Abstract
Objective
We wanted to assess the role of the popliteal lymph nodes for differentiating rheumatoid arthritis (RA) from osteoarthritis (OA), and we also wanted to investigate the relationship between the popliteal lymph nodes and the inflamed synovial volume (ISV) by using contrast enhanced (CE), fat suppressed, three dimensional-fast spoiled gradient echo (3D-FSPGR) MR imaging.
Materials and Methods
Contrast enhanced 3D-FSGPR MR imaging of 94 knees (21 with RA and 73 with OA) was analyzed. A lymph node was defined as being 'observed' if it could be seen in at least two planes of the three orthogonal reformatted planes. The number of observed lymph nodes, the mean of the smallest dimension of each lymph node and the existence of central fatty change were recorded. The OA group was graded according to the ISV calculated by a segmentation method: grade I was < 20 cm3; grade II ranged from 20 cm3 to 40 cm3; and grade III was > 40 cm3. Statistical analysis of the number and the mean size of the popliteal lymph nodes among the four groups (the RA group and the grade I-III OA groups) was performed.
Results
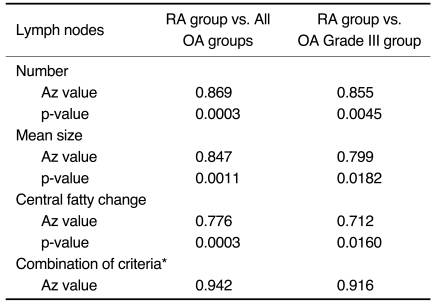
The prevalence of the observed popliteal lymph nodes was significantly different between all the OA groups or between the grade III OA group and the RA group (p < 0.0001, 0.0001, respectively). The popliteal lymph node was observed in 32 out of 73 OA cases, whereas it was visible in all of the 21 RA cases. The number (mean ± standard deviation) of lymph nodes in the grade I OA group, the grade II OA group, the grade III OA group and the RA group was 1.2 ± 0.4, 1.2 ± 0.4, 1.3 ± 0.5, and 2.7 ± 1.1, respectively. The mean size (mean ± standard deviation) of the lymph nodes was 3.8 ± 1.0 mm, 3.6 ± 1.1 mm, 4.1 ± 0.8 mm, and 5.4 ± 1.3 mm, respectively. The incidence of central fatty changes was significantly lower in the RA group than in all the OA groups and the grade III OA group. When differentiating RA from OA, and when the differentiation was confined to the RA group and grade III OA group, respectively, the criteria of the number of lymph nodes, their size, their central fatty change and a combination of all these three criteria showed statistical significance (Az values for the former were 0.869, 0.847, 0.776, and 0.942; Az values for the latter were 0.855, 0.799, 0.712, and 0.916). The number and mean size of the lymph nodes correlated with the ISVs (r = 0.49, p < 0.001; 0.50, 0.001, respectively).
Conclusion
The number, size and central fatty changes in the popliteal lymph nodes observed on the MR images might serve as simple and useful markers in differentiating RA disease from OA disease. These markers would be particular helpful in cases of severe synovial enhancement where the ISVs of both RA and OA overlap. The number and mean size of the lymph nodes also correlated well with the ISV.
Keywords: Knee, MR; Knee, arthritis; Lymph node, popliteal; Synovitis
It's reported in many anatomy textbooks that there are several popliteal lymph nodes (1, 2). These nodes are large in neonates and then they gradually decrease in size throughout life. Fatty changes may occur in the lymph nodes, particularly in the peripheral lymph nodes that receive little antigenic stimulation (3). The popliteal lymph nodes are not infrequently observed on routine knee MR imaging study; however, they have received little attention. In a previous study, the characteristics of the MR imaging-determined popliteal lymph nodes in patients having an internal derangement of the knee were established as to their prevalence, size and the occurrence of fatty changes (4). With aging, the number of popliteal lymph nodes was decreased, but the frequency of central fatty changes was increased (4). Since the regional lymph nodes are the primary target for preventing the spread of local inflammation, we assumed that the changes in the size, number and fatty composition of the lymph nodes were correlated with the severity of the synovitis of the joint. However, to our knowledge, the relationship between synovitis of the knee and the MR imaging-determined popliteal lymph nodes has not been reported on.
In contrast, regarding the evaluation of synovitis, the MR imaging-determined measurements of the quantitative synovial volume has proven to be useful for evaluating the disease activity, the severity and the treatment outcome for patients with rheumatoid arthritis (RA) (5-10). It has been reported that synovitis developed in osteoarthritis (OA) and that the synovial microscopic findings were not specific for differentiating RA disease from OA disease (11-15). For both RA and OA, the MR imaging-determined synovial volume was found to correlate with the synovial inflammatory activity (9). The synovial volume measurements would be of good value when interpreting the knee MR images of extensive synovial thickening without the physician having any knowledge of the clinical information, and this is particularly true for differentiating rheumatoid arthritis from osteoarthritis because the inflamed synovial volume was found to be higher for rheumatoid arthritis than for osteoarthritis, as was previously reported (9). However, these volume measurements take a lot of time. In an attempt to find an easier method, we assumed that the configuration and number of popliteal lymph nodes would correlate with the synovial inflammatory activity. For this purpose, we investigated the relationship between the popliteal lymph nodes and the inflamed synovial volume (ISV) by using contrast enhanced (CE), fat suppressed, three-dimensional fast gradient-recalled acquisition in the steady state with radiofrequency spoiling (3D-FSPGR) MR imaging. We report here on the role of the popliteal lymph nodes in distinguishing RA from OA.
MATERIALS AND METHODS
Patients Population
Ninety-four patients were enrolled in this retrospective study. The underlying disease was RA in 21 patients (18 women and three men ranging in age from 37 to 76 years [mean: 49.2 years, median: 48 years] and OA in 73 patients (55 women and 18 men ranging in age from 40 to 80 years [mean: 57.8 years, median: 58 years]) who came to our hospital and underwent MR from January 2000 to August 2003. The patients fulfilled either the American College of Rheumatology (ACR) 1987 classification criteria for RA (16) or the ACR 1986 classification criteria for OA of the knee (17). MRI, standard blood tests (including serum C-reactive protein [CRP] and the erythrocyte sedimentation rate [ESR]), as well as a clinical examination were performed for all the patients. Informed consents were obtained from all the patients prior to enrollment. The OA group was graded according to the ISV that was calculated using a segmentation method: In grade I, the ISV was < 20 cm3; in grade II, it ranged from 20 cm3 to 40 cm3; in grade III, the ISV was > 40 cm3.
MR Imaging Protocol
The MR imagings were performed on a 1.5-T imager (Horizon; General Electric Medical Systems, Milwaukee, WI) with a dedicated extremity coil. The patients were examined in the supine position with the afflicted knee placed in the neutral position. Fat suppressed 3D-FSPGR images were acquired with and without contrast enhancement in the sagittal plane for each patient; frequency-selective fat suppression was used for this sequence (TR/TE 20.9/2.2 msec, flip angle 15°). A 9-cm thick slab was partitioned into 60 sections, resulting in a section thickness of 1.5 mm. One signal was acquired, the matrix size was 256 × 192 and the field of view was 15 cm. This entire sequence took 4 minutes, 20 seconds to complete.
Determination of Popliteal Lymph Nodes
All the images were reviewed for the presence of popliteal lymph nodes by two of the authors (Y.H., H.S.), and a determination was then made by consensus. The nodes were considered to be present if they were observed in at least two imaging planes of the three orthogonal multiplanar reformatted planes. The lymph nodes appeared as discrete round or oval structures, and some of them showed a central fatty change. Hence, the fat suppressed, precontrast, 3D-FSPGR images showed the lymph nodes as low signal intensity (identical to fat), surrounded by a complete or incomplete rim of intermediate signal intensity. The non-fatty regions were enhanced on the postcontrast images. The blood vessels sometimes appeared as round or oval, but they could be easily distinguished from the lymph nodes by virtue of their anatomical location, the presence of an intraluminal signal void and their tubular, branching shape (18). The lymph node size was measured on the multiplanar reformatted images where they appeared the largest, and their size was expressed as their shortest dimension (19-22). The mean value of the lymph node size was obtained if multiple nodes were observed. The presence of central fatty changes was also determined.
Calculation of ISV
The ISV was calculated from the sagittal, fat-suppressed 3D FSPGR images by using a PC computer and 3D reconstruction software (VoxelPlus; Mevisys, Daejeon, Korea). The ISV was determined by using four separate steps that were modified from a previous study. First, a region conceivably believed to contain the synovium was manually traced using a mouse. The extra-articular hyperintense structures such as the arteries, veins and incompletely suppressed fat were not included in the region. Second, a segmentation algorithm based on the differences in the signal intensity values was used to distinguish the enhancing tissues from the non-enhancing tissues (5). The threshold signal intensity was defined as the midpoint between (a) the mean voxel value of the enhancing synovium minus 2 standard deviations and (b) the mean voxel value of the non-enhancing tissue (subcutaneous fat) plus 2 standard deviations (5). The mean values and standard deviations of the voxels in these regions of interest (ROIs) was obtained by drawing multiple ROIs over the enhancing synovium and the nonenhancing tissue (the subcutaneous fat). Third, the hyperintensities within the bone marrow above the threshold cut-off value were regarded as bone marrow edema. The bone marrow edema within the ROIs was manually erased by using a mouse. Fourth, a binary image was produced using a threshold cut-off value. The counted pixel value was transformed and this was expressed as a volume. It required approximately five minutes to calculate the volume in each patient.
Statistical Analysis
For the statistical analysis of each criterion for the popliteal lymph nodes (number, mean size and central fatty changes) between the RA group and all the OA groups or between the RA group and the grade III OA group, logistic regression analysis using a two-tier level was applied. First, the criteria for the presence or absence of popliteal lymph nodes were applied, and when the popliteal lymph nodes were found, the criteria for the number, the mean size and the central fatty changes were then used. The receiver operating characteristic (ROC) curves were used (by employing a logistic regression model) to assess the accuracy of differentiating RA from all the OA groups and also for differentiating the RA group from the grade III (having a large ISV) OA group. The area under each ROC curve (Az) was used to compare the overall performance of the criteria for the popliteal lymph nodes.
Each criterion of the popliteal lymph nodes was correlated with the ISVs by using the Pearson correlation method. p values < 0.05 indicated statistical significance. The SAS, version 8.1 software was used for the analysis.
The intra-observer and inter-observer variations in the ISVs' quantification and in the measurement of lymph node size were analyzed in the same way as reported previously (5). Two observers (A and B) determined the ISV and the lymph node size twice in the randomly chosen MR examinations (20 MR examinations), regardless of whether the images were from the RA or OA group. For the ISV, the signal intensity thresholds were re-calculated on each occasion while the manual tracing and erasing were re-performed. On the other hand, the shortest dimensions of the lymph node were re-determined on each occasion on the multiplanar reformatted views. The reproducibility error was expressed in two ways: 1) the mean percentage of the average absolute variation = 100 × ([value 1-value 2]/value1); and 2) the percentage coefficient of the variation = 100 × (standard deviation of absolute differences/mean value). Each of the intra-observer (A1 and A2; B1 and B2) and inter-observer (A and B) variations was computed by using the reproducibility error.
RESULTS
The prevalence of the observed popliteal lymph nodes was significantly different between all the OA groups or between the grade III OA group and the RA group (p < 0.0001, 0.0001, respectively) (Fig. 1). Popliteal lymph nodes were observed in 32 out of 73 OA cases (OA grade I, 9/24: grade II, 10/22; grade III, 13/27), whereas they were visible in all of the 21 RA cases (Fig. 2).
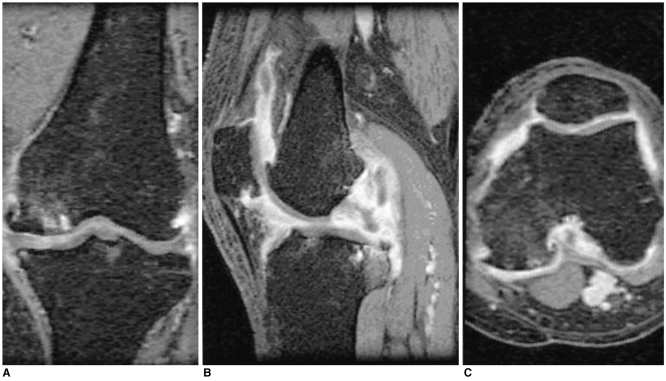
Fig. 1.
A 75-year-old woman with osteoarthritis
Coronal contrast enhanced, fat suppressed, 3D FSPGR image (A) and its reformatted (B) sagittal and (C) axial images in a separate workstation show the marked synovial membrane enhancement. Hence, this case was grouped with the Grade III osteoarthritis. The popliteal lymph node was not visible on all the knee MR images.
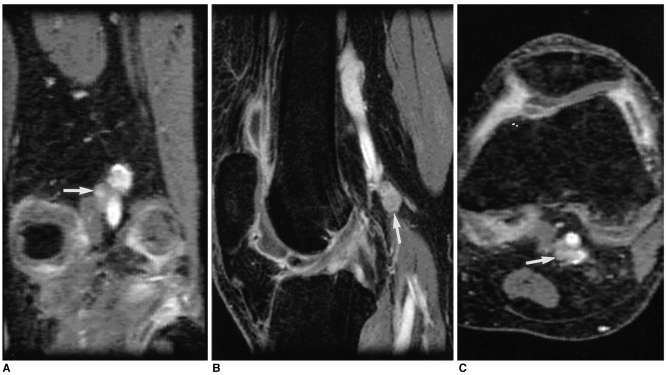
Fig. 2.
A 41-year-old woman with rheumatoid arthritis.
Coronal contrast enhanced, fat suppressed, 3D FSPGR image (A) and its reformatted (B) sagittal and (C) axial images in a separate workstation show an 8.6 mm sized (shortest diameter) lymph node (arrows) adjacent to the popliteal vessels. Central fatty changes cannot be seen. There was extensive synovial membrane enhancement in the knee joint.
The Az values and p-values for differentiating the RA group from all the OA groups and for differentiating the RA group from the grade III OA group are summarized in Table 1.
Table 1.
Accuracy of the Criteria for the Number and the Size of the Lymph Nodes in Differentiating the RA Group from All the OA Groups, or for Differentiating the RA Group from the Grade III OA Group Alone
Note.-*Applies the combination of each criterion as follows; the number, mean size and central fatty change of the lymph nodes.
The number and size of the popliteal lymph nodes of the RA group were significantly greater than those of all the OA groups and the grade III OA group (Fig. 2). The number (mean ± standard deviation) of lymph nodes of the four groups (OA grade I, n=9; grade II, n=10; grade III, n=13; RA group, n=21) was 1.2 ± 0.4, 1.2 ± 0.4, 1.3 ± 0.5, and 2.7 ± 1.1, respectively. The mean size (mean ± standard deviation) of the lymph nodes was 3.8 ± 1.0 mm, 3.6 ± 1.1 mm, 4.1 ± 0.8 mm, and 5.4 ± 1.3 mm, respectively. The incidence of central fatty changes was significantly lower in the RA group than in all the OA groups as well as the grade III OA group. The incidence of central fatty changes was seven out of nine in the grade I OA group, nine out of 10 in the grade II OA group and eight out of 13 in the grade III OA group, whereas the incidence of central fatty changes was four out of 21 in the RA group. For differentiating the RA group from the OA groups, the overall accuracy (Az values) was high when using each criterion of the number and size of the lymph nodes and the central fatty changes. When all three criteria were combined by using a logistic regression model, the Az value was slightly higher. Even when the analysis was limited to differentiating the RA group from the grade III OA group, the Az value was still high, but it slightly lower than the Az value for differentiating the RA group from all the OA groups.
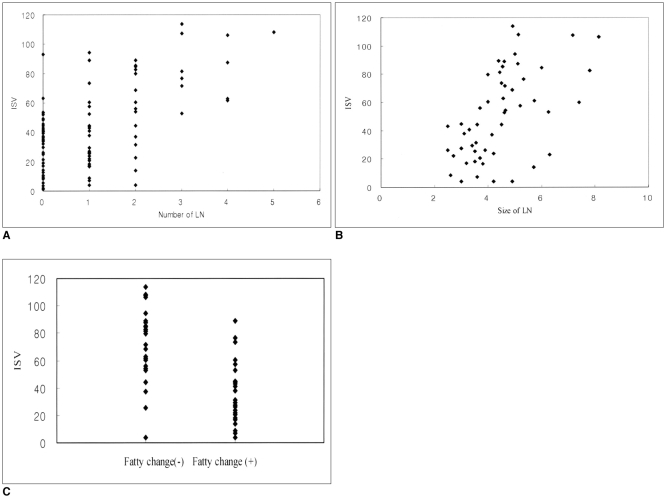
The number and mean size of the lymph nodes was correlated with the ISVs (r = 0.49, p < 0.001; 0.50, 0.001, respectively) (Fig. 3). The ISVs of the grade I, grade II, and grade III OA groups and the RA group were 9.8 ± 6.0 cm3, 29.3 ± 5.9 cm3, 53.6 ± 13.8 cm3, and 77.6 ± 23.0 cm3, respectively. The mean and standard deviation (arbitrary unit) of the signal intensity obtained from the ROIs were 323 ± 59 for the enhancing synovium and 55 ± 16 for the non-enhancing tissue. The Gaussian method revealed that the mean segmentation threshold value was 186 ± 35. The signal intensity values of the enhancing synovium and the fat did not overlap.
Fig. 3.
Scatter plots of (A) the number, (B) the mean size and (C) the central fatty changes in a popliteal lymph node as graphed against the inflamed synovial volumes (cm3). Each of these factors is statistically correlated with the inflamed synovial volume of the knee joint. The number and size of the lymph node means the number and mean size (mm) of the popliteal lymph nodes, respectively. Fatty changes (-) and (+) the mean absence and presence of central fatty changes in the popliteal lymph node, respectively.
This study showed high intra-observer agreement values. For the quantification of the ISVs, the mean percentage of the average absolute variations for observer A and observer B were 10% (0-30.29) and 13% (1.25-31.97), respectively. The coefficients of variation were 7% and 9%, respectively. The inter-observer mean percentage of the average absolute variation was 7% (range 0-21.53) and the coefficient of the variation was 5%, indicating a high reproducibility. For the measurement of the lymph node size, the mean percentages of average absolute variations for observer A and observer B were 4% (0-8.51) and 5% (0-15.56), respectively. The coefficients of variation were 3% and 3%, respectively. The inter-observer mean percentage of the average absolute variation was 7% (range 0-22.54) and the coefficient of variation was 5%, indicating a high reproducibility.
When a cut-off value of two or more lymph nodes was used for the discrimination between the OA group and the RA group, the sensitivity, specificity and accuracy of the CE 3D-FSPGR MR imaging for diagnosing RA were 86%, 89% and 88%, respectively. Meanwhile, when using a mean lymph node size of four or more millimeter, the sensitivity, specificity and accuracy were 95%, 82% and 85%, respectively.
DISCUSSION
In mammals, the lymph nodes constitute a major portion of the peripheral lymphoid tissues. Lymphatic fluid is drained from the anatomically distinct regions into a sentinel lymph node, and the node becomes the primary target site where interaction occurs between any antigenic material and the immune cells. This local immune and inflammatory reaction results in morphologic changes in the regional lymph nodes (3). The peripheral lymph nodes develop into their maximum size during the first year of life. At the same time, the popliteal lymph nodes have already begun to undergo central fatty changes at birth, and they have a considerable fat content by the first year (3). It's been described in several anatomy textbooks (1, 2) that there are about six or seven small popliteal lymph nodes. However, in a previous study (4) that investigated the popliteal lymph nodes by using MR imaging, it was found that the lymph nodes were visible in only 52% of the cases and the mean number of lymph nodes was 1.36. Moreover, with aging, the number of popliteal lymph nodes was decreased, but the frequency of central fatty change was increased. In this study, the mean number of lymph nodes per person was found to be 0.71, and this is fewer than would be expected based on the existing anatomic knowledge and literature.
Rheumatoid arthritis and osteoarthritis are common joint diseases in the elderly. Making the correct diagnosis and then initiating an appropriate therapy has a heavy influence the future joint function and the patient's satisfaction. Although the characteristic clinical and radiographic features are helpful in differentiating between these diseases, distinguishing between them on the basis of the imagings is sometimes difficult without knowledge of the patients' clinical information, especially for differentiating the severe synovitis caused by advanced osteoarthritis from that caused by rheumatoid arthritis on the MR imagings. Moreover, in the elderly patients, the superimposition of osteoarthritis with rheumatoid arthritis in the same patient makes the correct diagnosis difficult indeed because both of these diseases are common joint maladies and they both can occur together in the same anatomical location.
The synovium is the primary site of inflammation in the joints with RA. As RA is accompanied by an inflamed synovium, the joint's volume would be expected to provide some information on the disease activity and/or its severity. Several studies have recently reported that the ISVs correlate with the synovial inflammatory activity for OA as well as for RA, and that their synovial histopathology was not different qualitatively, but rather, it was different quantitatively (5-10). The ISVs, which corresponded to the synovial inflammatory activity, were found to be higher for RA than for OA (9). This study showed that the RA group and the three OA groups, as were determined by the ISV content, were significantly different in terms of the number, the mean size and the fatty changes in the lymph nodes. This suggests that the number, size and fatty change in the lymph nodes could reflect the synovial inflammatory activity of the knee joint and that these parameters could be good indicators for differentiating RA from OA. Moreover, these parameters can be simply applied to a routine clinical evaluation of arthritic knee joints. In particular, they will assist the physician in differentiating RA from OA in a knee joint with a large ISV.
The incidence of central fatty changes in the popliteal lymph nodes was significantly different between the RA group and all the OA groups, as well as being different between the RA group and grade III OA group. Of the OA groups, the grade III OA group had a lower rate of central fatty changes (62%) than did the grade I and II OA groups (84%). These results indirectly imply that the synovial inflammatory activity is high enough, in the case of the grade III OA group (with a large ISV), to cause an inflammatory reaction of the regional lymph nodes with a reconversion of the histological finding of fatty to non-fatty tissues when compared to the grade I and II OA groups.
The synovial volume measurement that was used to distinguish synovial enhancement from the joint fluid enhancement, as a result of a diffusion effect, is a subject of continuous debate. Therefore, this study selected the imaging parameters that would allow us to obtain the shortest acquisition time after an intravenous contrast injection, as was suggested in a previous study (23). If hyperemia or synovitis exists, the diffusion time can be reduced by a half. In contrast, if joint effusion is present, particularly with tension, the acquisition time should be at least doubled (24). Three dimensional imaging with a high z-axis resolution has the advantage of providing reformatted images that have good quality along any plane, and this enables the anatomic details to be clearly observed without any stepladder artifacts. The best imaging plane for the reviewing process should be properly selected according to the anatomic relationship between the popliteal lymph nodes and the popliteal vessels.
We believe that the volume quantitative method with size measurement using the multiplanar reformatted views is a reasonably acceptable method because it showed high intra-observer and inter-observer agreement.
This study had several limitations. First, the number of patients was small to obtain a cut-off value for differentiating the RA group from the OA groups, even though we have still presented our tentative results. Second, the compound estimations of the ISVs and enhanced joint fluid might inevitably occur on the contrast-enhanced MR imaging, as was mentioned above.
In summary, the number and the mean size of the lymph nodes correlated well with the ISV. The number, size and central fatty changes in the popliteal lymph nodes can serve as simple and useful markers (parameters?) for differentiating RA disease from OA disease. This method would be particularily helpful on the occasions when there is severe synovial enhancement where the ISVs of both the RA and OA overlap.
Footnotes
This study was supported by a grant (#HMP02-PJ1-PG10-20599-0004) of the 2002 Good Health R&D Project, Ministry of Health & Welfare, Korea.
References
- 1.Netter Frank H. The Ciba collection of medical illustrations. 3rd ed. Newjersey: Ciba-Geigy Corp; 1987. p. 121. [Google Scholar]
- 2.William PL, Warwick R, Dyson M, Bannister LH. Gray's Anatomy. 37th ed. London: Churchill Livingstone; 1989. pp. 848–849. [Google Scholar]
- 3.Luscieti P, Hubschmid T, Cottier H, Hess MW, Sobin LH. Human lymph node morphology as a function of age and site. J Clin Pathol. 1980;33:454–461. doi: 10.1136/jcp.33.5.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moon HJ, Suh JS, Lee SH. Magnetic resonance appearance of normal popliteal lymph nodes: location and relationship of number, fatty change, and size of the lymph nodes with aging. J Korean Radiol Soc. 2002;47:665–671. [Google Scholar]
- 5.Huh YM, Suh JS, Jeong EK, Lee SK, Lee JS, Choi BW, et al. Role of the inflamed synovial volume of the wrist in defining remission of rheumatoid arthritis with gadolinium-enhanced 3D-SPGR MR imaging. J Magn Reson Imaging. 1999;10:202–208. doi: 10.1002/(sici)1522-2586(199908)10:2<202::aid-jmri15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Palmer WE, Rosenthal DI, Schoenberg OI, Fischman AJ, Simon LS, Rubin RH, et al. Quantification of inflammation in the wrist with gadolinium-enhanced MR imaging and PET with 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology. 1995;196:647–655. doi: 10.1148/radiology.196.3.7644624. [DOI] [PubMed] [Google Scholar]
- 7.Ostergaard M, Hansen M, Stoltenberg M, Lorenzen I. Quantitative assessment of the synovial membrane in the rheumatoid wrist: an easily obtained MRI score reflects the synovial volume. Br J Rheumatol. 1996;35:965–971. doi: 10.1093/rheumatology/35.10.965. [DOI] [PubMed] [Google Scholar]
- 8.Ostergaard M, Hansen M, Stoltenberg M, Gideon P, Klarlund M, Jensen KE, et al. Magnetic resonance imaging-determined synovial membrane volume as a marker of disease activity and a predictor of progressive joint destruction in the wrists of patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:918–929. doi: 10.1002/1529-0131(199905)42:5<918::AID-ANR10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Ostergaard M, Stoltenberg M, Lovgreen-Nielsen P, Volck B, Jensen CH, Lorenzen I. Magnetic resonance imagingdetermined synovial membrane and joint effusion volumes in rheumatoid arthritis and osteoarthritis. Comparison with the macroscopic and microscopic appearance of the synovium. Arthritis Rheum. 1997;40:1856–1867. doi: 10.1002/art.1780401020. [DOI] [PubMed] [Google Scholar]
- 10.Conaghan P, Edmonds J, Emery P, Genant H, Gibbon W, Klarlund M, et al. Magnetic resonance imaging in rheumatoid arthritis: summary of OMERACT activities, current status, and plans. J Rheumatol. 2001;28:1158–1162. [PubMed] [Google Scholar]
- 11.Cooper NS, Soren A, McEwen C, Rosenberger JL. Diagnostic specificity of synovial lesions. Hum Pathol. 1981;12:314–328. doi: 10.1016/s0046-8177(81)80141-4. [DOI] [PubMed] [Google Scholar]
- 12.Gibson T, Fagg N, Highton J, Wilton M, Dyson M. The diagnostic value of synovial biopsy in patients with arthritis of unknown cause. Br J Rheumatol. 1985;24:232–241. doi: 10.1093/rheumatology/24.3.232. [DOI] [PubMed] [Google Scholar]
- 13.Goldenberg DL, Cohen AS. Synovial membrane histopathology in the differential diagnosis of rheumatoid arthritis of rheumatoid arthritis, gout, pseudogout, systemic lupus erythematosus, infectious arthritis and degenerative joint disease. Medicine (Baltimore) 1978;57:239–252. doi: 10.1097/00005792-197805000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Goldenberg DL, Egan MS, Cohen AS. Inflammatory synovitis in degenerative joint disease. J Rheumatol. 1982;9:204–209. [PubMed] [Google Scholar]
- 15.Fernandez-Madrid F, Karvonen RL, Teitge RA, Miller PR, An T, Negendank WG. Synovial thickening detected by MR imaging in osteoarthritis of the knee confirmed by biopsy as synovitis. Magn Reson Imaging. 1995;13:177–183. doi: 10.1016/0730-725x(94)00119-n. [DOI] [PubMed] [Google Scholar]
- 16.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 17.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 18.Grey AC, Carrington BM, Hulse PA, Swindell R, Yates W. Magnetic resonance appearance of normal inguinal nodes. Clin Radiol. 2000;55:124–130. doi: 10.1053/crad.1999.0330. [DOI] [PubMed] [Google Scholar]
- 19.Ingram CE, Belli AM, Lewars MD, Reznek RH, Husband JE. Normal lymph node size in the mediastinum: a retrospective study in two patient groups. Clin Radiol. 1989;40:35–39. doi: 10.1016/s0009-9260(89)80015-7. [DOI] [PubMed] [Google Scholar]
- 20.Dooms GC, Hericak H, Crooks LE, Higgins CB. Magnetic resonance imaging of the lymph nodes: comparison with CT. Radiology. 1984;153:719–728. doi: 10.1148/radiology.153.3.6093190. [DOI] [PubMed] [Google Scholar]
- 21.Dorfman RE, Alpern MB, Gross BH, Sandler MA. Upper abdominal lymph nodes: criteria for normal size determined with CT. Radiology. 1991;180:319–322. doi: 10.1148/radiology.180.2.2068292. [DOI] [PubMed] [Google Scholar]
- 22.Vinnicombe SJ, Norman AR, Nicholson V, Husband JE. Normal pelvic lymph nodes: evaluation with CT after bipedal lymphangioraphy. Radiology. 1995;194:349–355. doi: 10.1148/radiology.194.2.7824709. [DOI] [PubMed] [Google Scholar]
- 23.Huh YM, Suh JS, Lee JW, Song HT. Synovitis and soft tissue impingement of the ankle: assessment with enhanced 3D-FSPGR MR imaging. J Magn Reson Imaging. 2004;19:108–116. doi: 10.1002/jmri.10438. [DOI] [PubMed] [Google Scholar]
- 24.Steinbach LS, Palmer WE, Schweitzer ME. Special focus session. MR arthrography. Radiographics. 2002;22:1223–1246. doi: 10.1148/radiographics.22.5.g02se301223. [DOI] [PubMed] [Google Scholar]