Abstract
Sulfolobus acidocaldarius 2-keto-3-deoxygluconate aldolase (SacKdgA) displays optimal activity at 95 °C and is studied as a model enzyme for aldol condensation reactions. For application of SacKdgA at lower temperatures, a library of randomly generated mutants was screened for improved synthesis of 2-keto-3-deoxygluconate from pyruvate and glyceraldehyde at the suboptimal temperature of 50 °C. The single mutant SacKdgA-V193A displayed a threefold increase in activity compared with wild type SacKdgA. The increased specific activity at 40–60 °C of this mutant was observed, not only for the condensation of pyruvate with glyceraldehyde, but also for several unnatural acceptor aldehydes. The optimal temperature for activity of SacKdgA-V193A was lower than for the wild type enzyme, but enzymatic stability of the mutant was similar to that of the wild type, indicating that activity and stability were uncoupled. Valine193 has Van der Waals interactions with Lysine153, which covalently binds the substrate during catalysis. The mutation V193A introduced space close to this essential residue, and the increased activity of the mutant presumably resulted from increased flexibility of Lysine153. The increased activity of SacKdgA-V193A with unaffected stability demonstrates the potential for optimizing extremely thermostable aldolases for synthesis reactions at moderate temperatures.
Keywords: biocatalysis, directed evolution, enzyme, error-prone PCR, laboratory evolution, thermophile
Introduction
The aldol reaction leads to the formation of a new carbon–carbon bond, generating a polyhydroxylated product with at least one new chiral center. Aldolases are enzymes that reversibly catalyze the asymmetric aldol condensation reaction and are therefore of interest for bio-organic synthesis of chiral compounds (Dean et al. 2007, Bolt et al. 2008). In contrast to traditional organic synthesis, enzymatic aldol condensation is usually stereospecific, occurs under mild reaction conditions and requires no protection and deprotection steps. Unfortunately, applications of aldolases are often hampered by high enzyme production costs, limited protein stability, and the requirement for expensive (phosphorylated) substrates (Dean et al. 2007). The 2-keto-3-deoxygluconate aldolases (KdgA) found in Sulfolobus species are stable enzymes with high activities on non-phosphorylated (cheap) substrates (Buchanan et al. 1999, Wolterink-van Loo et al. 2007). These class I aldolases catalyze the reversible reaction of d,l-glyceraldehyde + pyruvate d,l-2-keto-3-deoxygluconate (KDG), in which a Schiff base intermediate is formed between the substrate and the catalytic lysine residue (Lamble et al. 2005b). Recently, 3D structures of S. solfataricus and S. acidocaldarius KdgA have been solved with covalently bound reaction intermediates in the active site (Theodossis et al. 2004, Wolterink-van Loo et al. 2007). It has been shown that these enzymes can accept a wide variety of (aldehyde) substrates, and therefore, have great potential for biotechnological applications (Lamble et al. 2003, Lamble et al. 2005a, Lamble et al. 2007, Wolterink-van Loo et al. 2007).
Enzymes have been modified by natural selection to perform optimally in certain biological contexts. Using these enzymes in industrial settings (e.g., in bioreactors), often requires optimization for activity, stability, selectivity and specificity (Reetz 2001). In past decades, several techniques, including rational design and random mutagenesis, have been developed to modify enzymes for specific applications (Sen et al. 2007). Enzyme catalytic rates are determined by general protein features and can be governed by long-range interactions, which are usually difficult to address by rational protein design (Kihara 2005). Therefore, the random approach of laboratory evolution seems particularly suitable for improving enzyme catalytic rates. Generally, diversity is created by error-prone PCR, followed by an activity screen or selection to identify the best mutants. Enzyme features that can be modified by random methods include stability (Shao et al. 1998, Brouns et al. 2005), substrate specificity (Xu et al. 2003, Fishman et al. 2004, van den Heuvel et al. 2004), enantioselectivity (van Loo et al. 2004, Lipovsek et al. 2007) and low-temperature catalysis (Lebbink et al. 2000, Merz et al. 2000, Sriprapundh et al. 2003, Machielsen et al. 2008). Many laboratory studies of aldolase evolution have been directed toward broadening substrate specificity improving enantioselectivity (Bolt et al. 2008) or increasing thermostability (Hao and Berry 2004).
In this study, random mutations were introduced into the thermostable KDG aldolase of Sulfolobus acidocaldarius using error-prone PCR (ep-PCR). A library of 1500 variants was screened for both enhanced condensation activity with pyruvate and d,l-glyceraldehyde at 50 °C and retention of kinetic stability. A single mutant, SacKdgA-V193A, showed enhanced activity and was analyzed in detail for its biochemical properties and stability, demonstrating the feasibility of optimizing extreme thermostable aldolases for application in cascade synthetic reactions with mesophilic enzymes. To our knowledge, it is the first report of increased low-temperature activity of (hyperthermophilic) aldolases through laboratory evolution.
Materials and methods
Chemicals, strains, plasmids, and media
All chemicals were of analytical grade and were purchased from Sigma-Aldrich (Zwijndrecht, Netherlands). A previously constructed vector pWUR193 (Wolterink-van Loo et al. 2007), a pET24d derivative containing the SacKdgA gene (Saci_0225), was used to produce SacKdgA. Escherichia coli XL1-Blue cells were used for cloning and E. coli BL21(DE3) cells were used for expression. The growth medium consisted of LB supplemented with 50 µg ml–1 kanamycin, unless stated otherwise. Cells were grown at 37 °C and liquid cultures were incubated in Erlenmeyer flasks in a rotary shaker at 100 rpm.
Construction of SacKdgA random library
Random mutations were introduced by PCR using two DNA polymerases in parallel to generate a library with balanced mutation ratio. Taq polymerase, which preferentially mutates As and Ts, and Mutazyme DNA polymerase, which mutates more Gs and Cs, were used. Error-prone PCR with Taq DNA polymerase was performed with skewed nucleotide concentrations, with the following primers: 5′-ctttaagaaggagatataccatg-3′, and 5′-ggccgcaaccttgtcgactta-3′ and the pWUR193 plasmid as template DNA. These primers flank the kdgA gene and include the start and stop codons. As such only the kdgA coding sequence was targeted in the ep-PCR procedure. The 50-µl reaction mixtures contained 10 ng template DNA, 200 ng forward primer, 200 ng reverse primer, 0.2 mM dATP and dGTP, 1 mM dCTP and dTTP, 0.5 mM MnCl2 and 5U REDTaq DNA polymerase (Sigma) in REDTaq reaction buffer. Additionally, the GeneMorph Random mutagenesis kit (Stratagene) was used for the ep-PCR with the Mutazyme DNA polymerase using the same primers and template DNA according to the manufacturer’s instructions, aiming for a mutation rate of 3–7 mutations per kb.
The PCR products obtained were purified, double digested with NcoI and SalI and ligated into equally digested pET24d with T4 DNA ligase. The different ligation mixtures were transformed to E. coli XL-1 blue cells, plated and grown overnight. We used this method of generating a library, because transformation to XL-1 blue cells appeared more efficient than to BL21(DE3) cells. Finally, two kdgA gene libraries with random mutations were obtained. One library consisted of 1084 clones originating from the Taq DNA polymerase PCR reaction. The other library consisted of 430 clones from the Mutazyme DNA polymerase PCR reaction. For each library, plasmid DNA was purified from pooled cell suspensions. Random sequencing of the constructs revealed a mean mutation rate of one base change per gene for both libraries.
Screening of random SacKdgA variants
For construction of the expression library, BL21(DE3) expression cells were transformed with the plasmid library, plated on Qtray LB-agar plates (25 cm × 25 cm) and incubated overnight. Single colonies were picked with a colony picker (QPix, Genetix) and used to inoculate 96-wells plates (Costar 3370; Corning) with 200 µl of medium per well supplemented with 0.1 mM IPTG. In each plate, two wells were manually inoculated with the negative control (pET24d in BL21(DE3)) and positive control (pWUR193 in BL21(DE3)). After 16 h of incubation at 37 °C, with shaking, the OD600 (Spectramax Plus384; Sopachem b.v.) was recorded. A replica plate was made by duplication of the induced plates in fresh 96-well plates containing 200 µl of medium with 10% glycerol per well, incubation overnight (37 °C, and shaking) and subsequent storage at –80 °C for future use. The induced plates were centrifuged to pellet the cells in a Sigma 4-15C centrifuge for 30 min at 1000 g. The pellets were stored at –20 °C until further use. Cells were lysed by addition of 100 µl 25% B-PER (Pierce) in 50 mM sodium phosphate buffer (pH 6.0) to the pellets and additional shaking at 700 rpm for 15 min at 37 °C.
Aldolase reactions were performed in 2-ml-deep well plates (Greiner) containing, per well, 250 µl of 50 mM sodium phosphate buffer, pH 6.0, 10 mM pyruvate, 20 mM d,l-glyceraldehyde, and 40 µl of lyzed cells. The plates were incubated for 60 min at 50 °C and then placed in a freezer (–20 °C) to stop the reaction. Reactions were diluted three times by addition of 500 µl of water. The remaining amount of pyruvate was determined by the l-lactate dehydrogenase (LDH) / NADH assay. Briefly, 190 µl of LDH assay buffer (containing 250 mM Tris-HCl buffer, pH 7.5, 0.2 mM NADH, 5 U ml–1, LDH (in excess)) was transferred to each well of a 96-well plate. The OD340 was measured, and then 10 µl of diluted sample of the aldolase reaction was added and incubated at 37 °C for 15 minutes. An end measurement of the OD340 was made to determine the difference in NADH absorption and hence the difference in pyruvate concentration of each sample.
DNA sequencing
Plasmids were isolated using the Qiagen plasmid isolation kit according to the manufacturer’s protocol. Inserts of the isolated plasmids were sequenced by single primer extension reaction with the T7 primer set (Forward: 5′-aatacgactcactatag-3′, reverse: 5′-gctagttattgctcagcgg-3′).
Production and purification of SacKdgA variants
SacKdgA and variants were produced to obtain a heat stable cell-free extract (HSCFE) and purified enzyme as described previously (Wolterink-van Loo et al. 2007). Fractions containing aldolase were pooled. Enzyme purity was checked by SDS-PAGE, staining the proteins with Coomassie brilliant blue. Protein concentrations were determined by the BioRad Bradford method.
Aldol condensation activity measurements
We assayed KDG aldolase activity by detection of the condensation product, 2-keto-3-deoxygluconate, with the thiobarbituric acid (TBA) assay as described previously (Wolterink-van Loo et al. 2007) and performing the standard assay at pH 6.0 and 50 °C, with 50 mM pyruvate and 20 mM d,l-glyceraldehyde. The temperature curve was made by using 10-min incubations for each temperature analyzed.
Aldolase activities with different aldehyde acceptors were measured by assaying the remaining pyruvate in an LDH assay in triplicate. Reactions of 150 µl volume containing 50 mM sodium phosphate buffer, pH 6.0, 50 mM pyruvate and 100 mM aldehyde and 0.5–1.5 µg enzyme were incubated at 50 °C for 10–60 min. The reactions were stopped by rapidly cooling the reaction mixtures on ice. From 985 µl of assay buffer (containing 100 mM Tris-HCl, pH 7.5, 0.16 mM NADH and ~3 units LDH) the starting absorbance at 340 nm was read. After addition of 15 µl of sample and incubation for 5 minutes the absorbance was remeasured. The absorbance difference in NADH (molecular extinction coefficient 6.22 mM–1 cm–1 (Horecker and Kornberg 1948)) was taken to calculate the amount of unconsumed pyruvate.
Thermostability of the enzymes at 90 °C was determined as follows. The KDG aldolase was diluted to 40 µg ml–1 in 50 mM sodium phosphate, pH 6.0, and 175-µl aliquots and were placed in sealed HPLC-vials. Vials were immersed in a 90 °C water bath and sampled at different times and immediately cooled on ice. Residual activity of each sample was measured by the TBA assay.
Results and discussion
Construction of a random mutant SacKdgA library
Synthetic cascade reactions in which aldolases are involved are performed either separately or as one-pot reactions (Sugiyama et al. 2007, Yu et al. 2006). Combinations of different enzymes often require establishing a compromise of the chemical and physical reaction conditions (Jennewein et al. 2006, Littlechild et al. 2007, Pollard and Woodley 2007, Straathof et al. 2002). When combining enzymes from thermophilic and mesophilic organisms, optimization of conditions for one type of enzyme or the other is required.
The KDG aldolase from S. acidocaldarius (SacKdgA) is an extremely stable pyruvate-dependent aldolase, with a broad substrate specificity and high affinity for non-phosphorylated substrates (Wolterink-van Loo et al. 2007). A potential application of this enzyme would be in a cascade with an available mesophilic alditol oxidase (Heuts et al. 2007), in which the latter enzyme provides the aldehyde substrate for the aldolase. The SacKdgA has maximal activity at 95 °C and at 37 °C its activity is reduced to approximately 5% of its rate at 95 °C. We therefore attempted to improve SacKdgA activity at suboptimal temperatures, while seeking to maintain its stability. As little is known about the molecular basis of catalytic temperature dependence, a random approach was used to optimize low-temperature activity of SacKdgA. Diversity was generated in the SacKdgA gene by ep-PCR, resulting in a library with a mean of one mutation per gene. A plasmid library of 1500 clones was isolated from E. coli XL-1 Blue cells, which was used in the transformation of E. coli BL21(DE3) for expression. A total of 5000 transformants were screened to ensure sufficient coverage of the genetic diversity in the kdgA mutant library.
Screening for increased condensation activity at 50 °C of SacKdgA
The desired operating temperature for bio-cascade reactions in combination with mesophilic enzymes is 37 °C (or lower). The incubation time necessary to detect any wild-type SacKdgA activity at this temperature was in the order of hours, which was deemed incompatible with a high-throughput screening strategy. Therefore, the mutant KdgA library was screened for aldol condensation activity at 50 °C, because at this temperature a small but significant amount of activity of the wild type could be detected in a relatively short assay time (60 min). The mutants were screened for their ability to convert d,l-glyceraldehyde and pyruvate to d,l-2-keto-3-deoxygluconate. Because protein expression levels were not taken into account, winners in the screening procedure would be more active than the wild type due to either increased activity or increased enzyme content, or both.
Determination of pyruvate consumption was chosen as a primary screen for KdgA activity for ease of execution and because it provided the opportunity to screen for activity with other aldehydes in the same assay. The activities of the KdgA variants in the libraries were corrected for effects of other pyruvate consuming or producing activities in the E. coli extract by subtracting the activity of BL21(DE3) harboring a pET24d vector without insert. This way, 162 mutants with up to 4-fold increased activities were identified. However, consecutive screening in triplicate (using the pyruvate depletion assay) proved most candidates to be false positives. This was attributed to the indirect screening method of measuring residual pyruvate, which resulted in more frequent errors compared with screening for condensation product. Finally 12 clones were selected for further testing and were recultivated, their plasmid DNA was isolated for sequencing, and used to re-transform BL21(DE3) to obtain the proteins for further analyses. Wild-type SacKdgA and the 12 selected clones were produced on a one-liter scale, heat-stable cell-free extracts (HSCFE) were prepared from the cultures, and the variants were compared with the activity of the wild type using the TBA assay (measuring the amount of condensation product formed).
Most clones had equal or less activity than wild-type KdgA and sequencing revealed that these clones had either no amino acid change or only a single mutation of a solvent exposed residue (data not shown). Variants with lower activity had less protein in the HSCFE (data not shown). Variant t34D12 appeared to be on average 1.5 times more active than wild-type KdgA, included no mutations. Variant t34B9 was 1.5 times more active and contained mutation N168D, located at the protein surface. The most active variant, t37F3 was 3 times more active than the wild-type SacKdgA, with a specific activity of 28 U mg–1 versus 9 U mg–1. This variant contained a single mutation of the buried Val193 to alanine.
Structural analysis of SacKdgA V193A
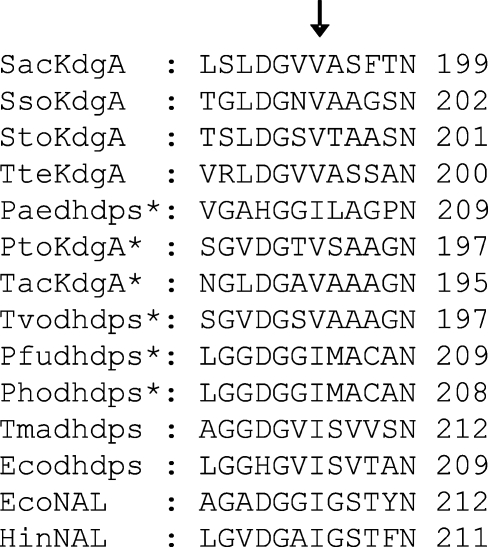
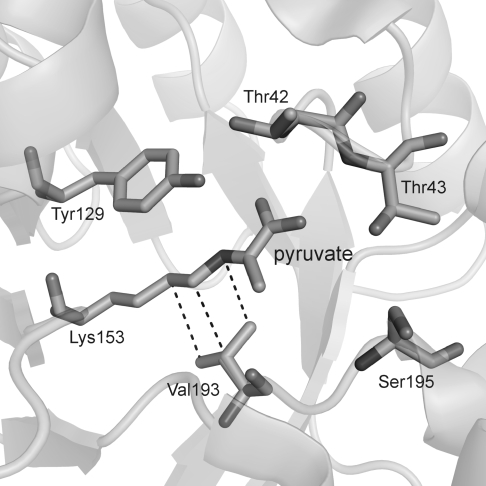
Variant t37F3, containing mutation V193A, was 3 times more active than the wild-type SacKdgA. Valine 193 is well conserved in KdgA aldolases from hyperthermophilic crenarchaea (Figure 1). A valine at position 193 in SacKdgA appears to be specific for KdgA-type aldolases from hyperthermophilic crenarchaea and euryarchaea. Homologous sequences of other (putative) pyruvate aldolases have an isoleucine at this position and have been characterized as, or predicted to be, dihydrodipicolinate synthases (DHDPS) and neuraminate lyases (NAL) (Figure 1). Other archaeal (both crenarchaeal and euryarchaeal) hypothetical KdgAs or hypothetical DHDPS have a very different genetic environment (Ahmed et al. 2005) and are unlikely to be true KdgAs. In wild-type SacKdgA, Val193 has Van der Waals interactions with the catalytic lysine (Lys153) that forms a Schiff base intermediate with the substrate (Figure 2). Mutation V193A created space in close proximity to Lys153 and possibly allowed a higher degree of mobility of the active site compared with wild-type SacKdgA. Similar mutations, creating space in close proximity to the active site, have resulted in increased activity at suboptimal temperatures of various proteins such as Pyrococcus furiosus β-glucosidase (Lebbink et al. 2000), Thermotoga neapolitana xylose isomerase (Sriprapundh et al. 2003), and Sulfolobus solfataricus indoleglycerol phosphate synthase (Merz et al. 2000). In addition, a natural cold-adapted elastase (Papaleo et al. 2006) has increased local flexibility (in loop regions) compared with its mesophilic counterparts.
Figure 1.
Partial alignment of different (hypothetical) pyruvate-dependent aldolases (KdgAs, dihydrodipicolinate synthases (DHDPS) and neuraminate lyases (NAL)) from Sulfolobus acidocaldarius (Sac), S. solfataricus (Sso), S. tokodaii (Sto), Thermoproteus tenax (Tte), Pyrobaculum aerophilum (Pae), Picrophilus torridus (Pto), Thermoplasma acidophilum (Tac), T. volcanium (Tvo), Pyrococcus furiosus (Pfu), P. horikoshii (Pho), Thermotoga maritima (Tma), E. coli (Eco) and Heamophilus influenzae (Hin). The valine residue (at the arrow) is conserved in crenarchaeal KdgAs and some euryarchaea (Thermoplasma) and otherwise conservatively replaced by an isoleucine in (hypothetical, indicated by an asterisk) dihydrodipicolinate synthases (DHDPS) and neuraminate lyases (NAL).
Figure 2.
Diagram of the active site of SacKdgA with pyruvate covalently bound to Lys153 (pdb: 2nuy). Van der Waals interactions between the Lys153 side chain and Val193 are indicated with dotted lines. The figure was generated with PyMol.
Optimal temperature for activity and kinetic stability
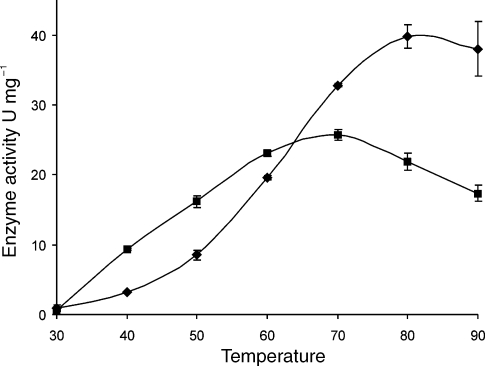
Wild-type SacKdgA and variant V193A were purified to homogeneity for characterization (data not shown). To study the effect of temperature on the activity of SacKdgA-V193A and to determine whether its activity was also increased at moderate temperatures, SacKdgA-V193A and wild-type SacKdgA were assayed in the temperature range of 30 °C to 90 °C (Figure 3). SacKdgA-V193A was more active than wild-type SacKdgA between 30 °C and 60 °C. The optimum temperature for activity shifted from 90 °C in wild-type SacKdgA to 70 °C for the mutant, similar to results obtained for a P. furiosus β-glucosidase CelB variant reported by Lebbink et al. (2000). Although at 30 °C the activities of both enzymes were just detectable above background level, it was found that mutant V193A was significantly more active between 40 and 60 °C. The highest relative increase in activity was found at 40 °C, close to the desired “mesophilic” 37 °C. In addition, it was concluded that V193 contributes significantly to the activity of SacKdgA at the optimal growth temperature of S. acidocaldarius (80 °C), which agrees well with the fact that it is well conserved in homologous enzymes from hyperthermophiles; however, in some orthologs valine is replaced by isoleucine, which probably gives comparable restricted flexibility of the active site lysine residue. An attractive feature of SacKdgA in biotechnological applications is its extreme thermostability. Improving the enzyme’s low-temperature activity should not interfere with protein stability. Variant SacKdgA-V193A remained soluble during purification, which included a heat incubation step of 30 min at 75 °C, indicating that the variant had retained significant residual thermostability. Furthermore, inactivation studies at 90 °C of both wild-type and variant aldolase showed that they had similar activity half-lives of 100 and 120 minutes, respectively. Therefore, mutation V193A affected only the activity of SacKdgA, demonstrating that activity and thermostability can be uncoupled for thermostable Class I aldolases. Previously, similar observations have been made for mesophilic class II fructose-bisphosphate aldolase; aldolase thermostability was improved by directed evolution without reduction in catalysis at mesophilic temperatures (Hao and Berry 2004). Moreover, a model has been proposed that describes the apparent uncoupling of stability and activity (Daniel et al. 2008) in enzymes, and in mutant V193A stability is unaffected, although activity had already decreased at temperatures above 70 °C.
Figure 3.
Temperature dependent activity of purified SacKdgA wild type (r) and SacKdgA-V193A (j). Activities were determined in 50 mM sodium phosphate buffer (pH 6.0), 10 mM pyruvate and 20 mM glyceraldehyde.
Kinetic characterization of mutant and wild-type SacKdgA at 50 °C
To study the effect of mutation V193A on catalysis, the kinetic reaction constants of SacKdgA-V193A and wild-type aldolase for condensation of pyruvate and d,l-glyceraldehyde were determined at 50 °C (Table 1). The turnover, kcat, of the mutant enzyme, had increased three times. The KM had increased as well, three times for glyceraldehyde and five times for pyruvate. The kinetic constants confirm the validity of the high-throughput screen, which was performed under saturating substrate conditions. Despite the increase in KM, the catalytic rate of the mutant enzyme, as a function of the substrate concentration, was always higher than the catalytic rate of the wild-type enzyme (not shown); only at low pyruvate concentrations (< 2 mM) did the wildtype enzyme shows a higher activity than the mutant enzyme. This mutant V193A would provide a benefit over wild-type SacKdgA in biotechnological applications, in which saturating substrate conditions are typically used.
Table 1.
Biochemical data for SacKdgA and mutant V193A, determined at 50 °C. Values between brackets are standard deviations.
| SacKdgA | SacKdgA-V193A | |
| Vmax (GA) (U/mg) | 8.1 (0.4) | 29.1 (0.9) |
| kcat (GA) (s–1) | 4.4 (0.2) | 15.7 (0.5) |
| KM(GA) mM | 2.1 (0.3) | 5.4 (0.5) |
| Vmax (pyr) (U/mg) | 10.1 (0.1) | 27.8 (1.0) |
| kcat (pyr) (s–1) | 5.5 (0.1) | 15.0 (0.5) |
| KM(pyr) mM | 0.8 (0) | 5.4 (0.6) |
Substrate specificity of mutant and wild type SacKdgA
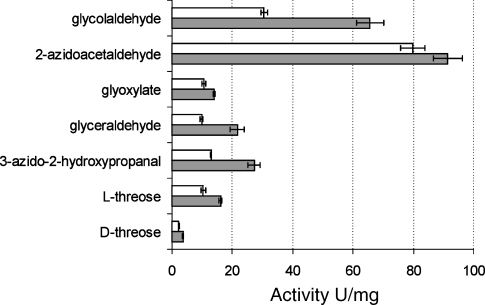
Sulfolobus acidocaldarius KdgA is part of the Entner-Doudoroff pathway for degradation of glucose (Selig et al. 1997) and probably galactose (Lamble et al. 2003) to pyruvate and glyceraldehyde. The enzyme displays broad substrate specificity and can accept various non-natural acceptors in the condensation reaction with pyruvate (Wolterink-van Loo et al. 2007). Therefore, we investigated the effect of mutation V193A on the condensation activity at 50 °C using pyruvate and alternative aldehyde acceptors (Figure 4). Under substrate conditions far above the KM (at least 9 times), mutant V193A was more active than the wild-type enzyme on all tested acceptor substrates. The highest relative increases in activity were measured with glyceraldehyde, glycolaldehyde and 3-azido-2-hydroxypropanal as acceptors. Glyceraldehyde was the acceptor used in the screening, and glycolaldehyde and 3-azido-2-hydroxypropanal are the tested acceptors that are most similar to glyceraldehyde. As such, this study confirms the first law of directed evolution “You get what you screen for” (Schmidt-Dannert and Arnold 1999).
Figure 4.
Activity of purified SacKdgA (white bars) and SacKdgA-V193A (gray bars) on different (unnatural) aldehyde substrates at 50 °C. Activities were determined in 50 mM sodium phosphate buffer (pH 6.0), 50 mM pyruvate and 100 mM aldehyde.
Conclusions
In this study, the extremely thermostable Class I KDG aldolase of S. acidocaldarius with maximal activity at 95 °C was successfully improved for catalysis at the suboptimal temperature of 50 °C to enhance its potential for use in one-pot synthesis reactions together with mesophilic enzymes. Screening of 1500 variants resulted in isolation of a single variant Sac-KDG-V193A that was three times more active than wild-type SacKdgA for condensation of pyruvate and glyceraldehyde at 40 °C. The mutation increased the activity of the enzyme but did not affect its extreme thermostability. Despite the relatively small size of the generated library, the chosen laboratory evolution approach has proved to be an efficient strategy for improving the desired property of SacKdgA. To our knowledge this is the first report on improving aldolase activity at suboptimal temperatures.
Acknowledgments
This research was performed as part of the IBOS Programme (Integration of Biosynthesis & Organic Synthesis) of Advanced Chemical Technologies for Sustainability (ACTS). We thank Harm Kloosterman for assistance in using the facilities at BioExplore (Groningen, the Netherlands).
References
- R1.Ahmed H., Ettema T.J., Tjaden B., Geerling A.C., Van Der Oost J., Siebers B. The semi-phosphorylative Entner-Doudoroff pathway in hyperthermophilic archaea: a re-evaluation. Biochem. J. 2005;390:529–540. doi: 10.1042/BJ20041711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R2.Bolt A., Berry A., Nelson A. Directed evolution of aldolases for exploitation in synthetic organic chemistry. Arch. Biochem. Biophys. 2008;474:318–330. doi: 10.1016/j.abb.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R3.Brouns S.J., Wu H., Akerboom J., Turnbull A.P., De Vos W.M., Van Der Oost J. Engineering a selectable marker for hyperthermophiles. J. Biol. Chem. 2005;280:11422–11431. doi: 10.1074/jbc.M413623200. [DOI] [PubMed] [Google Scholar]
- R4.Buchanan C.L., Connaris H., Danson M.J., Reeve C.D., Hough D.W. An extremely thermostable aldolase from Sulfolobus solfataricus with specificity for non-phosphorylated substrates. Biochem. J. 1999;343:563–570. [PMC free article] [PubMed] [Google Scholar]
- R5.Daniel R.M., Danson M.J., Eisenthal R., Lee C.K., Peterson M.E. The effect of temperature on enzyme activity: new insights and their implications. Extremophiles. 2008;12:51–59. doi: 10.1007/s00792-007-0089-7. [DOI] [PubMed] [Google Scholar]
- R6.Dean S.M., Greenberg W.A., Wong C.H. Recent advances in aldolase-catalyzed asymmetric synthesis. Adv. Synth. Catal. 2007;349:1308–1320. [Google Scholar]
- R7.Fishman A., Tao Y., Bentley W.E., Wood T.K. Protein engineering of toluene 4-monooxygenase of Pseudomonas mendocina KR1 for synthesizing 4-nitrocatechol from nitrobenzene. Biotechnol. Bioeng. 2004;87:779–790. doi: 10.1002/bit.20185. [DOI] [PubMed] [Google Scholar]
- R8.Hao J., Berry A. A thermostable variant of fructose bisphosphate aldolase constructed by directed evolution also shows increased stability in organic solvents. Protein Eng. Des. Sel. 2004;17:689–697. doi: 10.1093/protein/gzh081. [DOI] [PubMed] [Google Scholar]
- R9.Heuts D.P., Van Hellemond E.W., Janssen D.B., Fraaije M.W. Discovery, characterization, and kinetic analysis of an alditol oxidase from Streptomyces coelicolor . J. Biol. Chem. 2007;282:20283–20291. doi: 10.1074/jbc.M610849200. [DOI] [PubMed] [Google Scholar]
- R10.Horecker B.L., Kornberg A. The extinction coefficients of the reduced band of pyridin nucleotides. J. Biol. Chem. 1948;175:385–390. [PubMed] [Google Scholar]
- R11.Jennewein S., Schurmann M., Wolberg M., Hilker I., Luiten R., Wubbolts M., Mink D. Directed evolution of an industrial biocatalyst: 2-deoxy-D-ribose 5-phosphate aldolase. Biotechnol. J. 2006;1:537–548. doi: 10.1002/biot.200600020. [DOI] [PubMed] [Google Scholar]
- R12.Kihara D. The effect of long-range interactions on the secondary structure formation of proteins. Protein Sci. 2005;14:1955–1963. doi: 10.1110/ps.051479505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R13.Lamble H.J., Heyer N.I., Bull S.D., Hough D.W., Danson M.J. Metabolic pathway promiscuity in the archaeon Sulfolobus solfataricus revealed by studies on glucose dehydrogenase and 2-keto-3-deoxygluconate aldolase. J. Biol. Chem. 2003;278:34066–34072. doi: 10.1074/jbc.M305818200. [DOI] [PubMed] [Google Scholar]
- R14.Lamble H.J., Danson M.J., Hough D.W., Bull S.D. Engineering stereocontrol into an aldolase-catalysed reaction. Chem. Commun.: 2005;2005:124–126. doi: 10.1039/b413255f. [DOI] [PubMed] [Google Scholar]
- R15.Lamble H.J., Theodossis A., Milburn C.C., Taylor G.L., Bull S.D., Hough D.W., Danson M.J. Promiscuity in the part-phosphorylative Entner-Doudoroff pathway of the archaeon Sulfolobus solfataricus . FEBS Lett. 2005;579:6865–6869. doi: 10.1016/j.febslet.2005.11.028. [DOI] [PubMed] [Google Scholar]
- R16.Lamble H.J., Royer S.F., Hough D.W., Danson M.J., Taylor G.L., Bull S.D. A thermostable aldolase for the synthesis of 3-deoxy-2-ulosonic acids. Adv. Synth. Catal. 2007;349:817–821. [Google Scholar]
- R17.Lebbink J.H., Kaper T., Bron P., Van Der Oost J., De Vos W.M. Improving low-temperature catalysis in the hyperthermostable Pyrococcus furiosus beta-glucosidase CelB by directed evolution. Biochemistry (Mosc.) 2000;39:3656–3665. doi: 10.1021/bi991483q. [DOI] [PubMed] [Google Scholar]
- R18.Lipovsek D., Antipov E., Armstrong K.A., Olsen M.J., Klibanov A.M., Tidor B., Wittrup K.D. Selection of horseradish peroxidase variants with enhanced enantioselectivity by yeast surface display. Chem. Biol. 2007;14:1176–1185. doi: 10.1016/j.chembiol.2007.09.008. [DOI] [PubMed] [Google Scholar]
- R19.Littlechild J.A., Guy J., Connelly S., Mallett L., Waddell S., Rye C.A., Line K., Isupov M. Natural methods of protein stabilization: thermostable biocatalysts. Biochem. Soc. Trans. 2007;35:1558–1563. doi: 10.1042/BST0351558. [DOI] [PubMed] [Google Scholar]
- R20.Machielsen R., Leferink N.G., Hendriks A., Brouns S.J., Hennemann H.G., Daubetamann T., Van Der Oost J. Laboratory evolution of Pyrococcus furiosus alcohol dehydrogenase to improve the production of (2S,5S)-hexanediol at moderate temperatures. Extremophiles. 2008;12:587–594. doi: 10.1007/s00792-008-0164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R21.Merz A., Yee M.C., Szadkowski H., Pappenberger G., Crameri A., Stemmer W.P., Yanofsky C., Kirschner K. Improving the catalytic activity of a thermophilic enzyme at low temperatures. Biochemistry (Mosc.) 2000;39:880–889. doi: 10.1021/bi992333i. [DOI] [PubMed] [Google Scholar]
- R22.Papaleo E., Riccardi L., Villa C., Fantucci P., De Gioia L. Flexibility and enzymatic cold-adaptation: a comparative molecular dynamics investigation of the elastase family. Biochim. Biophys. Acta. 2006;1764:1397–1406. doi: 10.1016/j.bbapap.2006.06.005. [DOI] [PubMed] [Google Scholar]
- R23.Pollard D.J., Woodley J.M. Biocatalysis for pharmaceutical intermediates: the future is now. Trends Biotechnol. 2007;25:66–73. doi: 10.1016/j.tibtech.2006.12.005. [DOI] [PubMed] [Google Scholar]
- R24.Reetz M.T. Combinatorial and evolution-based methods in the creation of enantioselective catalysts. Angew. Chem. Int. Ed. Engl. 2001;40:284–310. [PubMed] [Google Scholar]
- R25.Schmidt-Dannert C., Arnold F.H. Directed evolution of industrial enzymes. Trends Biotechnol. 1999;17:135–136. doi: 10.1016/s0167-7799(98)01283-9. [DOI] [PubMed] [Google Scholar]
- R26.Selig M., Xavier K.B., Santos H., Schonheit P. Comparative analysis of Embden-Meyerhof and Entner-Doudoroff glycolytic pathways in hyperthermophilic archaea and the bacterium Thermotoga . Arch. Microbiol. 1997;167:217–232. doi: 10.1007/BF03356097. [DOI] [PubMed] [Google Scholar]
- R27.Sen S., Dasu V.V., Mandal B. Developments in directed evolution for improving enzyme functions. Appl. Biochem. Biotechnol. 2007;143:212–223. doi: 10.1007/s12010-007-8003-4. [DOI] [PubMed] [Google Scholar]
- R28.Shao Z., Zhao H., Giver L., Arnold F.H. Random-priming in vitro recombination: an effective tool for directed evolution. Nucleic Acids Res. 1998;26:681–683. doi: 10.1093/nar/26.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R29.Sriprapundh D., Vieille C., Zeikus J.G. Directed evolution of Thermotoga neapolitana xylose isomerase: high activity on glucose at low temperature and low pH. Protein Eng. 2003;16:683–690. doi: 10.1093/protein/gzg082. [DOI] [PubMed] [Google Scholar]
- R30.Straathof A.J., Panke S., Schmid A. The production of fine chemicals by biotransformations. Curr. Opin. Biotechnol. 2002;13:548–556. doi: 10.1016/s0958-1669(02)00360-9. [DOI] [PubMed] [Google Scholar]
- R31.Sugiyama M., Hong Z., Liang P.H., Dean S.M., Whalen L.J., Greenberg W.A., Wong C.H. D-Fructose-6-phosphate aldolase-catalyzed one-pot synthesis of iminocyclitols. J. Am. Chem. Soc. 2007;129:14811–14817. doi: 10.1021/ja073911i. [DOI] [PubMed] [Google Scholar]
- R32.Theodossis A., Walden H., Westwick E.J., Connaris H., Lamble H.J., Hough D.W., Danson M.J., Taylor G.L. The structural basis for substrate promiscuity in 2-keto-3-deoxygluconate aldolase from the Entner-Doudoroff pathway in Sulfolobus solfataricus . J. Biol. Chem. 2004;279:43886–43892. doi: 10.1074/jbc.M407702200. [DOI] [PubMed] [Google Scholar]
- R33.van den Heuvel R.H.H., van den Berg W.A.M., Rovida S., van Berkel W.J.H. Laboratory-evolved vanillyl-alcohol oxidase produces natural vanillin. J. Biol. Chem. 2004;279:33492–33500. doi: 10.1074/jbc.M312968200. [DOI] [PubMed] [Google Scholar]
- R34.van Loo B., Spelberg J.H., Kingma J., Sonke T., Wubbolts M.G., Janssen D.B. Directed evolution of epoxide hydrolase from A. radiobacter toward higher enantioselectivity by error-prone PCR and DNA shuffling. Chem. Biol. 2004;11:981–990. doi: 10.1016/j.chembiol.2004.04.019. [DOI] [PubMed] [Google Scholar]
- R35.Wolterink-van Loo S., Van Eerde A., Siemerink M.A., Akerboom J., Dijkstra B.W., van der Oost J. Biochemical and structural exploration of the catalytic capacity of Sulfolobus KDG aldolases. Biochem. J. 2007;403:421–430. doi: 10.1042/BJ20061419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R36.Xu H.F., Zhang X.E., Zhang Z.P., Zhang Y.M., Cass A.E.G. Directed evolution of E-coli alkaline phosphatase towards higher catalytic activity. Biocatal. Biotransform. 2003;21:41–47. [Google Scholar]
- R37.Yu H., Chokhawala H.A., Huang S., Chen X. One-pot three-enzyme chemoenzymatic approach to the synthesis of sialosides containing natural and non-natural functionalities. Nat. Protoc. 2006;1:2485–2492. doi: 10.1038/nprot.2006.401. [DOI] [PMC free article] [PubMed] [Google Scholar]