Abstract
Auxin efflux carrier PIN proteins have been intensively investigated as they are the first polar cargos to be identified in plants with a direct relevance for plant patterning. Based on their polar localization; PIN proteins direct the intercellular flow of signaling molecule auxin and thus bear a rate limiting effect on the formation of auxin activity gradients. With this influence on directionality and extent of auxin transport PINs play crucial roles in plant body organization. Many factors such as vesicle trafficking regulator ARF-GEF GNOM, a kinase PINOID, a retromer complex and membrane sterol composition influence polar PIN localization. Recent work uncovers the mechanism that generates default PIN polarity. Real time PIN tracking reveals that PIN polarity is generated from initially non-polar secretion via endocytosis and subsequent polar recycling. In addition, the Rab5 endocytic pathway emerges to be important for polar PIN localization as Rab5 interference causes non-polar distribution of PINs. This non-polar distribution of PINs during embryogenesis transiently alters auxin activity gradients and changes organ identity by transforming embryonic leaf cells to root fates. These findings for the first time link PIN polarity-based auxin activity gradient to cell fate decisions and thus demonstrate morphogen (a substance influencing cell fates on its concentration gradient) characters of auxin. They also suggest an auxin activity distribution-dependent sensing module that executes differential apical and basal developmental program during plant embryogenesis.
Key words: cell polarity, endocytosis, morphogen gradient, auxin, PIN proteins, Rab 5, embryogenesis
Cell polarity is one of the fundamental aspects of development. In unicellular organisms polarized molecules provide spatial cues for cell division and expansion whereas in multi-cellular organisms they provide developmental guidelines as early as upon fertilization of the egg. Polarized epithelial cells provide a model experimental system for analyzing cell polarity in mammals.1 They possess apical and basolateral plasma membrane domains those are physically separated by tight junction diffusion barriers.2 Plant cells posses up to four distinct plasma membrane domains inferred from differential localization of plasma membrane proteins at four distinct cell sides.3,4 In addition, plant cells lack physically indefinable tight junctions that separate plasma membrane domains by creating diffusion barriers. Furthermore, a single Golgi compartment is localized near the nucleus in mammalian cells, whilst plant cells possess several individual Golgi compartments that are distributed throughout the cell and display significant dynamicity.5 Finally, plant cells are encased in cellulose-containing cell wall compartments and bear high turgor pressure that can influence membrane composition and trafficking.6
Cell polarity plays essential roles in mammalian development as perturbations in cell polarity regulators directly influence patterning and development.7,8 The intimate relationship between cell polarity and development is perhaps even more prominent in plants as individual plant cell positions are fixed by their surrounding neighbors. Therefore, generation and perception of cell polarity is translated into oriented cell divisions, which fix tissue structure and thus determine plant architecture. In mammalian neurons the first manifestation of polarity is acquisition of axonal characteristics followed by remaining processes leading towards dendrite formation. However, in certain in vitro experimental conditions the axonal characteristics could be retained by altering the dendrite polarity characteristics back to axons.9 Whereas, the motile fibroblast apparently alter the front-rear polarity axis to change their crawling direction.10 Strikingly, plant cells often alter their polarities during development in response to internal cues and external stimuli without drastically altering fates or positions indicating existence of plant-specific polarity mechanisms.11–14 In order to better understand plant developmental programs or responses to the environment, it is crucial to understand the mechanistic basis of cell polarity generation and regulation in plants and to compare and contrast it with the established mechanisms in non-plant systems. Here, first I briefly review polarization mechanisms that have been established in mammalian cells to then propose mechanisms for cell polarity generation and regulation in plants in the light of recently published manuscripts14–16 on this topic.
Cell Polarity Regulation in Mammalian Cells
Mammalian cells possess distinct plasma membrane domains marked by specific protein complexes that not only first define the polar domains but also attract and/or retain polar cargo. Plasma membrane-destined proteins are first synthesized and later on sorted into specialized carrier vesicles that deliver the cargo to one particular plasma membrane domain. Cell polarity is regulated by interplay between three major components17: (i) intrinsic mechanisms sort membrane proteins into different post-Golgi and endosomal compartments for their delivery to different plasma membrane domains, (ii) protein complexes at the plasma membrane such as PAR,18,19 Crumbs and Scribble20,21 control the identity of particular plasma membrane domain that attracts a particular polar cargo and (iii) extrinsic cues such as cell-cell adhesion and tension control the orientation of cell polarity with respect to surrounding cells or the extracellular matrix. For the protein sorting-based intrinsic mechanism two basic scenarios exist: (a) a biosynthetic polarized delivery and (b) non-polar biosynthetic delivery followed by endocytic recycling towards the side of preference.22
Cell Polarity in Plants: PIN Proteins as Polarity Readouts or Polarity Regulators
Auxin efflux carrier PIN proteins are the first identified cell polarity markers in plants.23 Based on their polar localization they guide the cell to cell transport of signaling molecule auxin.24 PINs are the transmembrane proteins that show the ability of endocytic recycling.25 Under certain developmental or environmental-response situations they can alter their localization from one cell side to another11–14 thus they provide molecular tools for investigating cell polarity in plants. In contrast to mammalian cells where plasma membrane identity generators such as PAR, Crumbs and Scribble define cell polarity, no proteins that define cell polarity in plants have been described yet; neither do the sequenced plant genomes possess direct homologues of PAR, Crumbs and Scribble. Therefore, an important question is whether PIN trafficking machinery read out unidentified cell polarity cues for targeting PINs to appropriate cell side or whether PIN trafficking machinery itself define the default targeting of PINs to one particular side irrespective of influence of other cues and thus mark different plasma membrane domains in plant cells and then modify it by feedback regulating with other cues? The first option would require identification of the missing polarity factors that would be the dictators for docking of PIN to one defined cell side. To address the second ‘intrinsic PIN trafficking machinery itself in command’ option it first needs to be resolved whether intrinsic vesicle trafficking components can deliver PINs at proper plasma membrane domains by a trial and error based kinetic mechanism and whether manipulation of the intrinsic vesicle trafficking affect the polar localization of PINs.
A Two Step PIN Trafficking-Based Mechanism for PIN Polarity Generation
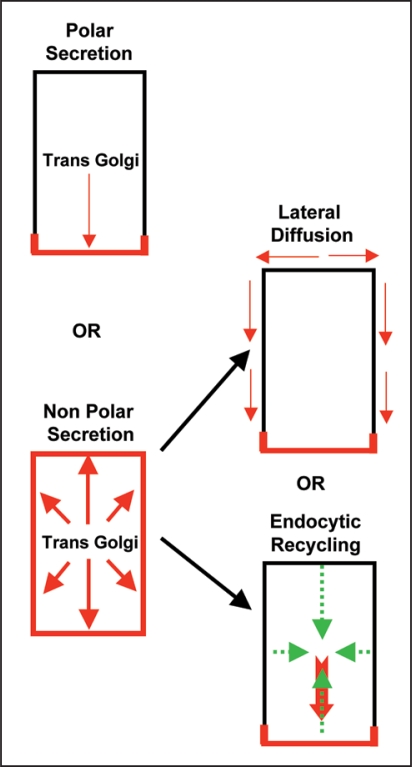
Recently the vesicle trafficking mechanism by which PIN polarity is generated in plant cells has been investigated by a real time microscopy applied to single polar cells in intact plants involving a two-pronged approach: (i) analysis of recovery of freshly synthesized Yellow fluorescent protein (YFP)-tagged PIN1 at the plasma membrane after its complete photo bleaching within entire cell and (ii) analysis of initial targeting of freshly synthesized PIN1 after its induced expression in cells in which PIN1 is generally not expressed.15 This analysis revealed that after synthesis PINs are first targeted to the plasma membrane in a random manner and are subsequently endocytosed and recycled to the side of preference (Fig. 1).15 Taking into account that PINs display little lateral diffusion15,26,27 and rapid endocytosis (as indirectly inferred from BFA treatment14,25,26,28) polarized endocytic recycling towards the side of preference (that may either involve a polarized docking/retaining factor for attracting recycling PIN vesicles or may involve polarized recycling pathway) can explain PIN polarity generation. When PIN internalization is impaired either by a short term increment in auxin (that has previously been shown to impair endocytosis29) or by interference with the Rab5-mediated endocytic pathway,15,30 PINs remain largely non-polar suggesting an important role for endocytosis in PIN polarity generation.15 As BFA sensitive ARF-GEF GNOM has been established as a key regulator for polarized recycling in plants25,28,31 the two step model of PIN polarity generation15 brings GNOM into a new perspective by placing it at the second postendocytic step. In gnom mutants or upon BFA treatment coordinated polar localization of PIN1 is strongly affected14,25,28,31 stressing that polarized recycling is as important as endocytosis for acquiring PIN polarity. In case of short term BFA treatment (presumably immediately locking GNOM functionality) PIN1 displays a less polar localization even before PIN-labeled BFA compartments attain their normal enlarged size, which may suggest that default non-polar secretion still occurs at early stages of BFA treatment. For this a BFA resistant intact secretary pathway must operate. In accordance BFA resistant ARF-GEF GNL1 operates at the Golgi for BFA-resistant secretion.32,33 Another previous study investigating relation between cell fate and PIN polarity during organ regeneration in plants also supports the initial default non-polar targeting as in case of cell fate alteration-mediated renewed expression, PIN molecules first arrive at the plasma membrane in a non-polar manner and then later on attains polar localization.34
Figure 1.
Mechanisms for default PIN polarity generation in plant cells. Two main scenarios exists: one-step mechanism involving polar secretion or a two-step mechanism involving non-polar secretion followed by either lateral diffusion or endocytic recycling. PIN localization at the plasma membrane is depicted in red.
The polarization of yeast and mammalian cells relies also on membrane sterols for polar targeting of proteins to the plasma membrane, their polar endocytic recycling and restricted lateral diffusion.35,36 It has been shown in plants that correct membrane sterol composition is essential for the acquisition of PIN polarity27,37 and the sterol composition of the plasma membrane can influence endocytosis that in turn influences PIN polarity.27 In addition, PINs display less lateral diffusion within plasma membrane15,26,27 and PINs reside in detergent resistant membrane domains and interact with another plasma membrane auxin transporter ABCB19 that is stably associated with sterol/sphingolipid-enriched membrane fractions.38 Together these findings suggest that lateral diffusion of PINs is restricted by its linking to less mobile membrane component(s) and thus takes away the possibility of membrane diffusion-based PIN polarity attainment (Fig. 1).
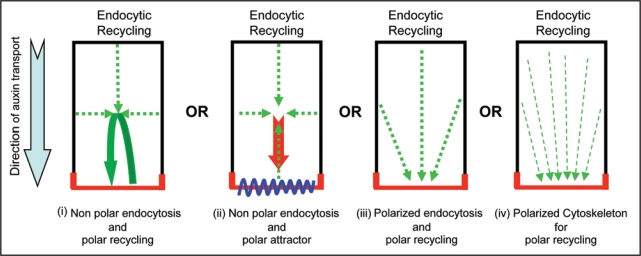
An important issue is how the side of preference builds up PIN localization at the expense of removal from all other cell sides. For this four scenarios can be envisaged (Fig. 2): (i) equal endocytosis from all cell sides followed by targeted recycling towards the side of preference so that the cargo localized at the side of preference will also be internalized and continuously replenished through directed recycling thus gradually increasing the extent of cargo at one particular side, (ii) equal endocytosis from all cell sides followed by targeted recycling towards the side of preference where a docking factor is present that preferably recognizes the recycling cargo (and not the freshly synthesized one), (iii) less endocytosis-based retrieval of cargo at the side of preference by a polarly localized stabilizer that retains the freshly synthesized cargo and also recognizes the recycling cargo or (iv) a polarized cytoskeleton component that favorably delivers only the recycling cargo (by recognizing some modified feature of internalized cargo such as phosphorylation) to a side of preference. So far no PIN docking factor or stabilizer that could also be polarly localized and thus would mark the cell side at which either freshly synthesized and initially delivered PIN should be retained or the internalized PIN from other cell sides should be targeted has been isolated. Microtubule plus ends has been proposed to provide the link between cell polarization and vesicle trafficking during cell division.39 However, polarized cytoskeleton and its relevance for PIN polarity in interphase cells have so far not been identified in plant cells. Therefore, equal endocytosis and active targeted recycling option seems more feasible. This option will also provide flexibility to alter polarized recycling without first changing the docking factor that could be relevant for many rapid PIN polarity alterations those are required for auxin transport-dependent plant response and developmental programs.
Figure 2.
Mechanisms for gradual delivery of PIN to the side of preference at the expense of its removal from other cell sides. PIN localization at the plasma membrane is depicted in red.
Another question that arises is whether PIN can perform the auxin efflux activity immediately after its initial non-polar targeting or does it acquire this capacity only after its endocytosis by some posttranslational modification such as phosphorylation. By seeing the effects of initial plasma membrane delivered PIN on auxin distribution in case of endocytic impairment in planta15 and effects of PINs in some cells where they display non polar localization (e.g., non-polar PIN1 during early stages of embryogenesis and PIN3 in columella cells) it seems that PINs are able to perform the auxin efflux function right away from their first default non-polar targeting. On the other hand endocytic recycling also has influence on auxin transport as in case of impairment of actin cytoskeleton (on which the PIN loaded vesicle traffic) the plasma membrane localized PIN alone is unable to efficiently perform the task of auxin efflux.40 How does the PIN topology on either a vesicle membrane or a plasma membrane can influence auxin efflux? In case of secretory delivery of PIN to the plasma membrane presumably the topology of PIN should be reversed in the secretory vesicle. Once this vesicle is fused with the plasma membrane then the topology of PIN at the plasma membrane should become normalized. After endocytosis the topology of PIN on the endocytic vesicle will be reversed again which may lead to the accumulation of cytosolic auxin in the endocytic vesicle (by PIN mediated efflux of cytosolic auxin inside endocytic vesicle). This endocytic vesicle accumulated auxin could be expelled out after the fusion of recycling endocytic vesicle with the plasma membrane in a mechanism that has been compared before to neurotransmitter-like secretion.41 Therefore, although PIN influences the directionality and extent of auxin transport a lot remains to be discovered on how exactly PIN performs auxin efflux.
Effect of Auxin on PIN Expression and PIN Localization
Auxin affects expression42,43 and localization15,44,45 of its carrier possibly through a canalization hypothesis where a substrate regulates its own trasporter.46 A comparable scenario exists in mammalian cells. In mammalian cells, hormone insulin promotes glucose uptake into muscle and adipose tissues through glucose transporter 4 (GLUT4). The insulin-sensitive glucose transporter GLUT4 is translocated to the plasma membrane in response to insulin and recycled back to the intracellular store(s) after removal of the hormone.47 In unstimulated cells, rapid endocytosis, slow exocytosis and dynamic or static retention cause GLUT4 to concentrate in early recycling endosomes, the trans-Golgi network and vesicle-associated protein 2-containing vesicles. In stimulated cells insulin modestly reduces GLUT4 endocytosis whereas it increases the rate of GLUT4 exocytosis and fusion with the plasma membrane. Perhaps auxin also utilizes such a dual-regulation mechanism by influencing PIN expression (and exocytosis) on one hand and retaining PIN at the plasma membrane by inhibiting PIN endocytosis. The effect of auxin on PIN expression and on its cellular abundance (and functionality) is definitely important as PIN mutants11,12,23,42,48,49 and overexpressions15,50 have typical auxin transport related defects. Whereas the effect of auxin on PIN localization could be very important as then by modulating PIN localization at particular cell side auxin can be transported in particular direction. The effect of auxin on PIN expression and localization seems more complex for two reasons: (i) under short term induction of auxin synthesis or auxin application PIN expression is enhanced, whereas in case of long term auxin synthesis or application PIN expression is repressed34,43 and (ii) auxin regulates PIN polarity in a different manner than GLUT protein regulation by insulin as GLUT proteins remain largely non-polar in polarized cells.51
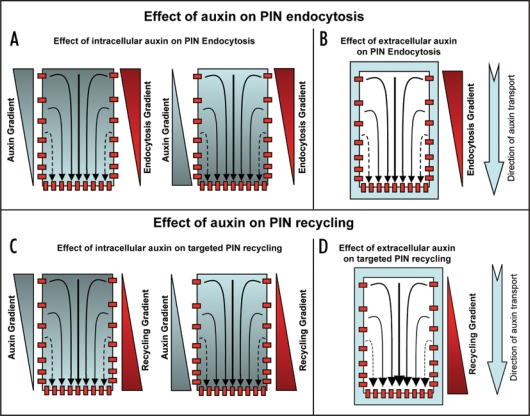
How auxin gradient is perceived and translated into the modulation of PIN polarity in individual cells still remains to be resolved, even though it is a crucial aspect of auxin-dependent self-organizing and reiterative developmental processes. Does auxin regulate PIN polarity by directly affecting PIN trafficking machinery or does auxin influence PIN polarity through cell fate and auxin response machinery regulation is still a matter of debate. The importance of endocytosis for PIN polarization15 together with experiments that causally link effect of auxin on endocytosis29 and on PIN polarity15,44,45 has interesting implications in terms of direct effect of auxin on PIN trafficking machinery. It provides a possible mechanism for feed-back regulation of PIN polarity by auxin itself that is a necessary component of many recent models of auxin-mediated development.52–56 Two different mechanisms of PIN polarization in response to auxin have been proposed: (i) a slower polarization away from the auxin source as shown for example during vascular tissue development44 and (ii) more rapid polarization towards the auxin maxima as shown during phyllotactic organ initiation.57 Both of these mechanisms might be explained further by taking into account the effect of auxin on PIN endocytosis and/or polarized recycling (Fig. 3). As shown in Figure 3, I propose multiple options that could operate either individually or together to obtain auxin-mediated polar PIN localization. It will now be important to ascertain which of the auxin-mediated PIN polarity mechanisms (by the influence of either differential endocytosis or targeted recycling) actually operate. Based on molecular modeling approaches coupled to real time in vivo analysis endocytosis dependent polarized targeting rather than a differential endocytosis has been recently reported for establishment of polarized localization of Cdc42 in budding yeast cells.58 Hence feedback loop-based and auxin-guided repeated targeting of PIN to one particular cell side for its gradual build up there seems to be a more favorable case. The effect of auxin on PIN polarity through its effect on cell fate34 and through its effect on auxin response factors44 has also been shown. However, in both of these cases auxin seems to have much slower effect on PIN polarity that could have certain limitations in terms of explaining rapid effects of auxin on PIN polarity those are required for fast alterations in auxin fluxes under certain situations.
Figure 3.
Mechanisms for effect of auxin to attain/maintain polar PIN localization. For this auxin (either intracellular, extracellular or both) could influence PIN polarity by influencing differential endocytosis, targeted recycling or both. (A) Intracellular auxin could trigger differential endocytosis positively (more endocyosis on the cell side with more auxin and less endocytosis on the cell side with less auxin, left) or negatively (more endocytosis on the cell side with less auxin and less endocytosis on the cell side with more auxin, right). (B) Extracellular auxin could trigger differential endocytosis in positive or negative manner depending on its extent. (C) Intracellular auxin could trigger targeted recycling positively (away from the cell side with more auxin towards cell side with less auxin, left) or negatively (away from the cell side with less auxin towards cell side with more auxin, right). (D) Extracellular auxin could trigger targeted recycling in positive or negative manner depending on its extent. Light blue color depicts auxin and red color depicts PIN localization at the plasma membrane (small red colored boxes) or PIN trafficking (red colored rectangles).
Rab5 Proteins: Common Endocytic Regulators in Animals and Plants
In mammalian cells, small GTPase Rab5 is a key regulator of endocytosis. Rab5 affects endocytosis via regulation of clathrin-coated vesicle formation at the plasma membrane, fusion of plasma membrane-derived vesicles to endosomes and fusion between endosomes.59,60 Rab5 localizes to endosomes and acts as molecular switch by cycling between the GDP-bound and GTP-bound states.61 In mammalian cells dominant negative (DN) mutants of Rab5 (GDP-locked Rab5) inhibit clathrin mediated endocytosis whereas dominant active (DA) Rab5 (GTP-locked Rab5) accelerate endocytosis suggesting the rate limiting effect of Rab5 activity on endocytosis.59,60,62
Arabidopsis possesses two direct homologues of Rab5 namely Ara7 and Rha1. Genetic analysis shows that single mutants of ara7 and rha1 do not display any defects suggesting functional redundancy, whereas the ara7rha1 double mutant is gametophytic lethal,15 indicating their vital role. In addition, two mutant alleles of activator of Ara7 and Rha1, AtVps9a that contains a highly conserved Vps9 domain which is a landmark for the Rab5 activator GEFs63 bear developmental defects. The null allele atvps9a-1 is embryo lethal and the leaky allele atvps9a-2 displays less severe postembryonic defects.15,64 These results show that Rab5 pathway is essential for plant development. Rab5 pathway affects PIN polarity via its effects on endocytosis.15 In Arabidopsis Rab5 homologues have been largely localized to endosomes30,65–67 and, therefore, how they could influence the early steps of PIN endocytosis is an obvious question. Similar scenario where Rab5 localizes mainly to endosomal compartments and still strongly influences clathrin mediated endocytosis at the plasma membrane bothered mammalian cell biologists until recently. Recently in worms RME-6 (a Rab5 activator) was found to load activated RAB-5 onto nascent clathrin-coated vesicles.68 Failure to load activated Rab5 on these vesicles render them fusion incompetent and thus trap them en route to endosomes. Based on the fact that PINs are internalized largely by clathrin mediated endocytic pathway,26 they pass through Rab5 positive compartments (our unpublished results) and that Rab5 interference affects PIN endocytosis,15 it could be hypothesized that Rab5 indirectly affects clathrin mediated endocytosis by inducing traffic jam of internalized vesicles between the plasma membrane and the endosomal compartments.
Endocytosis-Mediated Changes in PIN Polarity Affect Cell Fates and Organ Identity
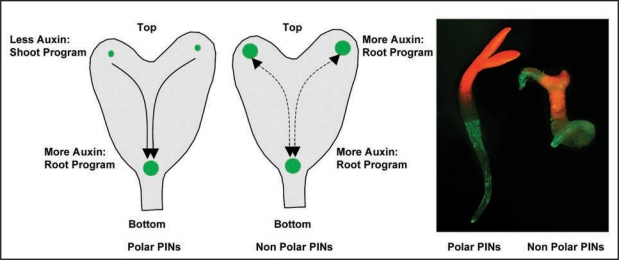
Interference with Arabidopsis Rab5 endocytic pathway disrupts PIN polarity and transiently modifies embryonic auxin distribution by increasing the auxin response maxima in cotyledon primordia. Interestingly, this appearance of ectopic auxin maxima results into expression of auxin pathway-associated molecular components, those are required for root formation,69,70 at the cotyledon regions. Accordingly, cells in cotyledons shift to root cell fate and roots are initiated (Fig. 4). This is the first example of vesicle trafficking process that connects PIN polarity changes to quantitative changes in auxin maxima and according changes in the cell fate-determining transcription factors. As altering the distribution of auxin subsequently alters cell fates these new results demonstrate morphogen (a substance influencing cell fates by its concentration gradient) characters of auxin. In case of gnom coordinated polarity of PIN1 is altered and PIN1 is largely localized to the apical cell side.14,31 This largely apical PIN localization enhances auxin accumulation in the apical half of the embryo and as a result the cotyledons are fused. A similar PIN1 apicalization happens in case of expression of PINOID kinase under the control of strong embryonic RPS5a promoter.13 However, neither gnom nor overexpressed PINOID causes organ identity alterations. Embryonic PIN polarity manipulation by sustaining default non-polar PIN localization (by endocytic impairment of PINs or by PIN overexpression) transiently alters auxin distribution that eventually leads to altered cell fates and organ identities.15 Plausible scenario for mechanism underlying the observed homeotic organ transformations is that endocytosis defects cause failure of PIN polarization leading to ectopic auxin accumulation at the place of auxin synthesis in the cotyledons,71–73 where downstream PLT expression ultimately leads to transformation of cotyledon fates into root fates. The failure of PIN polarization during embryogenesis apparently disrupts auxin flow from developing cotyledons to the root pole and consequently auxin levels from auxin production rises here leading to establishment of ectopic auxin response maxima at cotyledon positions. Embryonic cells are competent at this stage to interpret the elevated auxin response as a positional signal for root formation and accordingly they express root fate-promoting transcription factor PLT1,69,70 that sets up the initiation of roots at the positions of embryonic leaves. Although auxin accumulation by non polar PIN localization provokes fate changes and organ identity alterations in small number of significant cases, the exact mechanism how it does still remains unclear especially when drastic alterations in embryonic auxin distribution in gnom mutant and PINOID overexpressors do not show these phenotypes. Perhaps it is strictly required that alteration in auxin distribution should be transient in order to facilitate fate changes and in case of drastic auxin alterations embryonic cells just shut down the whole developmental program.
Figure 4.
Distribution-based morphogenic auxin activity gradients for embryo patterning in plants. Left two sketches show the schematics of auxin response maxima in control (with polar PINs) and in non-polar PIN case, where enhanced auxin response maxima at the cotyledons trigger root program. The picture on the right shows a control seedling (with normal cotyledons and root) and a seedling with PIN polarity manipulation (displaying root emergence from a cotyledon). Auxin response maxima are depicted in green.
Perspectives
In Drosophila, Wingless, Hedgehog and Decapentaplegic morphogen form gradients from their secretory source cells to the consuming sink cells by their regulated endocytosis in cells along their translocation paths and thus trigger their concentration-dependent developmental program.74,75 Upon endocytic inhibition, the morphogens spread randomly without forming a proper gradient and affects wing disc patterning.74,75 In analogy, recent results in plants indicate an important role for endocytosis in morphogenetic auxin activity gradient formation. However, in Drosophila wing discs morphogens are transported without a carrier and hence cell polarity alteration may not necessarily directly alter the trafficking of the morphogens. Recent findings in plants on the other hand reveal a strong interconnection between endocytosis, cell polarity and gradient-based patterning involving a relevance of the polarity of a carrier to the development of morphogen gradient. These findings also suggest that in embryo with less auxin accumulation a shoot program is initiated whereas with more auxin activity a root program is initiated. The new results are important steps in our understanding of the way plants grow and they also promise novel long-term future possibilities to modify the positioning of plant organs by localized transient alterations of PIN polarity and PIN polarity-based auxin activity gradients.
Acknowledgements
I am grateful to Ben Scheres, Niko Geldner, Kalika Prasad and Ari-Pekka Mahonen for helpful discussions and comments. Author's work is funded by Netherlands Scientific Organization (NWO)—VENI grant.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/7715
References
- 1.Gstraunthaler GJ. Epithelial cells in tissue culture. Ren Physiol Biochem. 1988;11:1–42. doi: 10.1159/000173147. [DOI] [PubMed] [Google Scholar]
- 2.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 3.Kleine-Vehn J, Friml J. Polar targeting and endocytic recycling in auxin-dependent plant development. Annu Rev Cell Dev Biol. 2008;24:447–473. doi: 10.1146/annurev.cellbio.24.110707.175254. [DOI] [PubMed] [Google Scholar]
- 4.Murphy AS, Bandyopadhyay A, Holstein SE, Peer WA. Endocytotic cycling of PM proteins. Annu Rev Plant Biol. 2005;56:221–251. doi: 10.1146/annurev.arplant.56.032604.144150. [DOI] [PubMed] [Google Scholar]
- 5.Nebenfuhr A, Staehelin LA. Mobile factories: Golgi dynamics in plant cells. Trends Plant Sci. 2001;6:160–167. doi: 10.1016/s1360-1385(01)01891-x. [DOI] [PubMed] [Google Scholar]
- 6.Meckel T, Hurst AC, Thiel G, Homann U. Endocytosis against high turgor: intact guard cells of Vicia faba constitutively endocytose fluorescently labelled plasma membrane and GFP-tagged K-channel KAT1. Plant J. 2004;39:182–193. doi: 10.1111/j.1365-313X.2004.02119.x. [DOI] [PubMed] [Google Scholar]
- 7.Nance J. PAR proteins and establishment of cell polarity during C. elegans development. Bioessays. 2005;27:126–135. doi: 10.1002/bies.20175. [DOI] [PubMed] [Google Scholar]
- 8.Wodarz A, Nathke I. Cell polarity in development and cancer. Nat Cell Biol. 2007;9:1016–1024. doi: 10.1038/ncb433. [DOI] [PubMed] [Google Scholar]
- 9.Dotti CG, Banker GA. Experimentally induced alteration in the polarity of developing neurons. Nature. 1987;330:254–256. doi: 10.1038/330254a0. [DOI] [PubMed] [Google Scholar]
- 10.Etienne-Manneville S. Polarity proteins in migartion and invasion. Oncogene. 2008;27:6970–6980. doi: 10.1038/onc.2008.347. [DOI] [PubMed] [Google Scholar]
- 11.Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, et al. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- 12.Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002;415:806–809. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- 13.Friml J, Yang X, Michniewicz M, Weijers D, Quint A, Tietz O, et al. A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science. 2004;306:862–865. doi: 10.1126/science.1100618. [DOI] [PubMed] [Google Scholar]
- 14.Kleine-Vehn J, Dhonukshe P, Sauer M, Brewer PB, Wisniewska J, Paciorek T, et al. ARF GEF-dependent transcytosis and polar delivery of PIN auxin carriers in Arabidopsis. Curr Biol. 2008;18:526–531. doi: 10.1016/j.cub.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 15.Dhonukshe P, Tanaka H, Goh T, Ebine K, Mahonen AP, Prasad K, et al. Generation of cell polarity in plants links endocytosis, auxin distribution and cell fate decisions. Nature. 2008;456:962–966. doi: 10.1038/nature07409. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Jaillais Y, Santambrogio M, Rozier F, Fobis-Loisy I, Miege C, Gaude T. The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell. 2007;130:1057–1070. doi: 10.1016/j.cell.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 17.Mellman I, Nelson WJ. Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol. 2008;9:833–845. doi: 10.1038/nrm2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson WJ, Grindstaff KK. Cell polarity: PAR for the polar course. Curr Biol. 1997;7:562–564. doi: 10.1016/s0960-9822(06)00282-x. [DOI] [PubMed] [Google Scholar]
- 20.Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta. 2008;1778:614–630. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 21.Martin-Belmonte F, Mostov K. Regulation of cell polarity during epithelial morphogenesis. Curr Opin Cell Biol. 2008;20:227–234. doi: 10.1016/j.ceb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Mostov K, Su T, ter Beest M. Polarized epithelial membrane traffic: conservation and plasticity. Nat Cell Biol. 2003;5:287–293. doi: 10.1038/ncb0403-287. [DOI] [PubMed] [Google Scholar]
- 23.Galweiler L, Guan C, Muller A, Wisman E, Mendgen K, Yephremov A, et al. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;282:2226–2230. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- 24.Wisniewska J, Xu J, Seifertova D, Brewer PB, Ruzicka K, Blilou I, et al. Polar PIN localization directs auxin flow in plants. Science. 2006;312:883. doi: 10.1126/science.1121356. [DOI] [PubMed] [Google Scholar]
- 25.Geldner N, Friml J, Stierhof YD, Jurgens G, Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature. 2001;413:425–428. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- 26.Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J, Stierhof YD, et al. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr Biol. 2007;17:520–527. doi: 10.1016/j.cub.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 27.Men S, Boutte Y, Ikeda Y, Li X, Palme K, Stierhof YD, et al. Sterol-dependent endocytosis mediates post-cytokinetic acquisition of PIN2 auxin efflux carrier polarity. Nat Cell Biol. 2008;10:237–244. doi: 10.1038/ncb1686. [DOI] [PubMed] [Google Scholar]
- 28.Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, et al. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell. 2003;112:219–230. doi: 10.1016/s0092-8674(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 29.Paciorek T, Zazimalova E, Ruthardt N, Petrasek J, Stierhof YD, Kleine-Vehn J, et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;435:1251–1256. doi: 10.1038/nature03633. [DOI] [PubMed] [Google Scholar]
- 30.Dhonukshe P, Baluska F, Schlicht M, Hlavacka A, Samaj J, Friml J, et al. Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis. Dev Cell. 2006;10:137–150. doi: 10.1016/j.devcel.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Steinmann T, Geldner N, Grebe M, Mangold S, Jackson CL, Paris S, et al. Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science. 1999;286:316–318. doi: 10.1126/science.286.5438.316. [DOI] [PubMed] [Google Scholar]
- 32.Ritcher S, Geldner N, Schrader J, Wolters H, Stierhof YD, Rios G, et al. Functional diversification of closely related ARF-GEFs in protein secretion and recycling. Nature. 2007;448:488–492. doi: 10.1038/nature05967. [DOI] [PubMed] [Google Scholar]
- 33.Teh OK, Moore I. An ARF-GEF acting at the Golgi and in selective endocytosis in polarized plant cells. Nature. 2007;448:493–496. doi: 10.1038/nature06023. [DOI] [PubMed] [Google Scholar]
- 34.Xu J, Hofhuis H, Heidstra R, Sauer M, Friml J, Scheres B. A molecular framework for plant regeneration. Science. 2006;311:385–388. doi: 10.1126/science.1121790. [DOI] [PubMed] [Google Scholar]
- 35.Keller P, Simons K. Cholesterol is required for surface transport of influenza virus hemagglutinin. J cell Biol. 1998;140:1357–1367. doi: 10.1083/jcb.140.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valdez-Taubas J, Pelham HR. Slow diffusion of proteins in the yeast plasma membrane allows polarity to be maintained by endocytic cycling. Curr Biol. 2003;13:1636–1640. doi: 10.1016/j.cub.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Willemsen V, Friml J, Grebe M, van den Toorn A, Palme K, Scheres B. Cell polarity and PIN protein positioning in Arabidopsis require STEROL METHYLTRANSFERASE1 function. Plant Cell. 2003;15:612–625. doi: 10.1105/tpc.008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Titapiwatanakun B, Blakeslee JJ, Bandyopadhyay A, Yang H, Mravec J, Sauer M, et al. ABCB19/PGP19 stabilises PIN1 in membrane microdomains in Arabidopsis. Plant J. 2009;57:27–44. doi: 10.1111/j.1365-313X.2008.03668.x. [DOI] [PubMed] [Google Scholar]
- 39.Dhonukshe P, Mathur J, Hulskamp M, Gadella TW., Jr Microtubule plus-ends reveal essential links between intracellular polarization and localized modulation of endocytosis during division-plane establishment in plant cells. BMC Biol. 2005;3:11. doi: 10.1186/1741-7007-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhonukshe P, Grigoriev I, Fischer R, Tominaga M, Robinson DG, Hasek J, et al. Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proc Natl Acad Sci USA. 2008;105:4489–4494. doi: 10.1073/pnas.0711414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baluska F, Samaj J, Menzel D. Polar transport of auxin: carrier-mediated flux across the plasma membrane or neurotransmitter-like secretion? Trends Cell Biol. 2003;13:282–285. doi: 10.1016/s0962-8924(03)00084-9. [DOI] [PubMed] [Google Scholar]
- 42.Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 43.Vieten A, Vanneste S, Wisniewska J, Benkova E, Benjamins R, Beeckman T, et al. Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development. 2005;132:4521–4531. doi: 10.1242/dev.02027. [DOI] [PubMed] [Google Scholar]
- 44.Sauer M, Balla J, Luschnig C, Wisniewska J, Reinohl V, Friml J, Benkova E. Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev. 2006;20:2902–2911. doi: 10.1101/gad.390806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scarpella E, Marcos D, Friml J, Berleth T. Control of leaf vascular patterning by polar auxin transport. Genes Dev. 2006;20:1015–1027. doi: 10.1101/gad.1402406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sachs T. Cellular interactions in tissue and organ development. Symp Soc Exp Biol. 1986;40:181–210. [PubMed] [Google Scholar]
- 47.Dugani CB, Klip A. Glucose transporter 4: cycling, compartments and controversies. EMBO Rep. 2005;6:1137–1142. doi: 10.1038/sj.embor.7400584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Friml J, Benkova E, Blilou I, Wisniewska J, Hamann T, Ljung K, et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell. 2002;108:661–673. doi: 10.1016/s0092-8674(02)00656-6. [DOI] [PubMed] [Google Scholar]
- 49.Luschnig C, Gaxiola RA, Grisafi P, Fink GR. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 1998;12:2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petrasek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertova D, et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science. 2006;312:914–948. doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- 51.Kalsi KM, Baker EH, Medina RA, Rice S, Wood DM, Ratoff JC, et al. Apical and basolateral localisation of GLUT2 transporters in human lung epithelial cells. Pflugers Arch. 2008;456:991–1003. doi: 10.1007/s00424-008-0459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Reuille PB, Bohn-Courseau I, Ljung K, Morin H, Carraro N, Godin C, et al. Computer simulations reveal properties of the cell-cell signaling network at the shoot apex in Arabidopsis. Proc Natl Acad Sci USA. 2006;103:1627–1632. doi: 10.1073/pnas.0510130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grieneisen VA, Xu J, Maree AF, Hogeweg P, Scheres B. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature. 2007;449:1008–1013. doi: 10.1038/nature06215. [DOI] [PubMed] [Google Scholar]
- 54.Jonsson H, Heisler MG, Shapiro BE, Meyerowitz EM, Mjolsness E. An auxin-driven polarized transport model for phyllotaxis. Proc Natl Acad Sci USA. 2006;103:1633–1638. doi: 10.1073/pnas.0509839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kramer EM. Computer models of auxin transport: a review and commentary. J Exp Bot. 2008;59:45–53. doi: 10.1093/jxb/erm060. [DOI] [PubMed] [Google Scholar]
- 56.Smith RS, Guyomarc'h S, Mandel T, Reinhardt D, Kuhlemeier C, Prusinkiewicz P. A plausible model of phyllotaxis. Proc Natl Acad Sci USA. 2006;103:1301–1306. doi: 10.1073/pnas.0510457103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuhlemeier C. Phyllotaxis. Trends Plant Sci. 2007;12:143–150. doi: 10.1016/j.tplants.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 58.Marco E, Wedlich-Soldner R, Li R, Altschuler SJ, Wu LF. Endocytosis optimizes the dynamic localization of membrane proteins that regulate cortical polarity. Cell. 2007;129:411–422. doi: 10.1016/j.cell.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, et al. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- 60.van der Bliek AM. A sixth sense for Rab5. Nat Cell Biol. 2005;7:548–550. doi: 10.1038/ncb0605-548. [DOI] [PubMed] [Google Scholar]
- 61.Vitale G, Alexandrov K, Ullrich O, Horiuchi H, Giner A, Dobson C, et al. The GDP/GTP cycle of Rab5 in the regulation of endocytotic membrane traffic. Cold Spring Harb Symp Quant Biol. 1995;60:211–220. doi: 10.1101/sqb.1995.060.01.024. [DOI] [PubMed] [Google Scholar]
- 62.Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M. Inhibition of Rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carney DS, Davies BA, Horazdovsky BF. Vps9 domain-containing proteins: activators of Rab5 GTPases from yeast to neurons. Trends Cell Biol. 2006;16:27–35. doi: 10.1016/j.tcb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Goh T, Uchida W, Arakawa S, Ito E, Dainobu T, Ebine K, et al. VPS9a, the common activator for two distinct types of Rab5 GTPases, is essential for the development of Arabidopsis thaliana. Plant Cell. 2007;19:3504–3515. doi: 10.1105/tpc.107.053876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumacher K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell. 2006;18:715–730. doi: 10.1105/tpc.105.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jaillais Y, Fobis-Loisy I, Miege C, Gaude T. Evidence for a sorting endosome in Arabidopsis root cells. Plant J. 2008;53:237–247. doi: 10.1111/j.1365-313X.2007.03338.x. [DOI] [PubMed] [Google Scholar]
- 67.Ueda T, Uemura T, Sato MH, Nakano A. Functional differentiation of endosomes in Arabidopsis cells. Plant J. 2004;40:783–789. doi: 10.1111/j.1365-313X.2004.02249.x. [DOI] [PubMed] [Google Scholar]
- 68.Sato M, Sato K, Fonarev P, Huang CJ, Liou W, Grant BD. Caenorhabditis elegans RME-6 is a novel regulator of RAB-5 at the clathrin-coated pit. Nat Cell Biol. 2005;7:559–569. doi: 10.1038/ncb1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, et al. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell. 2004;119:109–120. doi: 10.1016/j.cell.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 70.Galinha C, Hofhuis H, Luijten M, Willemsen V, Blilou I, Heidstra R, et al. PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature. 2007;449:1053–1057. doi: 10.1038/nature06206. [DOI] [PubMed] [Google Scholar]
- 71.Zhao Y. The role of local biosynthesis of auxin and cytokinin in plant development. Curr Opin Plant Biol. 2008;11:16–22. doi: 10.1016/j.pbi.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 72.Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, et al. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell. 2008;133:177–191. doi: 10.1016/j.cell.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 73.Tao Y, Ferrer JL, Ljung K, Pojer F, Hong F, Long JA, et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell. 2008;133:164–176. doi: 10.1016/j.cell.2008.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Entchev EV, Gonzalez-Gaitan MA. Morphogen gradient formation and vesicular trafficking. Traffic. 2002;3:98–109. doi: 10.1034/j.1600-0854.2002.030203.x. [DOI] [PubMed] [Google Scholar]
- 75.Fischer JA, Eun SH, Doolan BT. Endocytosis, endosome trafficking, and the regulation of Drosophila development. Annu Rev Cell Dev Biol. 2006;22:181–206. doi: 10.1146/annurev.cellbio.22.010605.093205. [DOI] [PubMed] [Google Scholar]