Abstract
Phylogenetic community ecology uses phylogenetic relationships among species to infer the dominant processes that shape community ecological structure. This field has particularly focused on habitat filtering and competition, the latter driving divergence and competitive exclusion. However, the effects of positive interactions among species of the same guild have rarely been considered in either empirical studies or theoretical models. We have recently documented a pervasive influence of mutualism in driving adaptive convergence in ecological niche. Müllerian mimicry in butterflies is one of the best-studied examples of mutualism, where unpalatable species converge in wing pattern locally to advertise their toxicity to predators. We showed that species that share similar wing patterns are more similar in their ecology than expected given the phylogeny and co-exist at a fine spatial scale, thereby maximizing the warning signal to local predators. Evidence for competition was detected only among species with distinct wing patterns, implying that mutualistic interactions outweigh the effects of competition. Positive interactions among potential competitors are common among plants and animals. We argue that such forces should be considered in the field of phylogenetic community ecology, along-side neutral processes, habitat filtering and competition.
Key words: community ecology, phylogeny, mutualism, positive interactions, competition, müllerian mimicry, butterflies, ithomiinae
A fundamental problem in community ecology is to unravel patterns of species co-existence. The occurrence of species is constrained by their fundamental niche, defined as the abiotic requirements necessary to maintain positive population growth rates, and by their dispersal abilities. In addition, interactions with other species in the local community can also affect the expansion of species ranges. Studies of these forces have tended to concentrate on coexistence of species in the same guild, i.e., species that are ecologically similar in a broad sense. There are two opposing predictions: first, species need to be adapted to a certain habitat in order to occupy a given locality, a force known as habitat filtering that tends to lead to ecologically similar species coexisting. Second, competitive interactions will tend to prevent species that are too similar to one another from coexisting.1
Over the past ten years, a new field of community ecology has emerged that investigates these phenomena by taking phylogenetic relationships among species into account.2–11 The basic rationale of this work is to use the genetic relatedness of species and the phylogenetic distribution of traits to better understand the dominant processes shaping community ecology over evolutionary time-scales. These studies have typically focused on competition and habitat filtering, as opposed to neutral processes such as dispersal or drift. A number of studies have shown phylogenetic overdispersion, or the tendency for co-occurring species to be less related than expected if species were distributed randomly. This result has generally been interpreted as a signature of the importance of competition, where competitive exclusion has prevented closely related species from co-occurring.5–7 The implicit assumption of this conclusion is that ecological niches are phylogenetically conservative, such that genetic similarity is a good predictor of ecological similarity.12,13 The converse pattern is also observed, whereby co-occurring species are more related than expected at random, and this is known as phylogenetic clustering. In this case the inference is that this is a signature of habitat filtering such that more ecologically similar species are best adapted to live in the same habitat.4,14
However, the common focus on competition or filtering during the interpretation of phylogenetic structure largely ignores the possibility of positive interactions among members of a guild. Positive interactions might counteract competition by allowing more species to co-exist on limited resources.15 Positive interactions should therefore promote co-existence and tighter association, as long as the balance of benefits and costs remain positive. At present, phylogenetic tests of the impact of positive interactions for species ecology and patterns of assemblage are rare, and until recently limited to plants.16,17
We have recently documented a pervasive influence for mutualism in causing adaptive convergence in ecological niche that can outweigh both common ancestry and competition.18 Müllerian mimicry is a form of mutualism whereby different species of distasteful butterflies have evolved the same brightly colored wing patterns that advertise their toxicity to predators.19–21 Naïve predators learn to avoid these patterns by sampling individuals, and co-occurring species benefit from converging on the same warning pattern because they share the density-dependent cost of educating predators. We surveyed a diverse community of clearwing butterflies (Nymphalidae: Ithomiinae or ‘ithomiines’). Ithomiine communities may consist of up to eight distinct mimicry complexes, formed by species sharing the same wing pattern. Mimicry diversity within a community is apparently enhanced by microhabitat partitioning among both mimicry rings and their avian predators, such that butterflies more frequently encounter birds that are familiar with their pattern, and that are more likely to avoid them. We therefore wanted to test the hypothesis that mimicry might promote adaptive convergence in microhabitat niche among species that share the same pattern (or co-mimics) to maximize warning signal overlap.
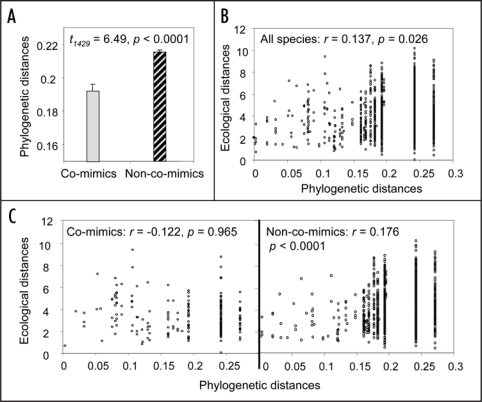
Our study community, located in Ecuador, was composed of 58 ithomiine species, belonging to eight distinct mimicry complexes (Fig. 1). We recorded five microhabitat variables (flight height, topography, and three variables representing forest structure) and generated a complete phylogeny of species in the community. We found a weak but significant phylogenetic signal in both mimicry (Fig. 2A) and microhabitat niche (Fig. 2B), meaning that closely related species share wing pattern and use similar microhabitats more often than expected at random. However, analyses controlling for the phylogeny showed that co-mimics were on average significantly more similar in their habitat use than would be expected given the phylogeny. Mutualistic interactions therefore drive adaptive ecological convergence, obscuring the phylogenetic signal in microhabitat use (Fig. 2C). We also found that species belonging to different mimicry complexes were more different in their microhabitat use than expected given the phylogeny, which could be a by-product of convergence among co-mimics, but could also be enhanced by competitive interactions (niche partitioning). Using niche complementarity as an indicator of competition, whereby species that are similar along one niche variable diverge along another, we further showed that competition along the variables measured is only detectable among non-co-mimics. Taken together, these results strongly suggest that although common ancestry and competition do contribute to shape the ecological structure of the community, their effects are outweighed by mutualistic interactions.
Figure 1.
The eight mimicry rings of the study community, illustrated by several species (eurimedia: Ithomia salapia, Pteronymia primula, Napeogenes inachia; hermias: Tithorea harmonia, Mechanitis polymnia, Melinaea satevis; lerida: Oleria gunilla, O. onega, Hyposcada illinissa; aureliana: Pseudoscada florula, Napeogenes sylphis, Hypoleria lavinia; agnosia: I. agnosia, Heterosais nephele, Pseudoscada timna; mamercus: Hypothyris mamercus, N. larina, H. moebiusi; confusa: Methona confusa, M. curvifascia, Callithomia lenea; mothone: Melinaea marsaeus, Mechanitis messenoides). Species with transparent wings are presented against a dark background.
Figure 2.
Relationships between phylogenetic and ecological distances, and mimicry. (A) Mean and standard error of phylogenetic distances among co-mimics and non-co-mimics. (B) Multidimensional ecological distances (Euclidean distances in the 5-dimension space formed by the five ecological variables measured) plotted against phylogenetic distances for all species, showing the correlation coefficient r and significance p (Mantel test). (C) Multidimensional ecological distances plotted against phylogenetic distances for co-mimics and non-co-mimics. Ecological distances observed among co-mimics are smaller than expected given the phylogeny (method controlling for phylogeny based on simulations: p = 0.030; method based on regression p < 0.0001), while ecological distances among non-co-mimics are greater that expected given the phylogeny (method based on simulations: p = 0.0013; method based on regression p = 0.0012).
Our study documents a prevalent role for mutualistic interactions in determining patterns of species co-existence, and argues that such forces should be considered in the field of phylogenetic community ecology. Similar positive interactions are well documented in other taxa, such as plant species that facilitate the establishment of others,22,23 or where observed synchronous flowering or seed set is interpreted as mutually beneficial.24,25 Positive interactions between competing animals are also well known. For instance, vertebrates commonly form mixed-species groups or flocks, thereby enhancing protection against predators26,27 and foraging efficiency.28
Considering positive interaction might lead to novel interpretations of existing data sets. For example, if mutualistic species tend to be distantly related, as is often the case in facilitation between plant species,16 then this might result in phylogenetic overdispersion. The latter has typically been interpreted as a result of competition between close relatives. Conversely, positive interactions could result in phylogenetic clustering if interacting species tend to be more closely related. The latter might be the case in plant species that benefit from attracting shared pollinators.29 We therefore suggest that existing theoretical models10 should be expanded to include positive interactions alongside neutral processes, habitat filtering and competition in generating testable predictions. In addition, empirical studies should take the possibility of positive interactions into account when interpreting the data. Finally, whenever possible it will be critical to measure the extent of phylogenetic conservatism of traits involved in both positive and negative interactions between species before deriving predictions to be tested.30 This will be considerably more challenging than making the simplistic assumption that competition decreases with phylogenetic distance, but ultimately should lead to a more fundamental understanding of how ecological communities are assembled.
Acknowledgements
We thank Olivier Hardy and Cam Webb for useful comments on this paper.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/7718
References
- 1.Armstrong RA, McGehee R. Competitive Exclusion. Am Nat. 1980;115:151–170. [Google Scholar]
- 2.Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. Phylogenies and community ecology. Annu Rev Ecol Syst. 2002;33:475–505. [Google Scholar]
- 3.Cavender-Bares J, Ackerly DD, Baum DA, Bazzaz FA. Phylogenetic overdispersion in Floridian oak communities. Am Nat. 2004;163:823–843. doi: 10.1086/386375. [DOI] [PubMed] [Google Scholar]
- 4.Kembel SW, Hubbell SP. The phylogenetic structure of a neotropical forest tree community. Ecology. 2006;87:86–99. doi: 10.1890/0012-9658(2006)87[86:tpsoan]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Lovette IJ, Hochachka WM. Simultaneous effects of phylogenetic niche conservatism and competition on avian community structure. Ecology. 2006;87:14–28. doi: 10.1890/0012-9658(2006)87[14:seopnc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Silvertown J, Dodd M, Gowing D, Lawson C, McConway K. Phylogeny and the hierarchical organization of plant diversity. Ecology. 2006;87:39–49. doi: 10.1890/0012-9658(2006)87[39:pathoo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Webb CO, Gilbert GS, Donoghue MJ. Phylodiversity-dependent seedling mortality, size structure and disease in a bornean rain forest. Ecology. 2006;87:123–131. doi: 10.1890/0012-9658(2006)87[123:psmssa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Hardy OJ, Senterre B. Characterizing the phylogenetic structure of communities by an additive partitioning of phylogenetic diversity. J Ecol. 2007;95:493–506. [Google Scholar]
- 9.Johnson MTJ, Stinchcombe JR. An emerging synthesis between community ecology and evolutionary biology. Trends Ecol Evol. 2007;22:250–257. doi: 10.1016/j.tree.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Kraft NJB, Cornwell WK, Webb CO, Ackerly DD. Trait evolution, community assembly, and the phylogenetic structure of ecological communities. Am Nat. 2007;170:271–283. doi: 10.1086/519400. [DOI] [PubMed] [Google Scholar]
- 11.Hardy OJ. Testing the spatial phylogenetic structure of local communities: statistical performances of different null models and test statistics on a locally neutral community. J Ecol. 2008;96:914–926. [Google Scholar]
- 12.Wiens JJ, Donoghue MJ. Historical biogeography, ecology and species richness. Trends Ecol Evol. 2004;19:639–644. doi: 10.1016/j.tree.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Donoghue MJ. A phylogenetic perspective on the distribution of plant diversity. Proc Natl Acad Sci USA. 2008;105:11549–11555. doi: 10.1073/pnas.0801962105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horner-Devine MC, Bohannan BJM. Phylogenetic clustering and overdispersion in bacterial communities. Ecology. 2006;87:100–108. doi: 10.1890/0012-9658(2006)87[100:pcaoib]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Gross K. Positive interactions among competitors can produce species-rich communities. Ecol Let. 2008;11:929–936. doi: 10.1111/j.1461-0248.2008.01204.x. [DOI] [PubMed] [Google Scholar]
- 16.Valiente-Banuet A, Verdu M. Facilitation can increase the phylogenetic diversity of plant communities. Ecol Let. 2007;10:1029–1036. doi: 10.1111/j.1461-0248.2007.01100.x. [DOI] [PubMed] [Google Scholar]
- 17.Valiente-Banuet A, Verdu M. Temporal shifts from facilitation to competition occur between closely related taxa. J Ecol. 2008;96:489–494. [Google Scholar]
- 18.Elias M, Gompert Z, Jiggins C, Willmott K. Mutualistic interactions drive ecological niche convergence in a diverse butterfly community. PLoS Biology. 2008;6:300. doi: 10.1371/journal.pbio.0060300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller F. Ituna and Thyridia: a remarkable case of mimicry in butterflies. Transact Entomol Soc London. 1879:1879. [Google Scholar]
- 20.Rowland HM, Ihalainen E, Lindstrom L, Mappes J, Speed MP. Co-mimics have a mutualistic relationship despite unequal defences. Nature. 2007;448:63–64. doi: 10.1038/nature05899. [DOI] [PubMed] [Google Scholar]
- 21.Sherratt TN. The evolution of Mullerian mimicry. Naturwissenschaften. 2008;95:681–695. doi: 10.1007/s00114-008-0403-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruno JF, Stachowicz JJ, Bertness MD. Inclusion of facilitation into ecological theory. Trends Ecol Evol. 2003;18:119–125. [Google Scholar]
- 23.Brooker RW, Maestre FT, Callaway RM, Lortie CL, Cavieres LA, Kunstler G, et al. Facilitation in plant communities: the past, the present and the future. J Ecol. 2008;96:18–34. [Google Scholar]
- 24.Boulter SL, Kitching RL, Howlett BG. Family, visitors and the weather: patterns of flowering in tropical rain forests of northern Australia. J Ecol. 2006;94:369–382. [Google Scholar]
- 25.Mduma SAR, Sinclair ARE, Turkington R. The role of rainfall and predators in determining synchrony in reproduction of savanna trees in Serengeti National Park, Tanzania. J Ecol. 2007;95:184–196. [Google Scholar]
- 26.Stensland E, Angerbjorn A, Berggren P. Mixed species groups in mammals. Mamm Rev. 2003;33:205–223. [Google Scholar]
- 27.Beauchamp G. Reduced flocking by birds on islands with relaxed predation. Proc R Soc B. 2004;271:1039–1042. doi: 10.1098/rspb.2004.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiley RH. Cooperative roles in mixed flocks of antwrens (Formicariidae) Auk. 1971;88:881. [Google Scholar]
- 29.Moeller DA. Facilitative interactions among plants via shared pollinators. Ecology. 2004;85:3289–3301. [Google Scholar]
- 30.Losos JB. Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol Let. 2008;11:995–1003. doi: 10.1111/j.1461-0248.2008.01229.x. [DOI] [PubMed] [Google Scholar]