Abstract
The Energide concept, as well as the endosymbiotic theory of eukaryotic cell organization and evolution, proposes that present-day cells of eukaryotic organisms are mosaics of specialized and cooperating units, or organelles. Some of these units were originally free-living prokaryotes, which were engulfed during evolutionary time. Mitochondria represent one of these types of previously independent organisms, the Energide, is another type. This new perspective on the organization of the cell has been further expanded to reveal the concept of a public milieu, the cytosol, in which Energides and mitochondria live, each with their own private internal milieu. The present paper discusses how the endosymbiotic theory implicates a new hypothesis about the hierarchical and communicational organization of the integrated prokaryotic components of the eukaryotic cell and provides a new angle from which to consider the theory of evolution and its bearing upon cellular complexity. Thus, it is proposed that the “selfish gene” hypothesis of Dawkins1 is not the only possible perspective for comprehending genomic and cellular evolution. Our proposal is that maternal mitochondria are the selfish “master” entities of the eukaryotic cell with respect not only to their propagation from cell-to-cell and from generation-to-generation but also to their regulation of all other cellular functions. However, it should be recognized that the concept of “master” and “servant” cell components is a metaphor; in present-day living organisms their organellar components are considered to be interdependent and inseparable.
Key words: complexity, embedded internal milieu, Energide, evolution, maternal mitochondria, prokaryote-eukaryote transition, order
Introduction
The concept of ‘Energide’, originally proposed in 1892 by Julius Sachs,2 demands revisions not only of the classical concept of cell,2–4 but also of the equally classical concept of the “internal milieu”.5 According to the most recent version of the Energide concept,2 the combination of nucleus and microtubular cytoskeleton is the fundamental and universal unit of eukaryotic life. The complementary endosymbiotic theory of the cell due to Margulis6 maintains that mitochondria were originally free-living prokaryotic organisms (α-proteobacteria), which became engulfed and integrated within either an archeal or a primitive eukaryotic host. The transition from being an autonomous proto-bacterium to becoming a subordinate host (nuclear)-controlled organelle was pivotal in the evolution of the eukaryotic cell7 (for a discussion of alternative hypotheses describing the origin of eukaryotic cell and evolution of mitochondria, see Gray et al.8).
Recent analyses of the genomes of eukaryotic nuclei and cytoplasmic mitochondria have revealed that the last universal common ancestors of the organellar components of eukaryotic cells were formed by cell-cell mergings or fusions. Now that we have a more complete and holistic view of eukaryotic cells, we can see that it is characterized by a distinct duality, or dialectic, recognizable in the complementarity of (originally the antagonism between) cellular structures and processes. Thus, at the level of the cell, the Energide (guest) is complemented by a Cell Periphery Complex (host). At the level of the genome, eubacterial features complement archaebacterial features; the cytoskeleton is composed of complementary tubulin (Guest) and actin (Host); membrane flow is comprised of exocytosis (Guest) and endocytosis (Host) which complement each other, and cell division integrates the complementary processes of mitosis and cytokinesis.2,3 At the level of the genome, eubacterial features complement archaebacterial features.
These complementary features strongly suggest that the most ancient eukaryotic cell was generated from the merger/fusion of at least three proto-cell organisms and that the eukaryotic genome is the result of the merger of at least two proto-genomes (reviewed in refs. 2–4). It has been suggested that this universal and ancestral “Host/Guest” cell consortium “enslaved” at least two other types of cells that entered it via a predatory phagocytosis.2,9 Thereby, a new even more complex endosymbiotic cell emerged consisting of diverse organelles.
Remarkable is that mitochondria, although enslaved by their host, have retained most of their prokaryotic biochemistry despite the fact that they harbour only a remnant of the genome which their eubacterial predecessor possessed. This view implies that two processes have paved the way for the evolution of eukaryotic cells which now have a greater degree of complexity than their ancestors. Importantly, this hypothesis has the consequence that such cells would disobey the usual tenets of Darwinian evolution due, notably, to the processes of lateral gene transfer (also called horizontal gene transfer) and endosymbiosis.2,4 Each of these processes, both individually and together, have played a crucial role in the evolution of complex eukaryotic cells and has endowed them with nuclei. Lateral gene transfer (LGT) is a process by which an organism incorporates genetic material from another organism without being the offspring of that organism. This phenomenon is still in operation in eukaryotic cells, and is a ubiquitous and continuing natural process which pervades the dynamics of nuclear DNA within and between diverse organisms. Moreover, it is still occurring between the mitochondria and the nucleus of cells.10–12 Analysis of genome DNA sequences reveals that ever since the incorporation of cellular organelles into a “host” cell, organellar DNA has constantly bombarded the nucleus of the host, and that DNA is transferred by LGT from the genome of organelles to the nucleus at frequencies that were previously thought impossible.10
As underlined by Baluska et al.2,4 evolution of complex eukaryotic cells not only provides us with an important paradigm for the elusive nature of living matter but also suggests why living entities should have evolved from a low level of complexity to one that is higher. Here we remark that the strength of the Darwinian evolutionary theory is its ability to explain how adaptation comes about, driven as it is by variation, competition and selection, but that the theory could also probably find important complements in the endosymbiotic theory and in Kauffman's proposal that there is a tendency inherent to living matter to acquire order and hence to develop more complex forms32 (see also below). Thus, a new interpretative frame has to be proposed in order to enlarge the idea of the classical Darwinian selection-based concept of evolution. Besides evolution towards increased fitness there is also evolution towards increased cellular complexity to consider. Merging, fusion and association of previously free-living cellular organisms not only relieves conflicts between organisms but also gives rise to more complex cellular organisms. This new interpretative frame thus enlarges upon the idea of the classical Darwinian selection-based concept of evolution which is oriented towards increased fitness (a process which acted upon even the ancient proto-cells), and accounts for the dramatic increase in cellular complexity during the prokaryote-eukaryote transition. To this end, it may be convenient to think of evolution as a “tinker”26 (see below) who, with the help of the ‘sieve of the natural selection’, creates the most efficient combinations of molecular modules at the sub-cellular level. At the cellular level there is a similar “sieving” of the results of “trial and error” tinkering events. In each case there is what appears to be a random walk led on by a hypothetical attractor, the attractor of order32 (see below)—i.e., the selective sieve that permits optimal associations of endosymbiotic organelles.
It appears that the most powerful source of innovation with regard to cellular complexity is due to the fusion, or merging, of individual cellular units into a new endosymbiotic unit which thereby acquires emergent properties not shared by the original parent cellular units. As Baluska et al.2–4 pointed out, this fusion of units is itself a permissible by-product of cellular competition. But this time it is not adaptive competition due to external factors in the sense posited by Darwinian adaptive evolution that leads to the establishment of a new life form, but is rather an outcome of an internal ‘predatory’ competition within a cellular structure that is the product of several successive endosymbioses. This alternative scenario leads to endosymbiotic evolution accompanied by an increased tendency towards, or potential for, cellular complexity. This, in turn, leads to the development of higher levels of organization due to modifications of cell boundary properties, including the means of cell-cell interactions and communications. Whereas ‘adaptive’ evolution leads only towards increased fitness at various levels of organization and is driven by the conflict of organisms within selectionist environments, evolution towards higher cellular complexity is driven by conflicts between ‘guest’ organisms within an endosymbiotic ‘host’ environment. If enough communication can be achieved across the boundaries of all the numerous unitary “guest” organelles within a “host” endosymbiotic cell, a balanced interplay of ‘forces’ may then be reached between what would otherwise simply be competition between organellar units within the same milieu. In the past of evolutionary time, this competitive endosymbiotic state allowed the development of a stable relationship between the Energide and other organellar guests and ultimately led to the ‘individuation’ of a new type of cell and the creation of not only the proposed higher level of cellular complexity but also the subsequent generation and multiplication of yet more complex cells. It should be noted that not much is known about mitochondria/Energide communication processes.13 Signaling, which certainly occurs between the two units, may be mediated by changes in external substances, in ion fluxes (e.g., calcium), and in structural changes to the organelle itself (e.g., mitochondrial fission/fusion homeostasis). Signaling may also arise as a property of the proton gradient at the mitochondrial inner membrane.9,14
The Present Hypothesis
The present hypothesis posits that the precursor of the Energide as well as several other small prokaryote bodies colonized a large proto-cell and found within it a suitable internal milieu. Then, as indicated above, there were intraorganellar forces that had to be balanced, namely the competitive forces of the ingested prokaryotic units within this shared internal milieu. Out of this new balanced state came the need for positive interactions between all those units which had been ingested in order to survive in an endosymbiotic relation with the host prokaryote. A second force also had to be reconciled: the often antagonistic relationship between the new composite organism (or cell) and its external and potentially hostile environment.
Wallace states that “Life is the interplay between structure, energy and information.”9 To this can be added the idea that these interrelated features realize their full meaning only within the framework of order. Inevitably, a further question is whether order is better conserved by containment of the system within a heterarchic or a hierarchic organizational structure: that is, whether all components of a ‘cell’ are of equal ‘strength’ and cooperate (heterarchy), or whether one component is dominant and regulates the function of subordinate components (hierarchy). A hierarchical order would necessarily invoke a master entity. To discern such an entity would require analysis of the inter-organellar symbiotic interactions and thereby discover cui maxime prodest.
On the basis of these premises, the hypothesis is put forward that, for multicellular organisms, cellular order is hierarchical, and that the “master” entity of the eukaryotic cell is not the Energide (i.e., nucleus with associated microtubules), as was previously supposed, but the maternal mitochondrion. Hence, a Copernican Revolution seems inevitable for the understanding of the biology of eukaryotic cells in multicellular organisms. The intracellular organelles no longer revolve around a nuclear “sun”, but everything revolves instead around a maternal mitochondrial “sun”. Thus, while Dawkins1 has formulated the important theory of the selfish gene (nuclear DNA), the present hypothesis maintains that, during the evolutionary descent of present-day eukaryotic animals and hence of humans, and perhaps plants also, the selfish entity that promotes its own conservation is the maternal mitochondrion.
Data and deductions.
Some data and deductions which support the present hypothesis centre around mitochondrial biology and are as follows:
Mammalian mtDNA is maternally inherited. This phenomenon was probably a late development in evolutionary time and did not apply in early eukaryotic organisms. However, in the modern eukaryotes the mitochondria of mammalian sperm are destroyed in the fertilized oocyte; they are ubiquitinated inside the oocyte cytoplasm and later subjected to proteolysis during pre-implantation development of the embryo. That mitochondria have an exclusive maternal origin, implies that mammalian organisms defend their gender-based singularity. Thus, the mitochondria move from one generation to the next unopposed by any recombination resulting from sexual mechanics. This is in contradistinction to the male Energide which mixes its nDNA with that of the female Energide following sexual fertilization of the oocyte. While this assumption is generally accepted, some authors have pointed out that, in some cases, a paternal inheritance of mitochondria is possible.15 Supporting this view is a case report describing a 28-year-old man with most of the mitochondria in his muscles inherited not from his mother but from his father.16 However, besides special cases such as the one mentioned, it should be accepted that males are dead-ends for mitochondria (due to their destruction at fertilization) and this has consequences for the human sex ratio.17
Plant mitochondria are maternally inherited. Recent work shows that in the thale cress, Arabidopsis thaliana, the mitochondria of sperm cells, brought into the egg cell during the double fertilization, are destroyed. Those that remain are of maternal origin.18 There are also observations from electron microscopy which show that, in barley, the mitochondria of the sperm are ejected from these cells before the sperm participate in fertilization.19
Mitochondria maintain power over the life and death of a cell because they have the controlling hand in programmed cell death by releasing proteins such as cytochrome c. This can explain why human cells dedicate well over 100 nuclear genes to the maintenance of mtDNA that encodes only 13 proteins.20 In fact, three main hypotheses have been put forward to explain why genes for certain mitochondrial membrane subunits of the oxidative phosphorylation complexes (OXPHOS) have been retained in the mtDNA.9 These hypotheses are not mutually exclusive and each has some experimental support. However, it should be considered, as mentioned above, that the mtDNA-encoded polypetides give overall control of the mitochondrial energy supply by which the eukaryotic cell is sustained, and even holds the key to cellular life and death and maybe to organismal life and death also. As Wallace points out, all mtDNA analyzed to date contain cob and cox1 genes, which are central to the coupling of electron transport with proton pumping via complexes III and IV.9 A consequence of our hypothesis is the proposal that, during evolution, mitochondria, while they have maintained possession of some crucial genes, they may nevertheless have transferred most of their mtDNA to the “servant” nDNA that is the product of the combined male and female Energide brought into being by sexual fertilization. In addition to hypothetical selfish action of the mitochondrial “master” unloading part of its genetic burden onto the nuclear “servant”, the transfer of endosymbiont genes to the host genome is also a consequence of the well-documented observation that, in isolated endoparasitic genomes, degenerative evolution and inevitable loss of DNA-based information is an unavoidable consequence of the increasing mutational load.
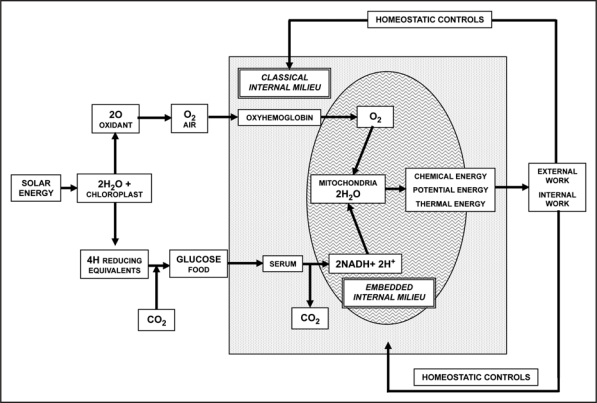
Mitochondria can evolve as colonies within the eukaryotic cell, i.e., in a privileged environment (Fig. 1). As beautifully stated by Wallace, “once the nuclear cytosol and mitochondrial endosymbiosis became established, the cytosol became the mitochondrial universe.”9 It follows that in a multi-cellular organism mitochondria enjoy their own “embedded milieu” (i.e., the internal environment of the prokaryotic mitochondrial ancestor that lies embedded within the internal milieu of the host cytoplasm), which protects them from the surrounding “classical internal milieu,” or cytosol, provided by the original host organelle, or cell.4 Obviously, this is true for the Energide and also for the other endosymbiotic organelles; each of their organellar “cytoplasms” is also an embedded milieu within the cytosol. Thus, the host's classical internal milieu harbours, embedded within it, one Energide with its cytoplasm, as well as a colony of interacting mitochondria each with its own internal milieu.
Mitochondrial DNA can mutate more rapidly than nDNA—animal mtDNAs have been found to have a high mutation rate, evolving about 20 times faster than nDNA sequences with analogous coding sequences.9 Mitochondria can more easily discard the unfavorable mutations since they are always organized as a dynamic colony, or chondriome, of 102 or 103 organelles within each eukaryotic cell. The relevance of the proper functioning of the chondriome is underlined by several findings of particular interest. For example, recent data show a cause-effect relationship between amyloid β over-production and alterations in mitochondrial dynamics. Thus, it has been demonstrated that the amyloid precursor protein (APP), through amyloid β production, causes an imbalance of mitochondrial fission/fusion that results in mitochondrial fragmentation and abnormal distribution. This, in turn, contributes to mitochondrial and neural dysfunction.21 Thus, not only might mitochondria be defective in supplying energy to neurons, but also alterations to the processes of mitochondrial fission can lead to apoptosis and neurodegeneration.22,23 These data may shed some light on the still unclear etiologies of sporadic Alzheimer's disease, Parkinson's disease and amyotrophic lateral sclerosis, which are among the most common neurodegenerative diseases and seem to have mitochondrial alterations as one of the causative factors.23,24
Transmission of heteroplasmic mtDNA deletions from a mother organism to its offspring is rare, whereas transmission of heteroplasmic point mutations is common in human pedigrees. The uneven distribution of mutated mtDNA among siblings has been attributed to a bottleneck phenomenon during mammalian oogenesis. This is still unexplained.
Mitochondria can sometimes move from one eukaryotic cell to another within one and the same organism and thereby reach a more favorable environment. Can mitochondria, for example, migrate from a dying cell to one that is more vital and with potentially a longer life span ahead of it? Can mitochondria of cancerous cells migrate into, and thereby infect, healthy cells? Actually, mitochondrial transfer between eukaryotic animal cells has been demonstrated and a hypothesis has been put forward of a “selfish” scenario in which it is surmised that mitochondria transfer occurs from predominantly respiration-deficient cells to respiration-competent cells, which, as new host cells, can then provide a more favorable environment for their selfish perpetuation.25
mtDNA and nDNA do not code for the entire assemblage of the mitochondrial envelope. Although some proteins of the outer mitochondrial membrane are in fact encoded by nDNA (interestingly, almost exclusively by genes of archaeal, i.e., host, and not of endosymbiont origin), but the membrane, as an entire structure, cannot be made de novo and is inherited by fission of the organelle and developed by self-assembly. In other words, the information carried by the two DNA genomes is insufficient to specify a eukaryotic cell in its entirety.20 Thus, mitochondria are unusual organelles in the sense that they cannot be made ex novo, but need a previous mitochondrion as a template to which new components are added. Almost the whole complement of proteins that constitute the mitochondria are encoded in the nucleus and synthesized in the cytosol as precursors of mitochondrial structure. These precursors are endowed with signals for their targeting to the surface of already existing mitochondria and for the transport and sorting to the various mitochondrial sub-compartments.13 Thus, each mitochondrion recruits new proteins from the cytosol, and these are added to the appropriate pre-existing compartments and protein complexes. Eventually, the expanding mitochondrion is divided by fission when a critical mass is attained.9
Figure 1.
Schematic representation of the energy flux from the sun to heterotrophic eukaryotic cells.9 As indicated in the scheme, chloroplasts fix carbon from CO2 and convert this carbon into high-energy organic molecules that constitute the basis of the global food chain. Mitochondria convert the energy of the chemical bonds of these compounds into adenosine triphosphate.7 The scheme indicates also the two internal milieux within a cell: the classical internal milieu and a protected embedded milieu belonging to the mitochondrial colony.
Evolution, Tinkering and Hierarchical Order
Two phenomena should be considered to broaden the more conservative form of neo-Darwinism of evolution, namely endosymbiosis and lateral gene transfer, LGT. They have, nevertheless, been of paramount importance in the acquisition of cellular complexity by eukaryotic cells. It is proposed that Jacob's far-reaching concept of evolution acting not as an engineer but as a tinker, using materials at its disposal to produce a new entity,26 can be extended from the molecular level up to the level of entire organisms.
This view is in agreement with the statement of Russell and Aloy,27 who propose that a key concept in biological system design is modularity; thus, modularity in relation to tinkering means that nature is continually reusing both the design principles as well as the modules of structure to which are coupled metabolic processes in the construction of new forms. This basic logic is apparent at nearly all levels, from the four bases making up the genetic code to the hierarchical organization of ecosystems.27 In the present paper, it is suggested that modularity is advantageous to the tinkering process by which the assemblage of endosymbiotic “contraptions” came into being during eukaryotic phylogeny. It is proposed, therefore, that different primitive organisms have been used by the evolutionary tinker as the modular building blocks for the assembly of a new endosymbiotic type of cell with an increased complexity and out of which more complex living systems can emerge. This mode of evolution recalls how “tesserae” are put together to construct a mosaic; or, at an even higher level, how intelligent animals consciously co-opt inanimate objects as tools for the enhancement of their mode of living.
Thus, present-day eukaryotic cells are a hierarchical mosaic of cooperating specialized units. It follows that a hierarchical order of modules within a cell (or at any level) would imply that there is a ranking of these constituent units (with respect to their complexity or bonding energy) in operation. Accordingly, criteria can be found to assess the leading or dominant component in a ranked series of organelles. We consider four such criteria:
Unbroken linear descendent through successive cell generations.
Control of energy production.
Possibility of by-passing, and therefore of surviving, cell death.
Regulation of cell death, life being the default position.
According to these criteria mitochondria are the “master” entities of the eukaryotic cells of multicellular organisms, and all other organelles are ‘servant’ entities (the Energide and everything else).
A fifth criterion is forthcoming from the answer to the question concerning which of the endosymbionts acquires the maximal advantage from the cooperation of organelles within a hierarchically ordered eukaryotic cell? Mitochondria represent the most numerous and cooperative of cellular organelles. Hence they can exploit, in an efficient way, all the other milieu embedded within an endosymbioitc cell. Basically, they have delegated to all the other organelles the task of maintaining a constant internal milieu—that cytosolic milieu which was originally supplied by the host cytoplasm. This they do with the aid of three controls: energy balance, fission with self-assembly of their own mitochondrial structure, and selective apoptosis of the cells containing them. Furthermore, mtDNA influences sex determination, as it has been shown that in early embryos mitochondria can kill or feminize males by initiating apoptosis in important gender-related cell lineages.17 Thus, a genetic conflict exists between mitochondrial genes, which are maternally inherited, and nuclear genes, which are biparentally inherited. A mitochondrion would prefer that all the organisms that propagate them should be female. But apart from thelotokous parthenogenesis (in animals) and apoxmixis (in plants), because of the prevalence of heterogametic male-femal sexual reproduction, this is obviously impossible. With an XY chromosomal sex-determining system, a gender-specifying locus on an X chromosome could bias the sex ratio, whereas an analogous locus on an autosome would favor an equal sex ratio. In other words, sexual reproduction prevents all nuclear and mitochondrial genes from realizing their evolutionary ideals or end-points, and differences in longevity between males and females could result from these types of conflicts.28 Genomic conflict can cause cytoplasmic male sterility in plants. In fact, conflict between maternally inherited mtDNA, which induce male sterility, and nuclear genes, which restore male fertility, has been described.29
Discussion
Basic epistemological considerations.
A starting point for our discussion is a consideration of scientific paradigms. According to Kuhn, a “paradigm” in the sphere of science has two essential characteristics: it is capable of attracting an enduring group of adherents away from competing modes of scientific activity, and it is sufficiently open-ended to raise new questions for the redefined group of practitioners to resolve.30 A paradigm plays a fundamental role in a given science because without it all the facts which pertain to the development of that science would seem to be equally important. A paradigm shift therefore provides a new focus to a science that is losing its interpretive and predictive power. A new paradigm gains status when it is found to be more successful than its competitors in solving problems that had previously been recognized, within the old paradigm, as critical “sticking points”. However, as Kuhn points out, all paradigms have a natural “life span”. Sooner or later each one is replaced by a new paradigm which explains not only new experimental evidence which previous theories contained within the former paradigm failed to account for, but also continues to account for everything that was formerly accommodated within the old paradigm.30,31
Evidently, a new scientific “paradigm” for the understanding of “the cell” is now gaining both credibility and adherents in the biological sciences. This emerging paradigm, as well as exposing and healing the limitations of the old cell concept, imposes a deep revision upon that concept as it stands at present. It follows that even the Darwinian evolutionary paradigm—that there is but one single selective force, i.e., ‘natural selection’—comes into question.
As indicated above, two types of evolutionary patterns can be recognized: (1) adaptive evolution where there is a tendency towards increased fitness driven by the conflict of organisms within their selectionist environment and where thresholds of survival have continually to be overcome and re-established at new levels, and (2) a predatory-endosymbiotic evolution that leads towards increased cellular complexity and which is powered by the conflicts between what were formerly independent heterotrophic unicellular organisms which now find themselves as enforced endosymbiotic partners embedded within a mosaic cell. It should be noticed that this second pattern is the outcome of intercellular tinkering whereby unitary cells have been merged together to form a new super-unit. Furthermore, two mechanisms—lateral gene transfer and endosymbiosis—have been described as paving the way towards the second of these patterns (endosymbiotic evolution). It should also be noticed that, while the first pattern (adaptive evolution) conforms to Darwinian evolution, the increase in complexity of eukaryotic cells and, hence, of the increased scope for organogenesis which can be achieved by the second pattern disobeys the tenets of Darwinian evolution. However, each pattern should be evaluated in the context of Kauffman's proposal that simple and complex systems exhibit order spontaneously.32
Stuart Kauffman suggests that Darwin's view is inadequate, since the source of order in the great branching tree of life itself is not the result of a single force: natural selection.32 We propose that the two above-mentioned processes (i.e., endosymbiosis and lateral gene transfer) should be analyzed against Kuaffman's proposal that selection achieves and maintains complex systems poised on the boundary, or edge, between order and chaos. These two processes could be viewed as instruments used by the “evolutionary tinker” since, as Jacob asserts, evolution tinkers together contraptions.33 Jacob's simile of tinkering provides a very suggestive insight of the way in which evolution might operate, but it leaves open one basic question: is tinkering founded only on the accidental encounter of various components within one and the same construction (same-level, intracellular tinkering)? Or could it be that two previously independent constructions (organisms) would fuse together (higher-level, intercellular tinkering)? Such an action would then lead to a single new assemblage which, in turn, would yield to further inter-cellular tinkering, but now with an increased range of parts that allows more efficient exploitation of the environment than was possible previously. In our opinion, it can be surmised that chance may be an important factor in this and in any other process. Although the nature of chance, or hazard, is mysterious, we can say that, in the present context, it regulates the rate at which independent organisms become tinkered, or fused, together.
However, chance operates within limits imposed by the order with which complex systems are inherently endowed.32 It may be surmised that there is even a propensity towards order (order is attractive), and that this ordering property, together with chance, contribute to some of the improbable phenomena revealed within a phylogeny. Nevertheless, at higher levels of living matter other factors besides chance and the attractiveness of order (which might be a metaphor for metabolic efficiency coupled with morphological plasticity) come into play. Let us, for example, consider again the scenario we have been pursuing—the evolutionary tinker's use of mitochondrion and Energide. In the atmosphere of the Earth, during the first phases of the emergence of life, there was a progressive enrichment of oxygen—a toxic element for anaerobic organisms. It may be surmised that a relatively privileged environment permitting the survival of anaerobic organisms would be created by enclosing within them of a prokaryotic pre-mitochondrial colony because the constituent organelles would consume oxygen and maintain its tension at a relatively low level. Obviously, this could have been one of the functional processes brought about by chance and the tinkering process which brought together the Energide and the mitochondria within a host cell. Chance lay in the predator-prey relationship of organisms which later generated a respective host-guest relationship within the newly tinkered endosymbiotic cell.
Final comments on the present hypothesis.
According to the present hypothesis, there has been not only a competitive coevolution between cytoplasmic genetic elements and nDNA, but also a master role has been conferred upon mitochondria. These have assumed a selfish control even of the human organism from fertilization through to the early-embryo stage and thence to the adult life. All these stages of development are biased to favor survival and propagation of the maternal mitochondria. The maternal mitochondria are nothing but prokaryotes which exert a powerful control over the endosymbiotic status of the eukaryotic cell.
A strong deduction can therefore be drawn from such a hypothesis: The increased complexity of multi-cellular organisms, during phylogeny, has been due to natural selection favoring adaptation to the external environment by means of the maintenance of the classical internal milieu of the endosymbiotic cell, using the mechanism of homeostasis. The classical internal milieu is obviously also an important requisite for the constancy of the proposed embedded milieu belonging to mitochondria, and vice versa.
It may also be surmised that, just as the physico-chemical parameters of the classical internal milieu are basic indices for monitoring the state of a multi-cellular organism, so the analogous parameters of the embedded milieu will have a similar importance for the assessment of the state of mitochondrial colonies. The relevance of this view is stressed by the demonstration that an increasing number of human diseases are caused by mutations in mtDNA.34,35 That is, the state of the embedded mitochondrial colony bears no longer an optimum relationship with the state of the classical internal milieu.
Because altered mitochondrial states have been proposed to play a role in ageing36,37 and in carcinogenesis,38 it may be conjectured that a new field of research regarding multicellular organisms will arise. It will inevitably include the investigation of how mitochondria, because of their role in controlling nDNA, affect cognition. In agreement with this view it has been demonstrated that the mitochondrial haplotype has a great impact on brain cognitive function.39 Furthermore, it has been shown that mtDNA, via interactions with nuclear DNA, can modify learning, exploratory behavior, sensory development and the anatomy of the brain. The effects of mtDNA alterations persist into old age, increasing in magnitude as organisms (there is evidence for this from mice) become older.40 Thus, it is not unexpected that complex patterns of behavior will emerge, e.g., the reproductive behaviors of females within a species or group because they are the primary bearers of mitochondria.17 It should be noted that female susceptibility to male manipulations may persist (and evolve) because of the indirect advantage to the female selection for those males which are good in manipulating females.41 Even propensities for male homosexuality and male infertility have been proposed to be governed by mitochondria.17
In conclusion, we are convinced that this new perspective on the eukaryotic cells will provide new clues to allow a better understanding not only of the logic of living systems but also of the logic of evolution and the attainment of higher biological complexity. It will also pose additional scientific questions for biology, as well as lead to the design of critical experiments which will bring us closer to the control of devastating diseases such as cancer, Alzheimer's, Parkinson's, and many more which, as indicated above, might eventually be discovered to be due to pathological alterations to the hierarchical order that governs mitochondria, Energides and the Cell Periphery Complexes within endosymbiotic eukaryotic cells.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/8320
References
- 1.Dawkins R. The Selfish Gene. Oxford: Oxford University Press; 1999. [Google Scholar]
- 2.Baluška F, Volkmann D, Barlow PW. Cell-cell channels and their implications for Cell Theory. In: Baluška F, Volkmann D, Barlow PW, editors. Cell-Cell Channels. Georgetown and New York: Landes Bioscience and Springer Verlag; 2006. pp. 1–18. [Google Scholar]
- 3.Baluška F, Volkmann D, Barlow PW. Cell bodies in a cage. Nature. 2004;428:371. doi: 10.1038/428371a. [DOI] [PubMed] [Google Scholar]
- 4.Baluška F, Volkmann D, Barlow PW. Eukaryotic cells and their Cell Bodies: Cell Theory revisited. Ann Bot. 2004;94:9–32. doi: 10.1093/aob/mch109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agnati LF, Fuxe K, Baluška F, Guidolin D. Implications of the ‘Energide’ concept for communication and information handling in the central nervous system. J Neurol Transm. 2008 doi: 10.1007/s00702-009-0193-1. In press. [DOI] [PubMed] [Google Scholar]
- 6.Margulis L. Serial endosymbiotic theory (SET) and composite individuality. Transition from bacterial to eukaryotic genomes. Microbiol Today. 2004;31:172–174. [Google Scholar]
- 7.Osteryoung KW, Nunnari J. The division of endosymbiotic organelles. Science. 2003;302:1698–1704. doi: 10.1126/science.1082192. [DOI] [PubMed] [Google Scholar]
- 8.Gray MW, Burger G, Lang BF. Mitochondrial evolution. Science. 1999;283:1476–1481. doi: 10.1126/science.283.5407.1476. [DOI] [PubMed] [Google Scholar]
- 9.Wallace DC. Why do we still have a maternally inherited mitochondrial DNA? Insights from evolutionary medicine. Annu Rev Biochem. 2007;76:781–821. doi: 10.1146/annurev.biochem.76.081205.150955. [DOI] [PubMed] [Google Scholar]
- 10.Timmis JN, Ayliffe MA, Huang CY, Martin W. Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nat Rev Genet. 2004;5:123–135. doi: 10.1038/nrg1271. [DOI] [PubMed] [Google Scholar]
- 11.Turner C, Killoran C, Thomas NS, Rosenberg M, Chuzhanova NA, Johnston J, et al. Human genetic disease caused by de novo mitochondrial-nuclear DNA transfer. Hum Genet. 2003;112:303–309. doi: 10.1007/s00439-002-0892-2. [DOI] [PubMed] [Google Scholar]
- 12.Wolf K. Insertion of mitochondrial DNA in the chromosomes—a cause for cancer and aging? Endocytob Cell Res. 1996;11:211–218. [Google Scholar]
- 13.Neupert W, Herrmann JM. Translocation of proteins into mitochondria. Annu Rev Biochem. 2007;76:723–749. doi: 10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- 14.Ryan MT, Hoogenraad NJ. Mitochondrial-nuclear communications. Annu Rev Biochem. 2007;76:701–722. doi: 10.1146/annurev.biochem.76.052305.091720. [DOI] [PubMed] [Google Scholar]
- 15.Bromham L, Eyre-Walker A, Smith NH, Smith JM. Mitochondrial Steve: paternal inheritance of mitochondria in humans. Trends Ecol Evol. 2003;18:2–4. [Google Scholar]
- 16.Schwartz M, Vissing J. Paternal inheritance of mitochondrial DNA. N Engl J Med. 2002;347:576–580. doi: 10.1056/NEJMoa020350. [DOI] [PubMed] [Google Scholar]
- 17.Zeh JA, Zeh DW. Maternal inheritance, sexual conflict and the maladapted male. Trends Genet. 2005;21:281–286. doi: 10.1016/j.tig.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Matsushima R, Hamamura Y, Higashiyama T, Arimura S, Sodmergen, Tsutsumi N, et al. Mitochondrial dynamics in plant male gametophyte visualized by fluorescent live imaging. Plant Cell Physiol. 2008;49:1074–1083. doi: 10.1093/pcp/pcn084. [DOI] [PubMed] [Google Scholar]
- 19.Mogenson HL, Rusche ML. Quantitative ultrastructural analysis of barley sperm I. Occurrence and mechanism of cytoplasm and organelle reduction and the question of sperm dimorphism. Protoplasma. 1985;128:1–13. [Google Scholar]
- 20.Schatz G. The magic garden. Annu Rev Biochem. 2007;76:673–678. doi: 10.1146/annurev.biochem.76.060806.091141. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, et al. Amyloid-β overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad USA. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newmeyer DD, Ferguson-Miller S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell. 2003;112:481–490. doi: 10.1016/s0092-8674(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 23.Schon EA, Manfredi G. Neuronal degeneration and mitochondrial dysfunction. J Clin Invest. 2003;111:303–312. doi: 10.1172/JCI17741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delivani P, Martin SJ. Mitochondrial membrane remodeling in apoptosis: an inside story. Cell Death Differ. 2006;13:2007–2010. doi: 10.1038/sj.cdd.4402049. [DOI] [PubMed] [Google Scholar]
- 25.Csordás A. Mitochondrial transfer between eukaryotic animal cells and its physiologic role. Rejuv Res. 2006;9:450–454. doi: 10.1089/rej.2006.9.450. [DOI] [PubMed] [Google Scholar]
- 26.Jacob F. Evolution and tinkering. Science. 1977;196:1161–1166. doi: 10.1126/science.860134. [DOI] [PubMed] [Google Scholar]
- 27.Russell RB, Aloy P. Targeting and tinkering with interaction networks. Nat Chem Biol. 2008;4:666–673. doi: 10.1038/nchembio.119. [DOI] [PubMed] [Google Scholar]
- 28.Rand DM. Mitochondrial genetics of aging: intergenomic conflict resolution. Sci Aging Knowl Environ. 2005;45:5. doi: 10.1126/sageke.2005.45.re5. [DOI] [PubMed] [Google Scholar]
- 29.Budar F, Touzet P, De Paepe R. The nucleomitochondrial conflict in cytoplasmic male sterilities revisited. Genetica. 2003;117:3–16. doi: 10.1023/a:1022381016145. [DOI] [PubMed] [Google Scholar]
- 30.Kuhn T. Chicago: The University of Chicago Press; 1996. The Structure of Scientific Revolutions. [Google Scholar]
- 31.Agnati LF, Genedani S, Leo G, Rivera A, Guidolin D, Fuxe K. One century of progress in neuroscience founded on Golgi and Cajal's outstanding experimental and theoretical contributions. Brain Res Rev. 2007;55:167–189. doi: 10.1016/j.brainresrev.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Kauffman SA. New York: Oxford University Press; 1993. The Origin of Order. [Google Scholar]
- 33.Jacob F. Molecular tinkering in evolution. In: Bendall DS, editor. Evolution from Molecules to Men. Cambridge: Cambridge University Press; 1983. [Google Scholar]
- 34.Zeviani M, Antozzi C. Mitochondrial disorders. Mol Hum Reprod. 1997;3:133–148. doi: 10.1093/molehr/3.2.133. [DOI] [PubMed] [Google Scholar]
- 35.Wallace DC, Brown MD, Lott MT. Mitochondrial DNA variation in human evolution and disease. Gene. 1999;238:211–230. doi: 10.1016/s0378-1119(99)00295-4. [DOI] [PubMed] [Google Scholar]
- 36.Wallace DC. Mitochondrial DNA in aging and disease. Sci Am. 1997;277:40–47. doi: 10.1038/scientificamerican0897-40. [DOI] [PubMed] [Google Scholar]
- 37.Pak JW, Herbst A, Bua E, Gokey N, McKenzie D, Aiken JM. Mitochondrial DNA mutations as a fundamental mechanism in physiological declines associated with aging. Aging Cell. 2003;2:1–7. doi: 10.1046/j.1474-9728.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- 38.Penta JS, Johnson FM, Wachsman JT, Copeland WC. Mitochondrial DNA in human malignancy. Mutat Res. 2001;488:119–133. doi: 10.1016/s1383-5742(01)00053-9. [DOI] [PubMed] [Google Scholar]
- 39.Roubertoux PL, Marcet B, Sluyter F, Verrier B. Mitochondrial DNA (mtDNA) and behavior: interaction between mitochondrial and nuclear genes, preliminary results from microarrays. Behav Genet. 2003;33:717. [Google Scholar]
- 40.Roubertoux PL, Sluyter F, Carlier M, Marcet B, Maarouf-Veray F, Chérif C, et al. Mitochondrial DNA modifies cognition in interaction with the nuclear genome and age in mice. Nat Genet. 2003;35:65–69. doi: 10.1038/ng1230. [DOI] [PubMed] [Google Scholar]
- 41.Cordero C, Eberhard WG. Female choice of sexually antagonistic male adaptations: a critical review of some current research. J Evol Biol. 2003;16:1–6. doi: 10.1046/j.1420-9101.2003.00506.x. [DOI] [PubMed] [Google Scholar]