Abstract
Objective
To compare the outcomes of 14-gauge automated biopsy and 11-gauge vacuum-assisted biopsy for the sonographically guided core biopsies of breast lesions.
Materials and Methods
We retrospectively reviewed all sonographically guided core biopsies performed from January 2002 to February 2004. The sonographically guided core biopsies were performed with using a 14-gauge automated gun on 562 breast lesions or with using an 11-gauge vacuum-assisted device on 417 lesions. The histologic findings were compared with the surgical, imaging and follow-up findings. The histologic underestimation rate, the repeat biopsy rate and the false negative rates were compared between the two groups.
Results
A repeat biopsy was performed on 49 benign lesions because of the core biopsy results of the high-risk lesions (n = 24), the imaging-histologic discordance (n = 5), and the imaging findings showing disease progression (n = 20). The total underestimation rates, according to the biopsy device, were 55% (12/22) for the 14-gauge automated gun biopsies and 36% (8/22) for the 11-gauge vacuum-assisted device (p = 0.226). The atypical ductal hyperplasia (ADH) underestimation (i.e., atypical ductal hyperplasia at core biopsy and carcinoma at surgery) was 58% (7/12) for the 14-gauge automated gun biopsies and 20% (1/5) for the 11-gauge vacuum-assisted biopsies. The ductal carcinoma in situ (DCIS) underestimation rate (i.e., ductal carcinoma in situ upon core biopsy and invasive carcinoma found at surgery) was 50% (5/10) for the 14-gauge automated gun biopsies and 41% (7/17) for the 11-gauge vacuum-assisted biopsies. The repeat biopsy rates were 6% (33/562) for the 14-gauge automated gun biopsies and 3.5% (16/417) for the 11-gauge vacuum-assisted biopsies. Only 5 (0.5%) of the 979 core biopsies were believed to have missed the malignant lesions. The false-negative rate was 3% (4 of 128 cancers) for the 14-gauge automated gun biopsies and 1% (1 of 69 cancers) for the 11-gauge vacuum-assisted biopsies.
Conclusion
The outcomes of the sonographically guided core biopsies performed with the 11-gauge vacuum-assisted device were better than those outcomes of the biopsies performed with the 14-gauge automated gun in terms of underestimation, rebiopsy and the false negative rate, although these differences were not statistically significant.
Keywords: Biopsies, technology; Breast, biopsy; Breast, US; Breast neoplasms, diagnosis
Image-guided core biopsy is considered as an accepted alternative to surgical biopsy for the histologic assessment of breast lesions. It is a fast, safe, accurate, economic technique, and it is effective for minimizing the patients' discomfort (1-5). Guidance for percutaneous biopsy is usually provided by stereotaxis or by sonography. The advantages of sonography as a guidance modality for percutaneous breast biopsy include the lack of ionization radiation, the use of nondedicated equipment and the real-time needle visualization (1-3). For those lesions amenable to either stereotatic or sonography guided biopsy, sonography guided biopsy is preferable in terms of patient comfort, procedure time and cost (2, 3, 5).
The shortcomings of percutaneous image-guided core biopsy, namely, the histologic underestimation and the false negative diagnoses, continue to persist despite the use of large core needles and the improvements of the biopsy device. The previously published data has reported a 20% to 56% atypical ductal hyperplasia (ADH) underestimation, a 16% to 35% ductal carcinoma in situ (DCIS) underestimation, and a 3% to 11% false-negative rate for the 14-gauge automated core biopsy method (6-8). In the case of stereotactically guided core biopsy, many studies have demonstrated the advantages of 11-gauge vacuum-assisted biopsy over the 14-gauge automated biopsy with respect to lower underestimation (9-12), the need for rebiopsies (13), and the higher calcification retrieval rates (14, 15), because of its ability to extract more tissue (16). The benefit of vacuum-assisted biopsy has also been emphasized by some investigators for sonographically guided core biopsy (17). However, the advantages of the 11-gauge vacuum-assisted biopsy over the 14-gauge automated biopsy have not been established for the sonographically guided core biopsy (18).
This study was undertaken to compare the outcomes of sonographically guided core biopsies that were performed with a 14-gauge automated gun with those performed by using an 11-gauge vacuum-assisted device. The primary objective was to compare the histologic underestimation rates, the rebiopsy rates and the false negative rates of these two sonographically guided core biopsy methods.
MATERIALS AND METHODS
Patients and Biopsy Procedures
From January 2002 to February 2004, sonographically guided breast biopsies were performed on 979 consecutive lesions in 940 patients (age range, 13-88 years; mean age, 46.7 years) at our institution. Of these biopsies, 562 biopsies in 525 patients were performed with using a 14-gauge automated gun (Pro-Mag 2.2, Manan Medical Products, Northbrook, IL). Of these 525 patients, 490 patients had a biopsy for one lesion, 33 patients had biopsies for two separate lesions and two patients had biopsies for three separate lesions. Four hundred and seventeen biopsies in 415 patients were performed with using an 11-gauge vacuum-assisted device (Mammotome; Ethicon-Endosurgery, Cincinnati, OH). Of these 415 patients, 413 patients had a biopsy of one lesion and two patients had biopsies for two separate lesions. For all the 979 lesions, the referring physicians reported that 180 of them (18%) were palpable. An informed consent was obtained from every patient.
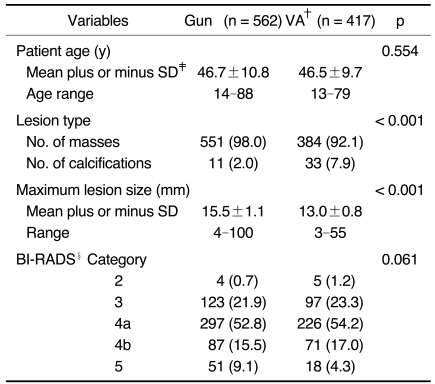
The breast biopsy device that was used depended primarily on the preference of the radiologist performing the biopsy, although the physician's and patient's preferences also affected this decision. The vacuum-assisted device was preferred for those lesions for which the potential benefits of the device were suggested (14, 17, 19); these lesions included calcified lesions, intraductal lesions and solid nodules of < 1.0 cm. The automated gun biopsy was preferred for the multifocal lesions and for the lesions in the subareolar or axillary area. There were no significant differences between the two study groups for the patients' ages, the lesion characteristics or histologic diagnoses (Tables 1, 2). The percentage of microcalcification lesions, as compared with that of the mass lesions, was significantly higher for the vacuum-assisted method than for the automated method (p < 0.001) (Table 1).
Table 1.
The Patient and Lesion Variables for 14-gauge Automated Biopsies and 11-gauge Vacuum-assisted Biopsies
Note.-The numbers in the parentheses are percentages. *Gun = 14-gauge automated gun biopsy, †VA = 11-gauge vacuum-assisted biopsy, ‡SD = standard deviation, §BI-RADS = Breast Imaging Reporting and Data System
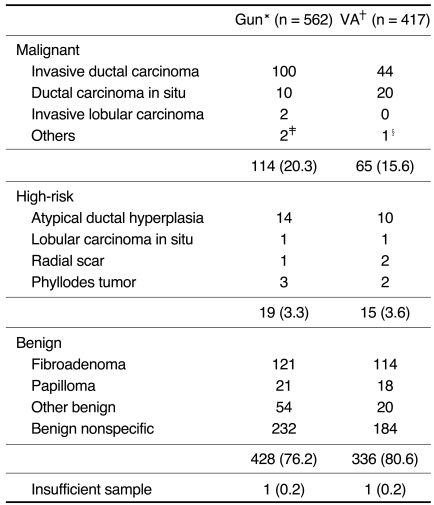
Table 2.
Histologic Diagnosis of the Core Biopsy Specimens
Note.-The numbers in parentheses are percentages. *Gun = 14-gauge automated gun biopsy, †VA = 11-gauge vacuum-assisted biopsy, ‡malignant lymphoma, granular cell tumor, §malignant spindle cell tumor
All biopsies were guided by using high-resolution sonography units with 10- or 12 MHz linear transducers (Kretz-Medison, Seoul; HDI 5000, Advanced Technology Laboratories, Bothell, WA) with the patient placed in the supine or oblique supine position. The mean number of core samples obtained with the 14-gauge automated gun was 4.5 (range, 3-10), and the mean number of core sample biopsies performed with the 11-gauge vacuum-assisted device was 10.2 (range, 6-30) (11, 17, 18). When a lesion that contained microcalcifications was biopsied, the specimen radiographs were obtained to document the presence of calcifications. Among the 44 lesions with calcifications, 75% of the calcifications were identified on the specimen radiographs. About 20% of the biopsies were performed by one of two attending radiologists who had 2-3 years experience with breast sonography and intervention. The other 80% of the biopsies were performed by four fellows after the first five to 10 biopsies were done under the supervision of one of the two attending breast imaging radiologists.
Imaging Evaluation
The characteristics of the lesions during mammography or sonography were described by consensus between the two radiologists prior to the biopsy in terms of the lesion type and size, and with using the guidelines of the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) final assessment categories (20, 21). All the lesions were classified as either calcifications or masses. If the calcifications were without an associated mass, architectural distortion or asymmetric density on the mammography, then they were classified as calcifications. Any mass or architectural distortion or asymmetric density seen on mammography, with or without calcifications, was classified as a mass. The maximum diameter of a lesion was measured by sonography. All the lesions were categorized according to the BI-RADS final assessment protocol as modified by Dr. Stavros. Category 4 was subclassified into 4a and 4b using the criterion of a 50% probability of malignancy (21). Thus, category 4a included the lesions with a risk from > 2% to < 50%, and category 4b included the lesions with a risk from > 50% to < 90%. Category 4 and 5 lesions were considered as indications for biopsy, and the category 3 lesions (probably benign) or category 2 lesions (benign), for which biopsies were requested by the physician or patient, were also biopsied.
Histology Evaluation and Imaging Follow-up
The pathologic lesions were classified as malignant (invasive ductal carcinoma, DCIS, invasive lobular carcinoma and other malignancies including lymphoma and malignant phyllodes tumor), high-risk (ADH, atypical papillary tumor, radial scar, benign phyllodes tumor, lobular carcinoma in situ and mucocele-like tumor), or benign (fibroadenoma, papilloma and nonspecific benign disease including fibrocystic change, benign mammopathy, adenosis and ductal epithelial hyperplasia, and specific benign disease including ductectasia, tubular adenoma, abscess and fat necrosis).
The patients with a biopsy result of malignancy and high-risk lesions received surgical excision. If a lesion had a highly suspicious sonographic finding, then surgical excision was recommended, despite a benign pathologic result. If a lesion's imaging was determined to be concordant with benign pathology, then six, 12 and 24 months follow-up was recommended.
Mammographic and sonographic follow-ups were performed for 450 (60%) of the 750 benign lesions, for 247 (61%) of the 406 benign lesions that received 14-gauge automated gun biopsy and for 203 (59%) of the 344 benign lesions that received 11-gauge vacuum-assisted biopsy. The mean duration of the follow-up was 9.2 months (range, 1-28 months) for the 14-gauge automated gun biopsy, and it was 8.8 months (range, 1-25 months) for the 11-gauge vacuum-assisted biopsy.
Outcome Analysis
Histologic underestimation was considered when the ADH at the core biopsy was upgraded to carcinoma at surgery (ADH underestimation), or when the DCIS at the core biopsy was upgraded to invasive carcinoma at surgery (DCIS underestimation). The underestimation rate of ADH or DCIS was determined by dividing the total number of lesions, for which excisional biopsy was performed, into the number of lesions that proved to be DCIS or invasive carcinoma upon excision.
When a repeat biopsy was performed, the time interval and the reason for the second biopsy were recorded. An immediate re-biopsy was considered when the repeat biopsy was performed before the first sonographic follow-up. A delayed rebiopsy was considered when the repeat biopsy was performed after a sonographic follow-up. The reasons for a second biopsy were a high-risk lesion, insufficient sampling, imaging-histologic discordance and disease progression, as noted by the imaging findings. Surgical excision was performed for 19 benign lesions due to the patient's request and these were excluded from the final analysis. The rebiopsy rate was determined by dividing the total number of core biopsies into the number of repeat biopsies.
A false-negative diagnosis was defined as a lesion that showed a benign pathology upon core biopsy, but it was malignant upon the subsequent surgical biopsy or repeat core biopsy. The false-negative rate was determined by dividing the total number of carcinomas found upon subsequent biopsy into the number of those lesions that were previously considered benign upon core biopsy (6). For those patients having a benign biopsy result without any follow-up imaging, cross-referencing with the national cancer registries was performed to find out if there was any additional false negative diagnosis.
Statistical Analysis
Statistical comparison was performed between the 14-gauge automated biopsy group and the 11-gauge vacuum-assisted biopsy group in terms of the underestimation, rebiopsy and the false-negative rates. We also analyzed whether there was any difference between these results according to the characteristics of the lesions, i.e., lesion type (mass or calcifications), lesion sizes and BI-RADS categories. We used 2 cm as the size criterion. Statistical analysis was performed using the chi-square test or Fisher's exact test. For all the analyses, the results were considered statistically significant if the p values were < 0.05.
RESULTS
Histologic Underestimation
Surgery was performed for 12 of the 14 ADH lesions and for 10 of the 10 DCIS lesions in the group that was biopsied with the 14-gauge automated gun, and surgery was performed for 5 of the 10 ADH lesions and for 17 of the 20 DCIS lesions in the group that was biopsied with the 11-gauge vacuum-assisted device.
The total underestimations, according to biopsy device, were 56% (12/22) for the 14-gauge automated gun biopsies and 36% (8/22) for the 11-gauge vacuum-assisted device. The ADH underestimation rate was 58% (7/12) for the 14-gauge automated gun biopsies, and it was 20% (1/5) for the 11-gauge vacuum-assisted biopsy device. The DCIS underestimation rate was 50% (5/10) for the 14-gauge automated gun biopsies versus 41% (7/17) for the 11-gauge vacuum-assisted biopsy. However, these differences were not statistically significant (p = 0.226).
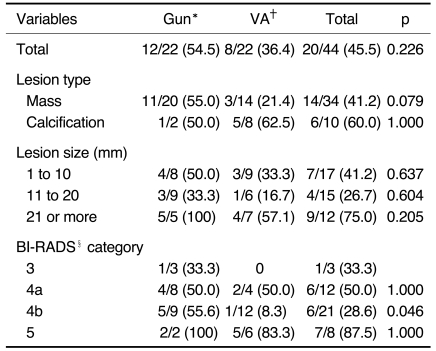
According to the lesion type, the histologic underestimation rate was 60% (6/10) for the calcifications and 41% (14/34) for the masses (p = 0.293). According to the lesion size, the histologic underestimation rate was 34% (9/12) for the lesions of 21 mm or more, and the histologic underestimation rate was 70% (11/32) for lesions less than 20 mm (p = 0.04). According to the BI-RADS categories, 88% (7/8) of the BI-RADS category 5 lesions were underestimated whereas 33% (1/3) of the category 3 lesions were underestimated, 50% (6/12) of the category 4a lesions were underestimated and 27% (6/21) of the category 4b were underestimated (p = 0.04). When the underestimation rates were compared for the two groups (Table 3), only the difference for the BI-RADS category 4b lesions was statistically significant (56% [5/9] and 8% [1/12]) (p = 0.046).
Table 3.
Underestimation Rates for the Two Biopsy Methods According to Lesion Characteristics
Note.-The numbers in parentheses are percentages. *Gun = 14-gauge automated gun biopsy, †VA = 11-gauge vacuum-assisted biopsy, §BI-RADS = Breast Imaging Reporting and Data System
Repeat Biopsy
Diagnostic repeat biopsies were performed in 49 (5%) of the 979 lesions: surgical biopsy was done for 48 lesions and vacuum-assisted biopsy was done for one gun-biopsied lesion. The repeat biopsy rate was 6% (33/562) for the 14 gauge-automated gun biopsies, and 4% (16/417) for the 11-gauge vacuum-assisted biopsies. This difference was not statistically significant (p = 0.19) (Table 4).
Table 4.
Reasons for Diagnostic Rebiopsy after Core Biopsy
Note.-*Gun: 14-gauge automated gun biopsy, †VA: 11-gauge vacuum-assisted biopsy
Immediate rebiopsy was performed for 4% (21/562) of the 14-gauge automated gun biopsies and for 2% (8/417) of the 11-gauge vacuum-assisted biopsies. Five of 21 immediate rebiopsies were due to imaging-histologic discordance for the 14-gauge automated gun biopsies, whereas none of the immediate rebiopsies were due to imaging-histologic discordance for the 11-gauge vacuum-assisted biopsies. Delayed repeat biopsy was performed for 2% (12/562) of the 14-gauge automated gun biopsies and for 2% (8/417) of the 11-gauge vacuum-assisted biopsies due to the imaging findings of disease progression. This difference was not statistically significant (p = 0.19).
False Negative Diagnosis
Of the 49 repeated biopsies, 18 (37%) of these lesions were diagnosed as malignant: 13 lesions (40%, 13/33 rebiopsies) that were sampled with 14-gauge automated gun biopsy and five lesions (31%, 5/16 rebiopsies) that were sampled with 11-gauge vacuum-assisted biopsy were diagnosed as malignant (Table 4). This difference was not statistically significant (p = 0.579). Eight ADH lesions were noted upon core biopsy (seven lesions by 14-gauge automated gun and one lesion by 11-gauge vacuum-assisted biopsy) and five phyllodes tumors were found to be malignant upon surgical excision; all these were considered to be histologic underestimates and not false-negative findings. Excluding the ADH underestimations and phyllodes tumors, four benign lesions by 14-gauge automated gun biopsy and one benign lesion by 11-gauge vacuum-assisted biopsy were found to be malignant upon surgical excision. Thus, four (3%) of the 128 lesions with a final diagnosis of carcinoma via 14-gauge automated gun biopsy, and one (1%) of the 69 lesions with a final diagnosis of carcinoma via 11-gauge vacuum-assisted biopsy constituted the false-negative diagnoses. No statistically significant difference was found between the two methods in terms of a false negative diagnosis (p = 0.659).
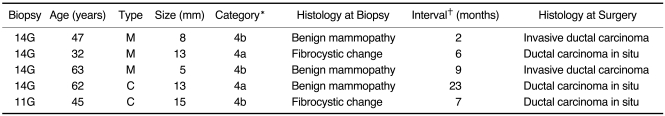
The five false-negative cases are summarized at Table 5. Immediate repeat biopsy with the 14-gauge automated gun was performed on a 0.8 cm sized category C4b mass with a nonspecific benign histology due to the imaging-histologic discordance, and invasive ductal carcinoma was finally found at surgery. Delayed repeat biopsies due to the progression of suspicious imaging findings revealed two DCIS lesions and one invasive ductal carcinoma in the 14-gauge automated gun biopsy group, and one DCIS in the 11-gauge vacuum-assisted biopsy group (Table 5). There were no additional false negative cases found by checking and cross-referencing with the national cancer registries.
Table 5.
The Patient and Lesion Variables for the Five False-Negative Lesions
Note.-*category according to Breast Imaging Reporting and Data System, †the time interval between the initial core biopsy and the repeat biopsy G = gauge, M = mass, C = calcifications
DISCUSSION
The results of our study showed that the outcomes of the sonographically guided core biopsies performed with the 11-gauge vacuum-assisted device were better than those biopsies performed with the 14-gauge automated gun in terms of underestimation, rebiopsy and the false negative rates. The underestimation rate was 55% (12/22) for the 14-gauge automated gun biopsies and 36% (8/22) for the 11-gauge vacuum-assisted device. The repeat biopsy rate was 6% (33/562) for the 14-gauge automated gun biopsies and 4% (16/417) for the 11-gauge vacuum-assisted biopsies. The false-negative rate was 3% (4/128) for the 14-gauge automated gun biopsies and 1% (1/69) for the 11-gauge vacuum-assisted biopsies; however, this difference for the false-negative rate was not statistically significant.
Histologic underestimation occurs when the percutaneous biopsy identifies the presence of a high risk or malignant lesion, but the pathology is incompletely characterized. Because most ADH and DCIS lesions contain calcifications, the histologic underestimates upon percutaneous biopsy are most often encountered for the calcific lesions (14, 15). In our study, the underestimation rate was 1.5 times more frequent for the 14-gauge automated gun biopsies than for the 11-gauge vacuum-assisted device, although the percentage of the calcified lesions versus mass lesions was significantly higher for the vacuum-assisted method (8%) than for the automated method (2%). According to the BI-RADS categories, 88% (7/8) of the BI-RADS category 5 lesions were underestimated, whereas 33% (1/3) of the category 3 lesions and 36% (12/33) of the category 4 lesions were underestimated (p = 0.04). When the underestimation rates were compared between the two groups (Table 3), the difference for the BI-RADS category 4b lesions was found to be significant (56% [5/9] and 8% [1/12]) (p = 0.046). The histologic underestimates for percutaneous biopsy seem to be related to the lesion's BI-RADS categories as well as to the type of lesion (5) and further investigation on this relationship is needed.
Philpotts et al. (18) have recently compared the outcomes of sonographically guided core biopsies that were performed with a 14-gauge automated gun with those that were performed with an 11-gauge vacuum-assisted device. Immediate rebiopsy was recommended for 14% (25/181) of the 14-gauge samplings and for 17% (17/100) of the 11-gauge vacuum-assisted sonographically guided core biopsies. These rates are much higher than our rebiopsy rates of 6% (33/562) and 4% (16/417), respectively. Their reasons for rebiopsy and the number of cores from the 11-gauge vacuum-assisted biopsy were notably different from those in our study. In their study, 65% (11/17) of the rebiopsies for the 11-gauge vacuum-assisted sonographically guided core biopsies were due to imaging-pathologic discrepancies, whereas none of the rebiopsies were due to imaging-pathologic discrepancies in our series. For the biopsies performed using the 11-gauge vacuum-assisted device, the mean number of core samples in Philpotts' study was 5.8 (range, 1-12), but the mean number of core samples in our study was 10.2 (range, 6-30). At our institution, the acquisition of greater amounts of tissue was encouraged by the more subtle sonographic findings such as calcified lesions, intraductal lesions and solid nodules less than 1.0 cm. Although complete lesion removal is generally not the goal of percutaneous biopsy, there is a much reduced chance of sampling error and of a consequent false-negative diagnosis with the complete or near complete removal of the sonographic evidence, i.e., the lesion (16, 17, 22).
The most obvious shortcoming of core biopsies of the breast is the possibility of a false-negative diagnosis. Percutaneous large-core breast biopsy carries with it an overall false-negative rate of 3% to 11% (23-26). Most of the false-negatives were due to the biopsy of calcifications, where the mass false-negative rate was 1%. The reported missed cancer rate for sonographically guided core biopsy is very low (1-5, 25): it was only 0.5% (3/687) for the 14-guage automated gun biopsies in Berg's study (26), and 0.5% (1/181) for the 14-guage automated gun biopsies and 1% (1/100) for the 11-gauge vacuum-assisted biopsies in Philpotts' study (18). Our results are comparable with those results of previous studies when using a similar biopsy technique and fellows as operators (18, 26). In our series, five (0.5%) of the 979 core biopsies were believed to have missed the lesion: one (0.2%) of the 417 vacuum-assisted biopsies and four (0.7%) of the 562 automated gun biopsies. All the carcinomas that were missed in our study were less than 1.5 cm, as observed upon sonography, and this finding was concordant with other studies (26). For very small lesions, sonographic images can make it appear as if the needle is within a lesion when the needle is, in fact, only adjacent to the lesion (4). Another notable finding in this study was that two (40%) of the five missed cancers were calcifications, whereas only 5% (44/979) of the total lesions were calcifications. For the two missed cancers, calcification retrieval was not confirmed by performing radiography. Therefore, to reduce the number of false negative diagnoses, an immediate repeat biopsy should be recommended if there is any imaging-histologic discordance or if the calcifications are not retrieved (15).
Our study has the following limitations. Imaging follow-ups by mammography and sonography were performed for only about 60% of the lesions that were benign upon core biopsy. The mean duration of follow-up was 9.0 months (1-28 months), and this was insufficient to accurately estimate the false negative rate, although we checked for these patients with using the national cancer registries. Another limitation was the inclusion of calcified lesions as a target for sonographically guided core biopsies, and also there was the preponderance of calcified lesions that were biopsied by 11-gauge vacuum-assisted biopsy over the 14-gauge automated gun biopsy. In our institution, if lesions are evident upon sonography, then the calcifications are also subjected to sonographically guided core biopsy because the sampling of the sonographically visible component often helps target the invasive component (27, 28). The calcified lesions are the main cause for histologic underestimation, missed cancer and repeat biopsy due to their discontinuities and their histologic heterogeneities. Further investigation on sonographically guided core biopsy of calcifications, including the randomization of the lesion type and size, is needed for making an accurate comparison.
In conclusion, sonographically guided core biopsy is an accurate method for evaluating those breast lesions that require the physician to perform tissue sampling. In our series, we believe that only five (0.5%) of the 979 core biopsies missed the target lesions. The outcomes of sonographically guided core biopsies performed with the 11-gauge vacuum-assisted device were better than those biopsies performed with the 14-gauge automated gun in terms of underestimation, rebiopsy and the false negative rate, although these differences were not statistically significant. Yet, this does not mean that we recommend vacuum-assisted biopsy rather than automated gun biopsy for all the sonographic masses seen in clinical practice. When deciding which biopsy method is the most effective, another factors such as cost, the time required for the biopsy and the complications, which were all beyond the scope of this study, should be taken into considered.
Footnotes
This study is supported by KISTEP and the Ministry of Science and Technology, Korea.
References
- 1.Parker SH, Jobe WE, Dennis MA, Stavros AT, Johnson KK, Yakes WF, et al. US-guided automated large-core breast biopsy. Radiology. 1993;187:507–511. doi: 10.1148/radiology.187.2.8475299. [DOI] [PubMed] [Google Scholar]
- 2.Liberman L, Feng TL, Dershaw DD, Morris EA, Abramson AF. Ultrasound-guided core breast biopsy: use and cost-effectiveness. Radiology. 1998;208:717–723. doi: 10.1148/radiology.208.3.9722851. [DOI] [PubMed] [Google Scholar]
- 3.Smith DN, Rosenfield Darling ML, Meyer JE, Denison CM, Rose DI, Lester S, et al. The utility of ultrasonographically guided large-core needle biopsy: results from 500 consecutive breast biopsies. J Ultrasound Med. 2001;20:43–49. doi: 10.7863/jum.2001.20.1.43. [DOI] [PubMed] [Google Scholar]
- 4.Fishman JE, Milikowski C, Ramsinghani R, Velasquez MV, Aviram G. US-guided core-needle biopsy of the breast: how many specimens are necessary? Radiology. 2003;226:779–782. doi: 10.1148/radiol.2263011622. [DOI] [PubMed] [Google Scholar]
- 5.Fajardo LL, Pisano ED, Caudry DJ, Gatsonis CA, Berg WA, Connolly J, et al. Stereotactic and sonographic large-core biopsy of nonpalpable breast lesions: results of the Radiologic Diagnostic Oncology Group V study. Acad Radiol. 2004;11:293–308. doi: 10.1016/s1076-6332(03)00510-5. [DOI] [PubMed] [Google Scholar]
- 6.Jackman RJ, Nowels KW, Rodriguez-Soto J, Marzoni FA, Jr, Finkelstein SI, Shepard MJ. Stereotactic, automated, large-core needle biopsy of nonpalpable breast lesions: false-negative and histologic underestimation rates after long-term follow-up. Radiology. 1999;210:799–805. doi: 10.1148/radiology.210.3.r99mr19799. [DOI] [PubMed] [Google Scholar]
- 7.Darling MLR, Smith DN, Lester SC, Kaelin C, Selland DL, Denison CM, et al. Atypical ductal hyperplasia and ductal carcinoma in situ as revealed by large-core needle breast biopsy: results of surgical excision. AJR Am J Roentgenol. 2000;175:1341–1346. doi: 10.2214/ajr.175.5.1751341. [DOI] [PubMed] [Google Scholar]
- 8.Han BK, Choe YH, Ko YH, Nam SJ, Kim JH, Yang JH. Stereotactic core-needle biopsy of non-mass calcifications: outcome and accuracy at long-term follow-up. Korean J Radiol. 2003;4:217–223. doi: 10.3348/kjr.2003.4.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burbank F. Stereotactic breast biopsy of atypical ductal hyperplasia and ductal carcinoma in situ lesions: improved accuracy with directional, vacuum-assisted biopsy. Radiology. 1997;202:843–847. doi: 10.1148/radiology.202.3.9051043. [DOI] [PubMed] [Google Scholar]
- 10.Jackman RJ, Burbank F, Parker SH, Evans WP, 3rd, Lechner MC, Richardson TR, et al. Stereotactic breast biopsy of nonpalpable lesions: determinants of ductal carcinoma in situ underestimation rates. Radiology. 2001;218:497–502. doi: 10.1148/radiology.218.2.r01fe35497. [DOI] [PubMed] [Google Scholar]
- 11.Philpotts LE, Lee CH, Horvath LJ, Lange RC, Carter D, Tocino I. Underestimation of breast cancer with 11-gauge vacuum suction biopsy. AJR Am J Roentgenol. 2000;175:1047–1050. doi: 10.2214/ajr.175.4.1751047. [DOI] [PubMed] [Google Scholar]
- 12.Jackman RJ, Burbank F, Parker SH, Evans WP, 3rd, Lechner MC, Richardson TR, et al. Atypical ductal hyperplasia diagnosed at stereotactic breast biopsy: improved reliability with 14-gauge, directional, vacuum-assisted biopsy. Radiology. 1997;204:485–488. doi: 10.1148/radiology.204.2.9240540. [DOI] [PubMed] [Google Scholar]
- 13.Philpotts LE, Shaheen NA, Carter D, Lange RC, Lee CH. Comparison of rebiopsy rates after stereotactic core needle biopsy of the breast with 11-gauge vacuum suction probe versus 14-gauge needle and automatic gun. AJR Am J Roentgenol. 1999;172:683–687. doi: 10.2214/ajr.172.3.10063860. [DOI] [PubMed] [Google Scholar]
- 14.Liberman L, Smolkin JH, Dershaw DD, Morris EA, Abramson AF, Rosen PP. Calcification retrieval at stereotactic, 11-gauge, directional, vacuum-assisted breast biopsy. Radiology. 1998;208:251–260. doi: 10.1148/radiology.208.1.9646821. [DOI] [PubMed] [Google Scholar]
- 15.Liberman L, Drotman M, Morris EA, LaTrenta LR, Abramson AF, Zakowski MF, et al. Imaging-histologic discordance at percutaneous breast biopsy. Cancer. 2000;89:2538–2546. doi: 10.1002/1097-0142(20001215)89:12<2538::aid-cncr4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Berg WA, Krebs TL, Campassi C, Magder LS, Sun CC. Evaluation of 14 and 11 gauge directional, vacuum-assisted biopsy probes and 14G biopsy guns in a breast parenchymal model. Radiology. 1997;205:203–208. doi: 10.1148/radiology.205.1.9314986. [DOI] [PubMed] [Google Scholar]
- 17.Parker SH, Klaus AJ, McWey PJ, Schilling KJ, Cupples TE, Duchesne N, et al. Sonographically guided directional vacuum-assisted breast biopsy using a handheld device. AJR Am J Roentgenol. 2001;177:405–408. doi: 10.2214/ajr.177.2.1770405. [DOI] [PubMed] [Google Scholar]
- 18.Philpotts LE, Hooley RJ, Lee CH. Comparison of automated versus vacuum-assisted biopsy methods for sonographically guided core biopsy of the breast. AJR Am J Roentgenol. 2003;180:347–351. doi: 10.2214/ajr.180.2.1800347. [DOI] [PubMed] [Google Scholar]
- 19.Mercado CL, Hamele-Bena D, Singer C, Koenigsberg T, Pile-Spellman E, Higgins H, et al. Papillary lesions of the breast: evaluation with stereotactic directional vacuum-assisted biopsy. Radiology. 2001;221:650–655. doi: 10.1148/radiol.2213010005. [DOI] [PubMed] [Google Scholar]
- 20.American College of Radiology. Breast imaging reporting and data system (BI-RADS™) 3rd ed. Reston, Va: American College of Radiology; 1998. [Google Scholar]
- 21.Stavros AT. Introduction to breast ultrasound. In: Stavros AT, editor. Breast ultrasound. 1st ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004. pp. 1–15. [Google Scholar]
- 22.Liberman L, Kaplan JB, Morris EA, Abramson AF, Menell JH, Dershaw DD. To excise or to sample the mammographic target: what is the goal of stereotactic 11-gauge vacuum-assisted breast biopsy? AJR Am J Roentgenol. 2002;179:679–683. doi: 10.2214/ajr.179.3.1790679. [DOI] [PubMed] [Google Scholar]
- 23.Parker SH, Burbank F, Jackman RJ, Aucreman CJ, Cardenosa G, Cink TM, et al. Percutaneous large-core breast biopsy: a multi-institutional study. Radiology. 1994;193:359–364. doi: 10.1148/radiology.193.2.7972743. [DOI] [PubMed] [Google Scholar]
- 24.Liberman L, Dershaw DD, Glassman JR, Abramson AF, Morris EA, LaTrenta LR, et al. Analysis of cancers not diagnosed at stereotactic core breast biopsy. Radiology. 1997;203:151–157. doi: 10.1148/radiology.203.1.9122384. [DOI] [PubMed] [Google Scholar]
- 25.Berg WA. Image-guided breast biopsy and management of high-risk lesions. Radiol Clin North Am. 2004;42:935–946. doi: 10.1016/j.rcl.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Berg WA, Berg AP, Ioffe OB. Initial success and frequency of rebiopsy after ultrasound-guided 14-g core breast biopsy [abstract] AJR Am J Roentgenol. 2003;180:10. [Google Scholar]
- 27.Soo MS, Baker JA, Rosen EL. Sonographic detection and sonographically guided biopsy of breast microcalcifications. AJR Am J Roentgenol. 2003;180:941–948. doi: 10.2214/ajr.180.4.1800941. [DOI] [PubMed] [Google Scholar]
- 28.Moon WK, Im JG, Koh YH, Noh DY, Park IA. US of mammographically detected clustered microcalcifications. Radiology. 2000;217:849–854. doi: 10.1148/radiology.217.3.r00nv27849. [DOI] [PubMed] [Google Scholar]