Abstract
The growth and development of rice (Oryzae sativa) seedlings was shown to be regulated epigenetically by a fungal endophyte. In contrast to un-inoculated (nonsymbiotic) plants, endophyte colonized (symbiotic) plants preferentially allocated resources into root growth until root hairs were well established. During that time symbiotic roots expanded at five times the rate observed in nonsymbiotic plants. Endophytes also influenced sexual reproduction of mature big sagebrush (Artemisia tridentata) plants. Two spatially distinct big sagebrush subspecies and their hybrids were symbiotic with unique fungal endophytes, despite being separated by only 380 m distance and 60 m elevation. A double reciprocal transplant experiment of parental and hybrid plants, and soils across the hybrid zone showed that fungal endophytes interact with the soils and different plant genotypes to confer enhanced plant reproduction in soil native to the endophyte and reduced reproduction in soil alien to the endophyte. Moreover, the most prevalent endophyte of the hybrid zone reduced the fitness of both parental subspecies. Because these endophytes are passed to the next generation of plants on seed coats, this interaction provides a selective advantage, habitat specificity, and the means of restricting gene flow, thereby making the hybrid zone stable, narrow and potentially leading to speciation.
Key words: symbiosis, fungal endophyte, symbiotic communication, epigenetic, intergenomic
Introduction
Plant growth and development, stress tolerance and sexual reproduction are critical aspects of plant fitness. Current evolutionary theory treats these parameters as if they were genetic processes exclusive to the plant genome (intragenomic).1–5 Optimal plant fitness in specific habitats is considered to involve slow adaptive processes as described by Darwin.6 In fact, the adaptive potential of plants has long been considered instrumental to phenotypic plasticity, distribution patterns and biogeography.7–9 Neglected in most plant ecological studies is the fact that all plants are thought to be symbiotic with fungal endophytes10,11 and the profound effects endophytes can have on plant stress tolerance and fitness.12–15 Here we demonstrate that the purview of Class 2 endophytes,15 extends beyond the realm of habitat-adapted stress tolerance12 to regulating plant growth, development and sexual reproduction. Results indicate that Class 2 endophytes represent an intergenomic epigenetic form of plant gene regulation.
Seedling Growth and Development
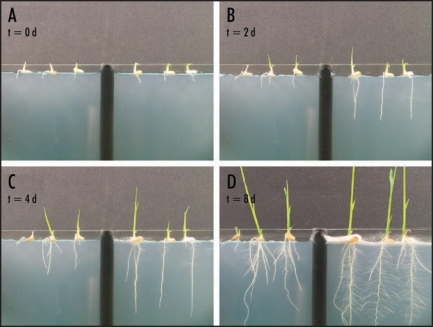
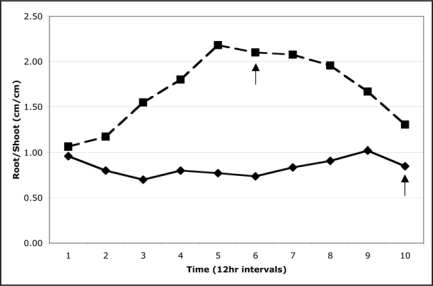
Numerous reports describe increased growth and biomass of plants colonized with fungal endophytes.15 However, documentation of endophyte impacts on plant growth rates, resource allocation and root development remains incomplete. Time-lapse image analysis of rice plants revealed that the Class 2 endophyte Fusarium culmorum increases root growth rate by up to five times compared to nonsymbiotic plants (Fig. 1). In addition, the allocation of resources was different with symbiotic plants expanding root systems before allocating significant amount of resource to stem growth and nonsymbiotic plants expanded both root and stem systems equivalently (Fig. 2). Symbiotic plants did not begin to invest significant resource into stem expansion until root systems produced extensive root hairs (Fig. 2). When grown in soil for 10 days,12 the biomass of 30 symbiotic and nonsymbiotic seedlings [104 mg and 62 mg, respectively (p < 0.05; SE < 0.01)] also reflected the impact of F. culmorum on growth. The data indicate that, in addition to providing stress tolerance,12 Class 2 endophytes may significantly improve the establishment of seedlings by increasing root growth and root hair development, and subsequently nutrient uptake and biomass.
Figure 1.
Growth of nonsymbiotic (left side of each image) and symbiotic (right side of each image) rice seedlings. Seedlings were placed on agar medium (1.5% agar, 1X hoaglands solution, 8 mM Ca2Cl) sandwiched between glass plates and grown under constant light (daylight balanced fluorescent bulbs, 22 µmol m−2 s−1) for 8 days (A–D). Growth of the endophyte (F. culmorum) and plant inoculations were performed as previously described.9
Figure 2.
Ratio of root and shoot growth in symbiotic and nonsymbiotic rice plants. Data are based on the average linear root and shoot growth of three symbiotic (dashed line) and three nonsymbiotic (solid line) plants. The arrow indicates the times of root hair development. Differences through time and between symbiotic and non-symbiotic plants were statistically significant (repeated measures ANOVA, between-subjects effect p = 0.03, within-subjects effect [quadratic] = 0.03, within-subjects interaction [quadratic] = 0.01)
Sexual Reproduction
Big sagebrush (Artemisia tridentata) is the most common shrub in North America. Throughout this range, several subspecies form stable hybrid zones in areas of contact,16 usually along elevation gradients coincident with temperature and soil differences.17 Two subspecies occur in Salt Creek Canyon, Utah, at elevations of <1,830 m (basin, A. t. ssp. tridentata) and >1,880 m (mountain, A. t. ssp. vaseyana) with a hybrid zone (1,850 m) occurring between the parental populations. Earlier studies showed that the parental taxa and hybrids were most fit in their native habitats and relatively unfit outside their native habitat—as predicted by the bounded hybrid superiority model.18 Unresolved was the nature of the adaptations and why such widespread taxa were restricted by elevation.
We conducted a double reciprocal transplant experiment in which parental, reciprocal F1 hybrid, and indigenous hybrid plants, along with their respective soils, were transplanted into three gardens at Salt Creek Canyon: (1) Mountain; (2) Basin; and (3) Hybrid. Plants were harvested three years later and assessed for root, shoot and reproductive tissue biomasses (Fig. 3). Class 2 fungal endophytes were isolated and characterized from the lower stems and crowns of all plants as previously described.12
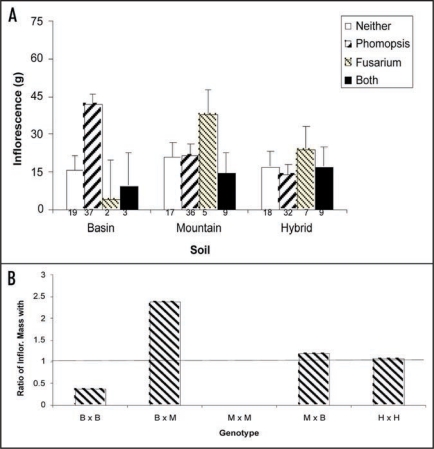
Figure 3.
Interaction between endophyte, sagebrush genotypes, soil and plant reproduction. (A) Plants were harvested at 3 years of age, divided into roots, shoot and reproductive tissue, dried at 65°C for = days and then the dried mass of each category was determined ± 1 mg. Data were analyzed using a MANOVA. Means and standard errors are shown. (B) Ratio of Inflorescence mass when plants are grown with and without Alternaria sp. This endophyte enhances only hybrid reproduction. Basin, Mountain and Hybrid plant varieties are designated B, M and H, respectively.
Endophytes segregated in a habitat-specific manner with indigenous plants in the basin, mountain and hybrid habitats containing a Phomopsis spp., Fusarium spp. and Alternaria sp., respectively. However, plants grown in the transplanted soils contained one or a combination of these endophytes. Plants performed best in basin soils when colonized with Phomopsis sp., whereas plants colonized with Fusarium sp. performed best in mountain soils—the native habitat of each endophyte. Plants with both of these endophytes performed poorly in all soils. Indigenous (multigenerational) hybrids did not exhibit enhanced growth when colonized with either Phomopsis spp. or Fusarium sp., but had increased fitness when colonized with Alternaria sp. (Fig. 3A). Remarkably, the Alternaria sp. enhanced hybrid reproduction an average of 154.9% and depressed parental reproduction by an average of 80.1% (Fig. 3B). Therefore, seed dispersed outside of a plant's indigenous habitat, will carry the associated endophyte, on the seed coat, into an alien soil and thus reduce the resulting offspring's fitness. This limits gene flow across the hybrid zone and inhibits parental taxa from invading the hybrid zone, or each other's habitat, potentially leading to speciation.
Our work indicates the potential importance of Class 2 fungal endophytes in the establishment and adaptation of plants in different environments. Endophyte induced acceleration of root growth and development may significantly increase the competitive ability of seedlings to acquire nutrients. Differential symbiotic regulation of sexual reproduction can ensure the fitness of plants indigenous to specific niches by decreasing the fitness of nonindigenous plants. Indeed, this type of intergenomic epigenetic regulation may provide insight into several unresolved plant biological phenomena such as phenotypic plasticity, biogeography, coexistence, facilitation and diversity-environment interactions.19–23 In addition, this phenomena can potentially cleave a species by restricting gene flow—a speciation process not envisioned by Darwin.6
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/7821
References
- 1.Schwaegerle KE. Quantitative genetic analysis of plant growth: biases arising from vegetative propagation. Evolution. 2005;59:1259–12567. [PubMed] [Google Scholar]
- 2.Smallwood MF, Calvert CM, Bowles DJ. Plant Responses to Environmental Stress. Oxford: BIOS Scientific Publishers Limited; 1999. p. 224. [Google Scholar]
- 3.Zhou L, Wang JK, Yi Q, Wang YZ, Zhu YG, Zhang ZH. Quantitative trait loci for seedling vigor in rice under field conditions. Field Crops Res. 2007;100:294–301. [Google Scholar]
- 4.Letort V, Mahe P, Cournede PH, Reffye PD, Courtois B. Quantitative genetics and functional-structural plant growth models: simulation of quantitative trait loci detection for model parameters and application to potential yield optimization. Ann Bot. 2008;101:1243–1254. doi: 10.1093/aob/mcm197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin X, Struik PC, Kropff MJ. Role of crop physiology in predicting gene-to-phenotype relationships. Trends Plant Sci. 2004;9:426–432. doi: 10.1016/j.tplants.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Darwin C. London: John Murray; 1859. On the origin of species by means of natural selection. [Google Scholar]
- 7.Clausen J, Keck DD, Hiesey WM. Regional differentiation in plant species. Am Nat. 1941;75:231–250. [Google Scholar]
- 8.Hiesey WM, Clausen J, Keck DD. Ecological aspects of evolution—relations between climate and intraspecific variation in plants. Am Nat. 1942;76:5–22. [Google Scholar]
- 9.Clausen J, Keck DD, Hiesey WM. Heredity of geographically and ecologically isolated races. Am Nat. 1947;81:114–133. doi: 10.1086/281507. [DOI] [PubMed] [Google Scholar]
- 10.Petrini O. Ecological and physiological aspects of host-specificity in endophytic fungi. In: Redlin SC, Carris LM, editors. Endopytic fungi in grasses and woody plants. St. Paul: APS Press; 1996. pp. 87–100. [Google Scholar]
- 11.Brundrett MC. Understanding the roles of multifunctional mycorrhizal and endophytic fungi. In: Schulz BJE, Boyle CJC, Sieber TN, editors. Microbial root endophytes. Springer-Verlag; 2006. pp. 281–293. [Google Scholar]
- 12.Rodriguez RJ, Henson J, Van Volkenburgh E, Hoy M, Wright L, Beckwith F, Kim Y, Redman RS. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008;2:404–416. doi: 10.1038/ismej.2007.106. [DOI] [PubMed] [Google Scholar]
- 13.Redman RS, Sheehan KB, Stout RG, Rodriguez RJ, Henson JM. Thermotolerance conferred to plant host and fungal endophyte during mutualistic symbiosis. Science. 2002;298:1581. doi: 10.1126/science.1072191. [DOI] [PubMed] [Google Scholar]
- 14.Baltruschat H, Fodor J, Harrach BD, Niemczyk E, Barna B, Gullner G, et al. Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol. 2008;180:501–510. doi: 10.1111/j.1469-8137.2008.02583.x. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez RJ, White JFJ, Arnold AE, Redman RS. Fungal endophytes: diversity and functional roles. New Phytol. 2008 doi: 10.1111/j.1469-8137.2009.02773.x. In Press. [DOI] [PubMed] [Google Scholar]
- 16.McArthur ED, Welch BL, Sanderson SC. Natural and artificial hybridization between big sagebrush (Artemisia tridentata) subspecies. J Hered. 1988;79:268–276. [Google Scholar]
- 17.Wang H, Byrd DW, Howard DL, McArthur ED, Graham JH, Freeman DC. Narrow hybrid zone between two subspecies of big sagebrush (Artemisia tridentata: Asteraceae) V. Soil properties. Int J Plant Sci. 1998;159:139–147. [Google Scholar]
- 18.Moore WS. An evaluation of narrow hybrid zones in vertebrates. Q Rev Biol. 1977;52:263–277. [Google Scholar]
- 19.Van Kleunen M, Fischer M. Constraints on the evolution of adaptive phenotypic plasticity in plants. New Phytol. 2005;166:49–60. doi: 10.1111/j.1469-8137.2004.01296.x. [DOI] [PubMed] [Google Scholar]
- 20.Crisci JV. The voice of historical biogeography. J Biogeog. 2001;28:157–168. [Google Scholar]
- 21.Silvertown J. Plant coexistence and the niche. Trends Ecol Evol. 2004;19:605–611. [Google Scholar]
- 22.Brooker RW, Maestre FT, Callaway RM, Lortie CL, Cavieres LA, Kunstler G, et al. Facilitation in plant communities: the past, the present and the future. J Ecol. 2008;96:18–34. [Google Scholar]
- 23.Ricklefs RE. Evolutionary diversification and the origin of the diversity-environment relationship. Ecol. 2006;87:3–13. doi: 10.1890/0012-9658(2006)87[3:edatoo]2.0.co;2. [DOI] [PubMed] [Google Scholar]