specific compartment, the Legionella-containing vacuole (LCV). Formation of LCVs is a complex process requiring the bacterial Icm/Dot type IV secretion system and more than 100 translocated effector proteins, which putatively subvert cellular signaling and vesicle trafficking pathways. Phosphoinositide (PI) glycerolipids are pivotal regulators of signal transduction and membrane dynamics in eukaryotes. Recently, a number of Icm/Dot substrates were found to anchor to the LCV membrane by binding to PIs. One of these effectors, SidC, specifically interacts with phosphatidylinositol-4 phosphate [PtdIns(4)P]. Using an antibody against SidC and magnetic beads coupled to a secondary antibody, intact LCVs were purified by immuno-magnetic separation, followed by density centrifugation. This purification strategy is in principle applicable to any pathogen vacuole that carries specific markers. The LCV proteome determined by LC-MS/MS revealed 566 host proteins, including novel components of the endosomal pathway, as well as the early and late secretory trafficking pathways. Thus, LCV formation is a robust process that involves many (functionally redundant) Icm/Dot substrates, as well as the interaction with different host cell vesicle trafficking pathways.
Key words: bacterial virulence, Dictyostelium, GTPase, macrophage, mass spectrometry, pathogen vacuole, phagocyte, phosphoinositide, vesicle trafficking
The Vacuolar Pathogen Legionella pneumophila
The Gram-negative bacterium Legionella pneumophila parasitizes environmental protozoa and grows in alveolar macrophages of the human lung, thus possibly causing the severe pneumonia Legionnaires' disease.1 L. pneumophila replicates in amoebae and macrophages within a unique compartment, the Legionella-containing vacuole (LCV),2 which in either phagocyte is formed by a seemingly conserved mechanism. Therefore, amoebae and in particular the genetically tractable social amoeba Dictyostelium discoideum, are valuable model systems to dissect L. pneumophila-phagocyte interactions on a molecular and cellular level.3
L. pneumophila promotes the uptake by phagocytes4,5 and LCV formation by means of the Icm/Dot type IV secretion system.6 To date, more than 100 Icm/Dot substrates have been identified, many of which modulate host cell vesicle trafficking.7 While some of these effector proteins subvert host cell GTP turnover or PI metabolism,8–10 most have not been characterized mechanistically. LCVs avoid fusion with lysosomes, interact with early secretory vesicles at endoplasmic reticulum (ER) exit sites and eventually fuse with the ER.2,11 Yet, multiple trafficking pathways converge to form a replicative LCV, several of which need to be inactivated to impair intracellular replication of L. pneumophila.12
A comprehensive description of LCV formation requires a catalogue of the factors involved. To determine the LCV proteome, we purified LCVs by a simple two-step protocol, using D. discoideum amoebae producing the ER marker calnexin-GFP and L. pneumophila labeled with the red fluorescent protein DsRed.13 Intact LCVs were enriched by immuno-magnetic separation with an antibody against the Icm/Dot substrate SidC (selectively binding to LCVs) and magnetic beads coupled to a secondary antibody, followed by density centrifugation. The proteome determined by LC-MS/MS revealed 566 host proteins, including factors associated with mitochondria, the endosomal pathway and the secretory pathway.
Endosomal Markers of LCVs
LCVs avoid fusion with lysosomes, but still interact with the endosomal pathway, as indicated by the presence of the late endosomal small GTPase Rab7,13,14 (Fig. 1). LCVs also acquire the D. discoideum inositol polyphosphate 5-phosphatase (IP5P) Dd5P4 and its mammalian homologue OCRL1 (oculocerebrorenal syndrome of Lowe).15 Dd5P4 plays a role in phagocytosis,16 and in its absence, L. pneumophila replicates much more efficiently in the amoebae. OCRL1 localizes to endosomes and the trans Golgi network (TGN), where it promotes (retrograde) trafficking between the two compartments.17
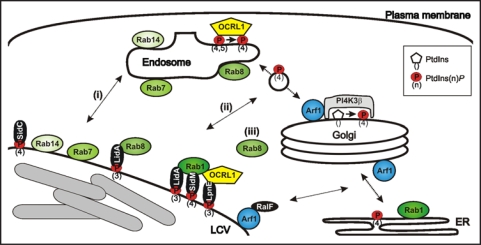
Figure 1.
Communication of LCVs with endosomal and secretory trafficking pathways. LCVs are decorated with endosomal and secretory markers, which might be acquired by (i) direct fusion with cell organelles, (ii) interaction with transport vesicles shuttling between compartments, or (iii) recruitment from the cytoplasm. L. pneumophila produces two distinct classes of Icm/Dot-translocated effector proteins, which interact with PtdIns(4)P (SidC, SidM)5,18,35 or PtdIns(3)P (LpnE, LidA),15,35 respectively.
Additional endosomal factors were identified in the LCV proteome, including the small GTPases Rab8 and Rab14, the putative copper transporter p80 and the coat protein clathrin.13,18 The GTPases and p80 are markers of LCVs containing wild-type but not icm/dot mutant L. pneumophila. While Rab14 accumulates on lysosomes,19,20 Rab8 localizes to endosomes, where the GTPase promotes fusion with vesicles from TGN exit sites in the late secretory pathway.21 The endosomal enzymes Rab8 and OCRL1 might be recruited to LCVs by (i) direct fusion of the two compartments, (ii) interaction of LCVs with transport vesicles at late secretory entry sites (Rab8) and early retrograde exit sites (OCRL1), or (iii) acquisition from the cytoplasm (Fig. 1).
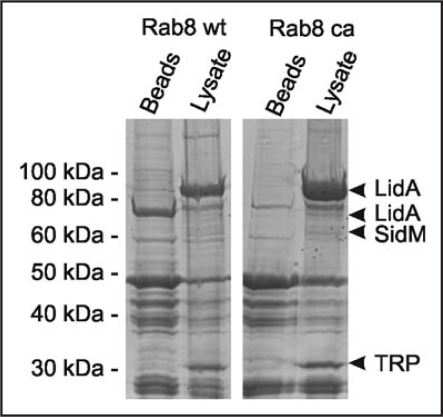
In pull-down experiments using GST-Rab8 coupled to glutathione beads and L. pneumophila lysate, we identified LidA as a Rab8-interacting protein (Fig. 2). The Icm/Dot substrate LidA localizes to the LCV membrane, promotes intracellular replication and avoidance of the endosomal pathway,22 interferes with the early secretory pathway23 and causes secretion defects upon production in the yeast Saccharomyces cerevisiae.7 Furthermore, LidA enhances the Rab1 guanine nucleotide exchange factor (GEF) activity of SidM/DrrA, and GST-LidA was found to bind Rab1, Rab6 and Rab8, which promote early secretory, Golgi to ER, or Golgi to plasma membrane transport, respectively.24 The promiscuous binding of LidA to different small GTPases suggests a role in subverting multiple host trafficking pathways.
Figure 2.
GST-Rab8 interacts with the Icm/Dot substrate LidA in L. pneumophila lysates. GST fusions proteins of Rab8 wild-type (wt) or constitutive active (ca; Rab8Q74L36) were used to precipitate binding partners in L. pneumophila lysates. Protein bands eluting from washed Rab8-coated beads but not from control beads were identified by LC-MS/MS as the Icm/Dot substrate LidA, the Rab1 GEF SidM and a putative tellurite resistance protein (TRP).
Secretory Markers of LCVs
LCVs acquire ER markers, including calnexin, calreticulin, protein disulfide isomerase, and peptides containing the KDEL/HDEL retrieval motif.2,11,13 The formation of LCVs involves interactions with the early secretory pathway at ER exit sites and depends on the activity of the small GTPases Arf1, Sar1 and Rab1.12,25–27 Arf1 and Rab1, as well as the v-SNARE Sec22b also localize to LCVs.
Arf1 and Rab1 are recruited to LCVs and activated by two Icm/Dot substrates, which function as GEFs: RalF is a GEF for Arf family GTPases,28 and SidM is a Rab1 GEF24,29 that also functions as a GDP dissociation inhibitor (GDI) displacement factor (GDF).30,31 Other Icm/Dot substrates promoting interactions of the LCV with the secretory pathway include SidJ, which is involved in the recruitment of ER to LCVs32 and SidC. The 106 kDa protein SidC and its paralogue SdcA anchor to LCVs by binding to phosphatidylinositol-4 phosphate [PtdIns(4)P] via a 20 kDa “P4C” [PtdIns(4)P-binding of SidC] domain near the C-terminus, which is unrelated to eukaryotic PI-binding folds.5,18 A 70 kDa N-terminal fragment of SidC is sufficient to bind ER vesicles, and correspondingly, L. pneumophila sidC-sdcA deletion mutants do no longer recruit ER vesicles to LCVs, wherein the bacteria replicate at wild-type rate. Thus, communication with the ER is dispensable for the formation of replication-permissive LCVs.18
PtdIns(4)P is a lipid component of LCVs5 and mediates exit of early secretory vesicles from the ER,33 but preferentially localizes to the TGN, where it is produced by an Arf1-dependent recruitment of PtdIns 4-kinase IIIβ (PI4K IIIβ) to promote trafficking along the secretory pathway.34 Depletion by RNA interference of Rab8, Arf1, PI4K IIIβ but not other small GTPases or PI4Ks reduces the amount of the PtdIns(4)P-binding effector SidC on LCV membranes.13,35 PtdIns(4)P might either accumulate on LCVs by direct fusion with (a) compartment(s) harboring this PI, or be synthesized on LCVs by PI4K IIIβ recruited by Arf1 from the cytoplasm (Fig. 1). Since an L. pneumophila ΔralF mutant strain is defective for Arf1 but not SidC acquisition,35 and since we failed to localize PI4K IIIβ on LCVs in D. discoideum and macrophages (unpublished observation), a mechanism involving the direct fusion of LCVs with a compartment decorated with PtdIns(4)P seems more likely.
Conclusions and Outlook
Formation of LCVs is a robust yet complex process involving more than 100 (functionally redundant) Icm/Dot-translocated effector proteins and multiple host cell vesicle trafficking pathways. While it is firmly established that LCVs interact with the early secretory pathway and ER, it is becoming apparent that this interaction is not required to form a replication-permissive vacuole. Moreover, LCVs also communicate with vesicle trafficking pathways between the TGN and endosomes, suggesting that the late secretory pathway, as well as retrograde endosome to TGN trafficking plays a role in LCV formation. A current challenge in the field is to define the minimal set of L. pneumophila effector proteins and host trafficking pathway required to form a replication-permissive vacuole.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/7713
References
- 1.Fields BS, Benson RF, Besser RE. Legionella and Legionnaires' disease: 25 years of investigation. Clin Microbiol Rev. 2002;15:506–526. doi: 10.1128/CMR.15.3.506-526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isberg RR, O'Connor TJ, Heidtman M. The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat Rev Microbiol. 20089;7:13–24. doi: 10.1038/nrmicro1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hilbi H, Weber SS, Ragaz C, Nyfeler Y, Urwyler S. Environmental predators as models for bacterial pathogenesis. Environ Microbiol. 2007;9:563–575. doi: 10.1111/j.1462-2920.2007.01238.x. [DOI] [PubMed] [Google Scholar]
- 4.Hilbi H, Segal G, Shuman HA. Icm/Dot-dependent upregulation of phagocytosis by Legionella pneumophila. Mol Microbiol. 2001;42:603–617. doi: 10.1046/j.1365-2958.2001.02645.x. [DOI] [PubMed] [Google Scholar]
- 5.Weber SS, Ragaz C, Reus K, Nyfeler Y, Hilbi H. Legionella pneumophila exploits PI(4)P to anchor secreted effector proteins to the replicative vacuole. PLoS Pathog. 2006;2:46. doi: 10.1371/journal.ppat.0020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segal G, Feldman M, Zusman T. The Icm/Dot typeIV secretion systems of Legionella pneumophila and Coxiella burnetii. FEMS Microbiol Rev. 2005;29:65–81. doi: 10.1016/j.femsre.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Heidtman M, Chen EJ, Moy MY, Isberg RR. Large-scale identification of Legionella pneumophila Dot/Icm substrates that modulate host cell vesicle trafficking pathways. Cell Microbiol. 2009;11:230–248. doi: 10.1111/j.1462-5822.2008.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ninio S, Roy CR. Effector proteins translocated by Legionella pneumophila: strength in numbers. Trends Microbiol. 2007;15:372–380. doi: 10.1016/j.tim.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Brüggemann H, Cazalet C, Buchrieser C. Adaptation of Legionella pneumophila to the host environment: role of protein secretion, effectors and eukaryotic-like proteins. Curr Opin Microbiol. 2006;9:86–94. doi: 10.1016/j.mib.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Hilbi H. Modulation of phosphoinositide metabolism by pathogenic bacteria. Cell Microbiol. 2006;8:1697–1706. doi: 10.1111/j.1462-5822.2006.00793.x. [DOI] [PubMed] [Google Scholar]
- 11.Shin S, Roy CR. Host cell processes that influence the intracellular survival of Legionella pneumophila. Cell Microbiol. 2008;10:1209–1220. doi: 10.1111/j.1462-5822.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- 12.Dorer MS, Kirton D, Bader JS, Isberg RR. RNA interference analysis of Legionella in Drosophila cells: exploitation of early secretory apparatus dynamics. PLoS Pathog. 2006;2:34. doi: 10.1371/journal.ppat.0020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urwyler S, Nyfeler Y, Ragaz C, Lee H, Müller L, Aebersold R, et al. Proteome analysis of Legionella vacuoles purified by magnetic immuno-separation reveals secretory and endosomal GTPases. Traffic. 2008 doi: 10.1111/j.1600-0854.2008.00851.x. In press. [DOI] [PubMed] [Google Scholar]
- 14.Clemens DL, Lee BY, Horwitz MA. Mycobacterium tuberculosis and Legionella pneumophila phagosomes exhibit arrested maturation despite acquisition of Rab7. Infect Immun. 2000;68:5154–5166. doi: 10.1128/iai.68.9.5154-5166.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber SS, Ragaz C, Hilbi H. The inositol polyphosphate 5-phosphatase OCRL1 restricts intracellular growth of Legionella, localizes to the replicative vacuole and binds to the bacterial effector LpnE. Cell Microbiol. 2009;110:76–87. doi: 10.1111/j.1462-5822.2008.01266.x. [DOI] [PubMed] [Google Scholar]
- 16.Loovers HM, Kortholt A, de Groote H, Whitty L, Nussbaum RL, van Haastert PJ. Regulation of phagocytosis in Dictyostelium by the inositol 5-phosphatase OCRL homolog Dd5P4. Traffic. 2007;8:618–628. doi: 10.1111/j.1600-0854.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- 17.Lowe M. Structure and function of the Lowe syndrome protein OCRL1. Traffic. 2005;6:711–719. doi: 10.1111/j.1600-0854.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 18.Ragaz C, Pietsch H, Urwyler S, Tiaden A, Weber SS, Hilbi H. The Legionella pneumophila phosphatidylinositol-4 phosphate-binding type IV substrate SidC recruits endoplasmic reticulum vesicles to a replication-permissive vacuole. Cell Microbiol. 2008;10:2416–2433. doi: 10.1111/j.1462-5822.2008.01219.x. [DOI] [PubMed] [Google Scholar]
- 19.Buczynski G, Bush J, Zhang L, Rodriguez-Paris J, Cardelli J. Evidence for a recycling role for Rab7 in regulating a late step in endocytosis and in retention of lysosomal enzymes in Dictyostelium discoideum. Mol Biol Cell. 1997;8:1343–1360. doi: 10.1091/mbc.8.7.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris E, Wang N, Wu Wl WL, Weatherford A, De Lozanne A, Cardelli J. Dictyostelium LvsB mutants model the lysosomal defects associated with Chediak-Higashi syndrome. Mol Biol Cell. 2002;13:656–669. doi: 10.1091/mbc.01-09-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henry L, Sheff DR. Rab8 regulates basolateral secretory, but not recycling, traffic at the recycling endosome. Mol Biol Cell. 2008;19:2059–2068. doi: 10.1091/mbc.E07-09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conover GM, Derre I, Vogel JP, Isberg RR. The Legionella pneumophila LidA protein: a translocated substrate of the Dot/Icm system associated with maintenance of bacterial integrity. Mol Microbiol. 2003;48:305–321. doi: 10.1046/j.1365-2958.2003.03400.x. [DOI] [PubMed] [Google Scholar]
- 23.Derre I, Isberg RR. LidA, a translocated substrate of the Legionella pneumophila type IV secretion system, interferes with the early secretory pathway. Infect Immun. 2005;73:4370–4380. doi: 10.1128/IAI.73.7.4370-4380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Machner MP, Isberg RR. Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev Cell. 2006;11:47–56. doi: 10.1016/j.devcel.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Kagan JC, Roy CR. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat Cell Biol. 2002;4:945–954. doi: 10.1038/ncb883. [DOI] [PubMed] [Google Scholar]
- 26.Kagan JC, Stein MP, Pypaert M, Roy CR. Legionella subvert the functions of rab1 and sec22b to create a replicative organelle. J Exp Med. 2004;199:1201–1211. doi: 10.1084/jem.20031706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Derre I, Isberg RR. Legionella pneumophila replication vacuole formation involves rapid recruitment of proteins of the early secretory system. Infect Immun. 2004;72:3048–3053. doi: 10.1128/IAI.72.5.3048-3053.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagai H, Kagan JC, Zhu X, Kahn RA, Roy CR. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science. 2002;295:679–682. doi: 10.1126/science.1067025. [DOI] [PubMed] [Google Scholar]
- 29.Murata T, Delprato A, Ingmundson A, Toomre DK, Lambright DG, Roy CR. The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat Cell Biol. 2006;8:971–977. doi: 10.1038/ncb1463. [DOI] [PubMed] [Google Scholar]
- 30.Machner MP, Isberg RR. A bifunctional bacterial protein links GDI displacement to Rab1 activation. Science. 2007;318:974–977. doi: 10.1126/science.1149121. [DOI] [PubMed] [Google Scholar]
- 31.Ingmundson A, Delprato A, Lambright DG, Roy CR. Legionella pneumophila proteins that regulate Rab1 membrane cycling. Nature. 2007;450:365–369. doi: 10.1038/nature06336. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Luo ZQ. The Legionella pneumophila effector SidJ is required for efficient recruitment of endoplasmic reticulum proteins to the bacterial phagosome. Infect Immun. 2007;75:592–603. doi: 10.1128/IAI.01278-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blumental-Perry A, Haney CJ, Weixel KM, Watkins SC, Weisz OA, Aridor M. Phosphatidylinositol 4-phosphate formation at ER exit sites regulates ER export. Dev Cell. 2006;11:671–682. doi: 10.1016/j.devcel.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Godi A, Pertile P, Meyers R, Marra P, Di Tullio G, Iurisci C, et al. ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol. 1999;1:280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- 35.Brombacher E, Urwyler S, Ragaz C, Weber SS, Kami K, Overduin M, et al. Rab1 guanine nucleotide exchange factor SidM is a major PtdIns(4)P-binding effector protein of Legionella pneumophila. J Biol Chem. 2009 doi: 10.1074/jbc.M807505200. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powell RR, Temesvari LA. Involvement of a Rab8-like protein of Dictyostelium discoideum, Sas1, in the formation of membrane extensions, secretion and adhesion during development. Microbiology. 2004;150:2513–2525. doi: 10.1099/mic.0.27073-0. [DOI] [PubMed] [Google Scholar]