Abstract
Apical proteins are sorted and delivered from the trans-Golgi network to the plasma membrane by a mechanism involving sphingolipid–cholesterol rafts. In this paper, we report the effects of changing the levels of VIP17/MAL, a tetraspan membrane protein localized to post-Golgi transport containers and the apical cell surface in MDCK cells. Overexpression of VIP17/MAL disturbed the morphology of the MDCK cell layers by increasing apical delivery and seemingly expanding the apical cell surface domains. On the other hand, expression of antisense RNA directed against VIP17/MAL caused accumulation in the Golgi and/or impaired apical transport of different apical protein markers, i.e., influenza virus hemagglutinin, the secretory protein clusterin (gp80), the transmembrane protein gp114, and a glycosylphosphatidylinositol-anchored protein. However, antisense RNA expression did not affect the distribution of E-cadherin to the basolateral surface. Because VIP17/MAL associates with sphingolipid–cholesterol rafts, these data provide functional evidence that this protein is involved in apical transport and might be a component of the machinery clustering lipid rafts with apical cargo to form apical transport carriers.
The polarized delivery of newly synthesized proteins to the apical and the basolateral membranes of epithelial cells involves different mechanisms (1, 2). Sorting signals for basolateral membrane proteins are localized to the cytosolic domains (3) whereas apical proteins have their sorting determinants in their membrane anchors or in their ectodomains (4). Sorting information is decoded in the trans-Golgi network (TGN) of epithelial Madin–Darby canine kidney (MDCK) cells, where apical and basolateral proteins are sorted and packaged into separate containers for delivery to the cell surface (5). Apical delivery is thought to be based on the partitioning of apical proteins into lipid microdomains superimposed by protein–carbohydrate and protein–protein interactions that stabilize the protein–lipid assembly before release from the TGN (2). The lipid microdomains are constituted by liquid-ordered phases, or sphingolipid–cholesterol rafts, floating freely in the liquid-disordered phase of the lipid bilayer (6, 7). One important feature of lipid rafts is that they are small (<70 nm in diameter), i.e., the size is considerably smaller than the apical containers that deliver their cargo to the apical membrane (8, 9). This small size means that rafts have to associate with each other in the membrane of the TGN to form the containers destined for the apical cell surface. Elucidating the machinery involved in this packaging process is one of the main goals of our studies.
Several proteins are known to participate in apical protein transport. VIP21/caveolin (10) was identified as a 21-kDa membrane protein present in TGN-derived transport vesicles isolated from the epithelial MDCK cell. This protein is present in a large complex along with influenza virus hemagglutinin (HA) (11). Its cholesterol-binding property was suggested as a functional mechanism in microdomain formation during membrane trafficking (12). More recently, it was shown that large caveolin-1 homooligomers are targeted to the apical side and play a role in apical transport of influenza virus whereas caveolin-1/-2 heterooligomers are sorted into basolateral vesicles in the TGN (13). Annexin XIIIb also was identified as a component that is highly enriched in immunoisolated apical vesicles with HA and localized to the apical plasma membrane (14). Antibodies specific for annexin XIIIb significantly inhibited the transport of influenza virus HA from the TGN to the apical plasma membrane in permeabilized MDCK cells (14). Annexin XIIIb associates with lipid rafts during its function in apical delivery (15). Vectorial transport to the apical membrane involves transport along microtubules with both dynein and kinesin as microtubule motor proteins whereas the basolateral surface transport depends on kinesin alone (16). Also, myosin-I has been implicated in apical transport of Golgi-derived vesicles in epithelial cells (17). The synaptosomal-associated 23-kDa protein (SNAP) receptor (SNARE) machinery such as syntaxin 3, SNAP-23, α-SNAP, and tetanus toxin-insensitive vesicle-associated membrane protein (TI-VAMP) is involved in apical plasma membrane trafficking (18, 19). Syntaxin 3 is localized apically (20), and its overexpression causes an inhibition of TGN to apical transport and apical recycling, leading to an accumulation of small vesicles underneath the apical plasma membrane (18). Botulinum neurotoxin E, which cleaves SNAP-23, and antibodies against α-SNAP inhibit both TGN-apical and TGN-basolateral transport in a reconstituted in vitro system (18). TI-VAMP is localized to apical vesicles and to the apical plasma membrane and form N-ethylmaleimide-dependent complexes with syntaxin 3, SNAP23, and cellubrevin (19).
In this paper, we report analyzing the effects of increasing and decreasing the concentration of VIP17/MAL in MDCK cells. This protein was first identified as a component of post-Golgi transport containers in MDCK cells (21). The canine VIP17 turned out to be a tetraspan membrane protein, almost identical to the human MAL, a protein identified as a marker of T cell maturation (22). This protein has a narrow tissue distribution, being present only in kidney, spinal cord, brain, the stomach, and the caecum of the large intestine (23–25). During differentiation of oligodendrocytes, VIP17/MAL is expressed concomitantly to the rise in myelin production (23, 24). VIP17/MAL becomes incorporated into sphingolipid–cholesterol rafts as judged by the presence of the protein in detergent-insoluble glycolipid-enriched membranes (DIGs) after the Triton X-100 extraction at 4°C in all of the investigated cell types expressing VIP17/MAL (21, 23, 26, 27). In MDCK and other epithelial cells the protein is preferentially localized to the apical membrane (21, 26, 28). Because of its presence in apical transport containers and apical plasma membrane, VIP17/MAL is a candidate for apical targeting machinery. After expression of antisense RNA directed against VIP17/MAL, we have now been able to demonstrate that lowering of VIP17/MAL levels in MDCK cells impairs the targeting of proteins to the apical membrane. These studies establish VIP17/MAL as a component of the protein machinery responsible for the sorting and delivery of proteins from the TGN to the apical cell surface.
MATERIALS AND METHODS
Cell Culture and Transfection.
Two strains of MDCK cells were used: MDCK strain II cells (5) and MDCK(lac) cells that were originally constructed by transfecting the Lac repressor vector into MDCK cell (29) to introduce an inducible promoter. Both cell lines were maintained at 37°C in 5% CO2 in MEM supplemented with 10% FCS, penicillin (100 units/ml)/streptomycin (100 μg/ml), and 2 mM glutamine (all from GIBCO/BRL). Cells were seeded on 1.2-cm diameter Transwell filters (Corning Costar) by plating 2.5 × 105 cells per filter (30).
BHK 21 cells were maintained in Glasgow MEM supplemented with 5% FCS, 10% tryptose phosphate, penicillin (100 units/ml)/streptomycin (100 μg/ml), and 2 mM glutamine. Cells were seeded in 2 ml of complete growth medium on glass coverslips. Per dish, 1–2 μg of each expression plasmid was used for cotransfection. Transfections were performed in Opti-MEM (GIBCO/BRL) with 2 μl of Lipofectamine (GIBCO/BRL) according to the instructions of the manufacturer. Green fluoescent protein (GFP)-tagged VIP17 DNA was constructed by introducing the NheI–XmaI fragment of enhanced GFP (EGFP) sequence from pEGFP-C3 (CLONTECH) and the NcoI(blunt)–NsiI fragment from pBAT-V17HA into pOPRSVI-1 vector.
The adenovirus packaging cell line 293 was maintained in MEM supplemented with 10% FCS, penicillin (100 units/ml)/streptomycin (100 μg/ml), and 2 mM glutamine, and was used to produce recombinant adenoviruses (31). Approximately 1.5 × 106 cells were plated in a 25 cm2 flask 24 hr before transfection, by which time they had reached 70% confluence. Cells were transfected with 4 μg of PacI-digested adenoviral recombinant plasmids and 20 μl of Lipofectamine.
Lac-Inducible Expression of Epitope-Tagged VIP17 and Antisense RNA Against VIP17 in MDCK(lac) Clones.
A DNA fragment from pBAT-VIP17 or pBAT-VIP17HA in which HA 12CA5 epitope (13 aa) was introduced at the N terminus of VIP17 by using PCR (21) was cut with NcoI and NsiI and cloned into the operator vector pOPRSVI-1 (pOPRSVI-VIP17 or pOPRSVI-VIP17HA) which has a neomycin-resistance selection marker. POPRSVI-1 has been constructed from the Stratagene pOPRSVI-CAT of the LacSwitch by removal of the CAT gene and insertion of one synthetic multicloning site (GCGGCCGTCTAGAGTGATATCGGTACCCGGGCCCACCATGCATGGT GGCGGCCGC). HindIII fragments of pOPRSVI-VIP17 were obtained and reconstructed into pOPRSVI to produce antisense RNA against the 5′-terminal 318 nt of VIP17 (pOPRSVI-@-VIP17). Lac repressor-positive MDCK(lac) cells were selected with 0.2 mg/ml hygromycin and were transfected with pOPRSVI-VIP17HA [VIP17(lac) clones] or pOPRSVI-@-VIP17 [Anti(lac) clones] in MEM (GIBCO/BRL) with 5 μl of SuperFect (Qiagen, Chatswoth, CA) and 2 μg of DNA. Several stable clones were selected with 0.5 mg/ml. Five strongly positive VIP17 (lac) clones were screened by immunofluorescence of 48 clones, and 1 clone (no. 48) was finally selected and used for this paper. Four Anti(lac) clones were selected by HA transport assay from 7 positive clones, of 68 stable clones, and 2 (nos. A5A3 and A1C4) were used in this paper. Selected clones were incubated either with or without 5 mM isopropylthio-β-d-galactoside (IPTG) for indicated times to induce the transcription of the gene under lac operator control.
Adenoviral Expression in MDCK Cells.
Recombinant adenoviruses expressing glycophosphatidylinositol (GPI)-anchored yellow fluorescent protein (YFP) (YFP-GL-GPI), VIP17, and antisense RNA against VIP17 were produced as described (31). Construction of GPI-anchored YFP (YFP-GL-GPI) will be described in detail elsewhere (P. Keller, D. Toomre, and K.S., unpublished data). DNA fragments from pOPRSVI-VIP17 containing the ORF of the VIP17 gene or from pOPRSVI-@-VIP17, harboring the 5′-terminal 318 nt of VIP17 in the antisense direction, were introduced into pShuttle-CMV vector, and recombinant adenoviral plasmids were generated by homologous recombination with pAdEasy-1 (31). Transfection of 293 cells was performed as described above. Adenoviruses were purified by CsCl gradient centrifugation and stored at −20°C in storage buffer (5 mM Tris, pH 8.0/50 mM NaCl/0.1% BSA/25% glycerol). Filter-grown MDCK(lac) clones with 5 mM IPTG were infected with YFP-GL-GPI-producing adenoviruses for 1 hr in the infection medium (MEM with 0.2% BSA and 2 mM glutamine) at a multiplicity of infection of 20–100 plaque-forming units per cell and then incubated overnight. For filter-grown MDCK II cells, multiplicity of infection of 5–25 PFU per cell was used for 1 hr of infection to express VIP17 or antisense RNA against VIP17, and cells were incubated for 3 days.
Influenza HA Transport Assay.
The HA transport assay was performed as described (32). MDCK(lac) clones were infected for 1 hr at 37°C with the influenza viruses in infection medium. After aspiration of the medium, fresh infection medium was added, and the cells were incubated for 2 hr at 37°C. Infected cells were labeled for 8 min at 37°C, and incubated for 75 min at 19.5°C to accumulate HA in the TGN. The cells were then incubated for indicated times: 0 min, 15 min, and up to 30 min at 37°C. Finally, trypsin was added to the apical or basolateral surface of the cells to cleave surface-transported HA to HA1 and HA2. Each band density was quantified by phosphoimager, and the surface transport was calculated as described (33).
SDS/PAGE and Western Blot Analysis.
MDCK(lac) clones selected with 0.5 mg/ml G418 after the transfection with pOPRSVI-VIP17HA [VIP17(lac)] were grown and treated with 5 mM IPTG for indicated times. Aliquots of cell lysates were separated on a 15% polyacrylamide gel, and proteins were transferred to a nitrocellulose membrane. After staining with Ponceau red, the blot was probed with mAb against the 12CA5 epitope (34) and visualized by using a horseradish peroxidase-conjugated secondary antibody and enhanced chemiluminescence detection kit (Amersham Pharmacia).
Detergent Extraction and Two-Dimensional Gel Electrophoresis.
CHAPS extractions and two-dimensional gel electrophoresis were performed as described (21, 35). MDCK(lac) clone selected with 0.5 mg/ml G418 after the transfection with pOPRSVI-@-VIP17 were grown in 90 cm2 dishes with or without 5 mM IPTG for 3 days. After chasing at 37°C for 3 hr with 1 mCi (1 Ci = 37 GBq) [35S]methionine per dish, the cells were scraped, and the postnuclear supernatant was prepared with SHE (250 mM sucrose/10 mM Hepes, pH 7.4/2 mM EGTA) containing a mixture of protease inhibitors (chymostatin, leupeptin, antipain, pepstatin; 10 μg/ml each). For the preparation of CHAPS-insoluble complexes, equal volume of 2× CHAPS extraction buffer (40 mM CHAPS/100 mM Mes⋅KOH/200 mM NaCl/10 mM CaCl2) was added to the postnuclear supernatant and incubated for 1 hr on ice. After the sample was centrifuged at 235,000 × g for 1 hr at 4°C, the pellet was dissolved at 37°C in 0.75× IEF lysis buffer (7.35 M urea/1.5% ampholines LKB 7–9/3% Nonidet P-40/75 mM DTT). The proteins were loaded on Bio-Rad Mini-Protein II two-dimensional cell according to the manufacturer’s guide and resolved in two dimensions by IEF and SDS/PAGE.
Immunofluorescence Microscopy.
Cells grown on coverslips or filters were treated with 5 mM IPTG or adenoviruses as described above. Fixation and all procedures were performed at room temperature according to Fiedler et al. (14). Antibodies used were the mouse mAb rr1 against E-cadherin (36), a rabbit polyclonal antibody against gp80 (37), and a mouse mAb against gp114 (38). Images were taken by using an inverted confocal laser scanning microscope (LSM510; Zeiss).
RESULTS
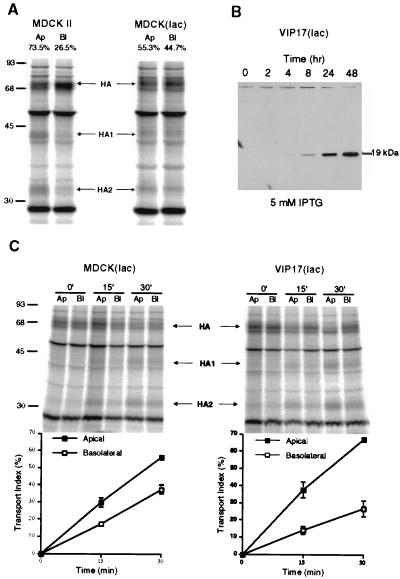
In the first set of studies, we used an MDCK cell line containing an inducible protein expression system to address the function of VIP17 in polarized MDCK cells because we found that stable VIP17 overexpression was toxic to the cells. MDCK(lac) cells, which were used for the Lac switchable system, was originally constructed by transfecting the Lac repressor vector into MDCK cells (29). During this study, we found that the Lac repressor-producing MDCK(lac) cells have a lesser capacity for apical transport than the MDCK strain II cell line. When we used influenza virus HA as an apical marker (32), more HA was observed in the Golgi and was mistargeted to the basolateral membrane in MDCK(lac) cells (Fig. 1A). In MDCK II cells, ≈75% of surface-transported HA was routed to the apical plasma membrane, whereas 25% was transported basolaterally. In contrast, in MDCK(lac) cells, only ≈55% of surface-transported HA was delivered to the apical membrane.
Figure 1.
Increased apical delivery of influenza virus HA after induced expression of epitope-tagged VIP17 in MDCK(lac) cells. (A) Comparison of influenza virus HA transport in MDCK strain II and Lac repressor producing MDCK(lac) cells. The cells were infected with influenza virus and incubated for 2 hr to produce viral proteins. Infected cells were labeled for 8 min with [35S]methionine at 37°C and incubated at 19.5°C for 75 min. After release from the 19.5°C block to allow TGN to plasma membrane transport of HA by incubation for 20 min at 37°C, trypsin was added to cleave cell surface HA to HA1 and HA2 on the apical (Ap) or on the basolateral (Bl) surface. In MDCK II cells, 73.5% of HA was delivered to the apical cell surface, whereas only 55.3% of HA was targeted to the apical membrane in MDCK(lac) cells. Molecular weights (kDa) are marked on the left. (B) Expression of epitope-tagged VIP17 under the control of the lac promoter in the VIP17(lac) clone 48, derived from MDCK(lac) cells after transfection of pOPRSVI-VIP17HA. Epitope-tagged VIP17 was detected by mAb 12CA5 as a 19-kDa polypeptide, after induction with 5 mM IPTG for the indicated times. (C) Kinetics of HA transport in MDCK(lac) cells after induced expression of epitope-tagged VIP17 with 5 mM IPTG for 3 days. Influenza-virus infected cells were pulse-labeled with [35S]methionine for 8 min and then kept at 19.5°C for 75 min. After release from the 19.5°C block by increasing the temperature to 37°C for the indicated times, trypsin was added to cleave cell surface HA to HA1 and HA2 on the apical (Ap) or on the basolateral (Bl) surface. Upper images show the SDS/PAGE gels and the Lower images show quantitation of the gels to depict surface delivery of HA to the apical and the basolateral sides. VIP17(lac) clone 48 showed increased apical transport and decreased basolateral mistargeting of HA, as compared with control MDCK(lac) cells.
Expression of Epitope-Tagged VIP17 Increased Apical Transport of Influenza HA in MDCK(lac) Cells.
Next, we overexpressed epitope-tagged VIP17 in MDCK(lac) cells (i) to compare the localization of VIP17 in MDCK(lac) cells and (ii) to analyze whether the overexpressed VIP17 has an effect on apical transport of influenza HA. We transfected the pOPRSVI-1 vector, which has a neomycin-resistance selection marker and contains the VIP17 DNA sequence, tagged with the HA epitope (pOPRSVI-VIP17HA). Stable clones [named VIP17(lac)] were selected with 0.5 mg/ml G418, and a clone (VIP17(lac) clone 48) was chosen that overexpressed epitope-tagged VIP17, regulated by the inducible promoter. After the cells were grown in the medium containing 5 mM IPTG for the indicated times, the expression was determined by Western blot using mAb 12CA5 (34) recognizing the HA epitope (Fig. 1B). This result showed that the expression of epitope-tagged VIP17 in VIP(lac) clone 48 increased in the presence of 5 mM IPTG.
The overexpressed VIP17 was preferentially localized to the apical plasma membrane in VIP17(lac) clone 48 (data not shown), as previously demonstrated in MDCK II cells (21). We next assayed the effect of overexpressed VIP17 on polarized transport (Fig. 1C). MDCK(lac) cells (Left) and VIP17(lac) clone 48 (Right) were compared to verify the specific effect of VIP17 expression. The cells were grown on filters with 5 mM IPTG for 3 days, and then infected with influenza virus. VIP17(lac) clone 48 (Right) showed increased apical delivery and decreased basolateral mistargeting of influenza HA when compared with control MDCK(lac) cells. There was no effect of IPTG treatment on HA transport of MDCK(lac) cells, and no increase of apical transport was detected without IPTG induction in VIP17(lac) clone 48 (data not shown).
Induced Expression of Antisense RNA Against VIP17 Inhibited Apical Transport of HA in MDCK(lac) Cells.
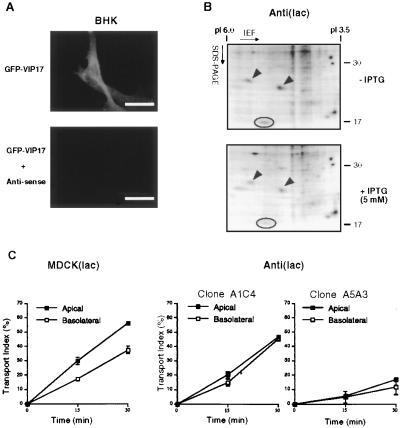
We next analyzed the function of VIP17 in MDCK(lac) cells by blocking the synthesis of endogenous VIP17 protein. For this purpose, we inserted 2/3 of VIP17 gene in the antisense direction (see Materials and Methods) and used this vector to produce antisense RNA against endogenous VIP17 transcripts to reduce VIP17 expression in MDCK(lac) cells. We first tested the effect of antisense RNA expression in BHK cells by cotransfection with pOPRSVI-EGFP-VIP17, which expresses GFP-tagged VIP17 (Fig. 2A, Upper). We observed that in BHK cells transfected with pOPRSVI-@-VIP17, which harbors antisense DNA, the GFP signal almost completely disappeared as compared with the control cells (Fig. 2A, Lower). When we coexpressed pOPRSVI-EGFP-VIP17 with other sense vectors containing VIP17 or HA epitope-tagged VIP17 DNA, the GFP signal was not abolished, indicating that VIP17-GFP expression was specifically blocked by antisense expression.
Figure 2.
Decreased apical transport of influenza virus HA after induced expression of antisense RNA against VIP17 in MDCK(lac) cells. (A) Immunofluorescence images of BHK cells that had been transfected with a vector that expressed GFP-tagged VIP17 alone (pOPRSVI-EGFP-VIP17) (Upper) and cotransfected with the vectors that express GFP-VIP17 and antisense RNA against VIP17 (pOPRSVI-EGFP-VIP17 + pOPRSVI-@-VIP17) (Lower). Twenty-four hours after transfection, the cells were fixed and observed in the fluorescence microscope. Expression of GFP-VIP17 was specifically inhibited by cotransfection with the vector expressing antisense RNA. (B) Two-dimensional gel electrophoresis of CHAPS-insoluble proteins from MDCK(lac) cells with (+IPTG) or without (−IPTG) induction of antisense RNA against VIP17. Anti(lac) clones were derived from MDCK(lac) cells after transfection with pOPRSVI-@-VIP17 and selection with G418. After Anti(lac) clone A3A5 was grown with (+IPTG) or without (−IPTG) induction for 3 days, the cells were lysed and processed for two-dimensional gel electrophoresis. VIP17 spot is indicated by a circle and is clearly reduced in antisense-expressing cells as compared with noninduced cells. α- and β-caveolin-1 are marked by arrowheads. (C) Kinetics of HA transport in MDCK(lac) clones, Anti(lac) A1C4 and A5A3 after induction of antisense RNA against VIP17 for 3 days. Both Anti(lac) clones showed reduced apical transport of HA, compared with the control MDCK(lac) cells. [Bar = 10 μm (A).]
Because we did not have an antibody detecting endogenous VIP17, we analyzed the levels of VIP17 expression of Anti(lac) clones by two-dimensional gel electrophoresis (e.g., Fig. 2B for clone A5A3). Cells were metabolically labeled with [35S]methionine for 3 hr to detect the newly synthesized proteins. We used the CHAPS pellet (see Material & Methods) for our two-dimensional gel analysis because VIP17 is insoluble after extraction of cell lysates with CHAPS. Fig. 2B Upper shows CHAPS-insoluble proteins before the induction with IPTG, and Fig. 2B Lower shows the same spots after the induction of antisense RNA against VIP17 with 5 mM IPTG. This experiment was repeated for several cell clones, and when the density of VIP17 spot was quantitated, there was usually a 50–70% reduction of VIP17 spot after inducing antisense expression. Based on this result, the endogenous expression of VIP17 was shown to be inhibited by antisense RNA expression in MDCK(lac) cells. Of interest, the intensity of several spots, including VIP21/caveolin-1 (arrowheads), were reduced from CHAPS-insoluble pellets, whereas many other proteins were unaffected after induction of antisense. This result suggests that these detergent-insoluble proteins were solubilized along with the reduction of VIP17.
We then proceeded to analyze the effect of antisense RNA expression in MDCK(lac) cells. Several Anti(lac) clones were assayed to investigate how IPTG induction of antisense expression affected transport of HA to the apical surface after influenza virus infection of the cells. We quantitated how much newly synthesized HA was delivered to the apical cell surface after a 19.5°C block. Delivery varied from 20% to 45% of the HA transported apically after 30 min at 37°C (Fig. 2C). In all selected clones transfected with pOPRSVI-@-VIP17, less HA was transported to the apical cell surface as compared with control MDCK(lac) cells (Left) or with noninduced cells (data not shown). In Anti(lac) clone A1C4 (Left), HA was mistargeted to the basolateral plasma membrane and showed no accumulation in the Golgi. In contrast, clone A5A3 (Right) showed much less HA transport both to the apical and basolateral cell surface and accumulation in the Golgi.
Apical Protein Transport Is Blocked by Antisense RNA Expression Against VIP17 Without Affecting Basolateral Distribution in MDCK(lac) Cells.
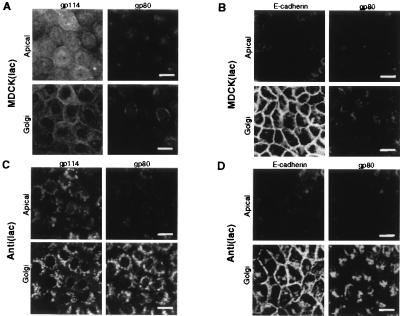
We also analyzed the effect of antisense RNA expression against VIP17 on the apical transport and intracellular localization of the endogenous apical membrane protein gp114 (38) and the secretory protein clusterin (gp80) (32, 37, 39) by using confocal immunofluorescence microscopy (Fig. 3). Upper images are optical sections at the level of the apical cell surface, and Lower images are at the level of the Golgi regions, 1 μm below the apical surface. Normally, the apical membrane protein gp114 localizes mainly to the apical membrane, but this protein can transcytose back and forth to the basolateral membrane (38). The apical secretory protein gp80 showed staining in the Golgi region and cytoplasmic puncta, typical of secretory proteins in MDCK cells (Fig. 3A). When antisense RNA against VIP17 was expressed in Anti(lac) clone A5A3, gp114 and gp80 accumulated and colocalized in the Golgi region (Fig. 3C). Thus, VIP17 was shown to be required for the transport of these endogenous apical proteins in MDCK(lac) cells.
Figure 3.
Intracellular localization of endogenous apical and basolateral proteins in MDCK(lac) cells, expressing antisense RNA against VIP17. MDCK(lac) (A and B) and MDCK(lac), expressing antisense RNA against VIP17 [Anti(lac) clone A5A3] (C and D) were grown on coverslips, fixed, and processed for immunofluorescence microscopy and observed under the Zeiss LSM510 confocal microscope. (A and C) Double-immunofluorescence image using antibodies against the apical membrane protein gp114 and the apically secreted protein gp80 in control MDCK(lac) cells and in the Anti(lac) clone A5A3 3 days after induction of antisense expression with IPTG. (B and D) Double-immunofluorescence image using antibodies against basolateral E-cadherin and apically secreted gp80. Upper images show apical surface, and the Golgi region (1 μm below the apical membrane) is visualized in the Lower images in each set. Only apical protein delivery was affected by expressing antisense RNA against VIP17 in MDCK(lac) cells. (Bar = 20 μm.)
In contrast, the basolateral distribution of E-cadherin, an endogenous basolateral protein, was not affected by antisense RNA expression against VIP17 in clone A5A3 (compare Fig. 3 C and D Left). Thus, we conclude that VIP17 is required only for apical surface transport, but not for basolateral delivery in MDCK(lac) cells.
Targeting of Adenoviral GPI-Anchored Membrane Protein Is Affected by VIP17 in MDCK(lac) Cells.
We next analyzed whether the different VIP17 expression levels in filter-grown MDCK(lac) cells affect the cell surface transport of a newly synthesized GPI-anchored protein. We expressed the GPI-anchored YFP (see Material and Methods) in MDCK(lac) cells by using recombinant adenoviruses. GPI-anchored proteins are routed apically in MDCK cells (40, 41), and adenovirally expressed GPI-anchored YFP (YFP-GL-GPI) was localized on the apical membrane in MDCK II cells (data not shown).
After recombinant adenoviral infection of MDCK(lac) cells, the localization of YFP-GL-GPI was analyzed by using confocal microscopy (Fig. 4). Bottom images are optical sections from the apical surface to the bottom of the cells, and the Top images are the lateral view of MDCK(lac) cells. In the control MDCK(lac) cell, GPI-GL-YFP was localized to the apical membrane but was also observed on the basolateral membrane, as was expected from the results of HA transport in this cell line (Fig. 1A). When VIP17(lac) cells (clone 48) were treated with 5 mM IPTG to overexpress epitope-tagged VIP17, we could see increased apical delivery and decreased basolateral targeting of YFP-GL-GPI as compared with the control MDCK(lac) cells (Fig. 4B). However, in Anti(lac) clone A5A3, we could hardly see any apical YFP-GL-GPI after 3-day induction of antisense expression with 5 mM IPTG. Most of the GPI-anchored protein was in the Golgi region below the apical membrane and was mistargeted to the basolateral membrane (Fig. 4C). Taken together, these results show that VIP17 is required for the apical delivery of GPI-anchored proteins in MDCK(lac) cells. The reason that the GPI-anchored protein is mistargeted to the basolateral surface and the other apical proteins gp114 and gp80 are accumulating in the Golgi complex is unclear at present and will require further studies.
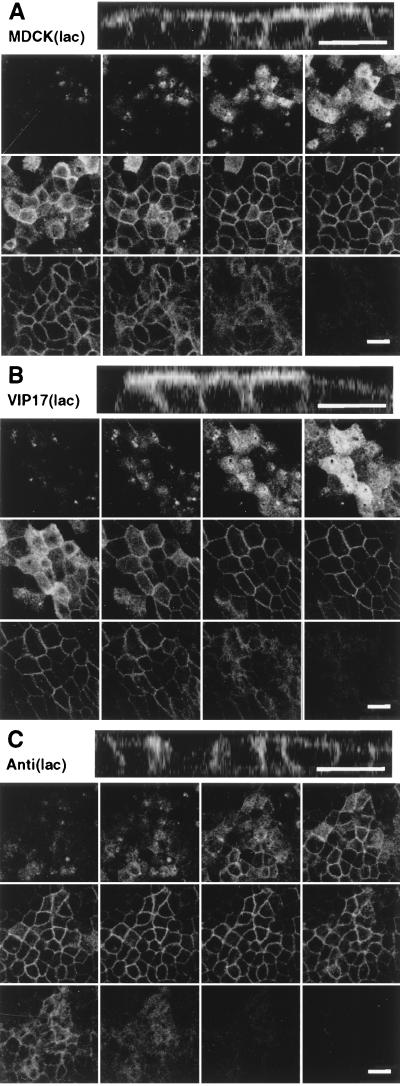
Figure 4.
Localization of a fluorescent GPI -anchored protein in MDCK (lac) cells. Filter-grown MDCK(lac) cells (control cell line, VIP17(lac) clone 48 and Anti(lac) clone A5A3) were induced with 5 mM IPTG for 3 days and then infected with recombinant adenovirus for 1 hr to express YFP-GL-GPI and incubated overnight. Cells were fixed with 4% paraformaldehyde and observed under a Zeiss LSM510 microscope. Twelve optical sections (0.5 μm) along the z axis from the apical cell surface to the base of the cell layer are shown, and the Top image is the x–z view of the same cells. (A) MDCK(lac) cells infected with recombinant adenoviruses to express YFP-GL-GPI. YFP-GL-GPI localized to the apical and basolateral plasma membrane. (B) VIP17(lac) clone 48, induced for 3 days to express epitope-tagged VIP17, was infected with recombinant adenovirus to express YFP-GL-GPI. YFP-GL-GPI shows stronger signal on the apical membrane when compared with control MDCK(lac) cells. (C) MDCK(lac) cells [Anti(lac) clone A5A3], induced to express antisense RNA against VIP17 for 3 days, were infected with recombinant adenovirus to express YFP-GL-GPI. YFP-GL-GPI was mistargeted to the basolateral membrane. (Bar = 20 μm.)
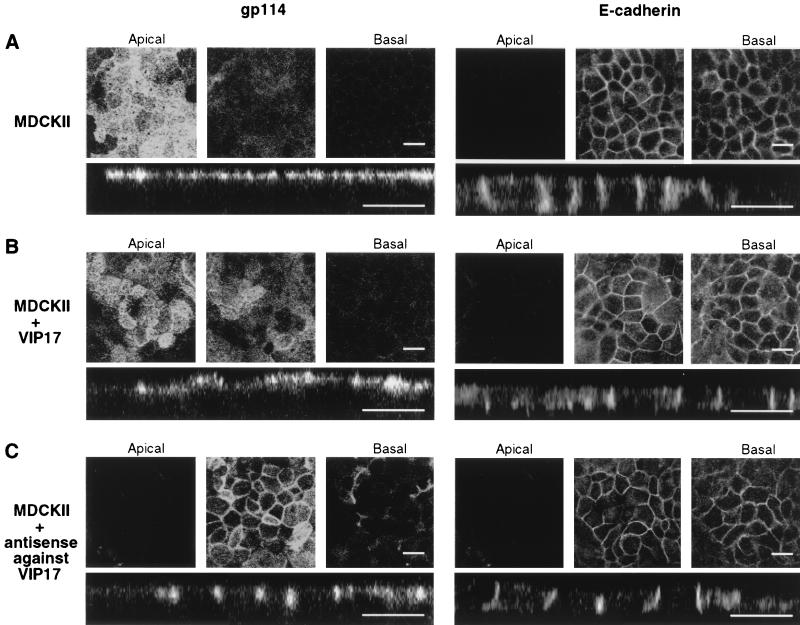
Apical Protein Distribution Is Affected by VIP17 Levels in MDCK II Cells.
Finally, we tested whether VIP17 also regulates apical protein distribution in MDCK II cells as well as in MDCK(lac) cells. For this purpose, we overexpressed VIP17 (Fig. 5B) or antisense RNA against VIP17 (Fig. 5C) by using the adenoviral expression system. We analyzed the localization of apical gp114 and basolateral E-cadherin on the filter-grown MDCK II cells after recombinant adenovirus infection by using confocal immunofluorescence microscopy. The Upper images are optical sections from the apical cell surface to the basal, and the Lower images show the lateral view of MDCK II cells. As shown in Fig. 5A, gp114 was localized to the apical and E-cadherin to basolateral plasma membrane, as expected. In MDCK II cells that had been infected with recombinant adenoviruses to overexpress VIP17 (Fig. 5B), the targeting of gp114 and E-cadherin was not changed, although the height of apical surface seemed to fluctuate as a result of the overexpression of VIP17. More careful analysis suggested that the apical surface was increased in size. In contrast, MDCK II cells infected with recombinant adenoviruses that express antisense RNA against VIP17 showed an apparent basolateral mistargeting of gp114. The localization of E-cadherin was not affected by adenoviral expression of VIP17 or antisense RNA against VIP17 (Fig. 5B). Apical secretory gp80 was accumulated in the Golgi as in Fig. 3 after antisense expression in MDCK II cells (data not shown). In addition, we obtained the same results as those shown in Fig. 4 when VIP17-expressing adenovirus or antisense RNA-expressing adenovirus was coinfected with adenoviral YFP-GL-GPI in MDCK II cells (data not shown). Altogether, these results strongly imply that VIP17 is a component of the general apical machinery required for the apical protein transport in polarized MDCK cells.
Figure 5.
Localization of apical gp114 and basolateral E-cadherin after adenoviral expression of VIP17 or antisense RNA against VIP17 in MDCK II cells. MDCK II cells were grown on filters and infected with recombinant adenoviruses to express VIP17 or antisense RNA against VIP17 for 1 hr and incubated for 3 days. Cells were fixed and processed by using antibodies against gp114 (Left) and E-cadherin (Right) for immunofluorescence microscopy as in Materials and Methods, and then observed under a Zeiss LSM510 microscope. In each set, three optical x–y sections from the apical cell surface to the basal (Upper) and the x–z sections (Lower) are shown. (A) In control MDCK II cells, gp114 was localized to the apical and E-cadherin to basolateral plasma membrane. (B) In cells overexpressing VIP17, the apical surface was enlarged, but the targeting of gp114 and E-cadherin was not changed. (C) In cells expressing antisense RNA against VIP17, gp114 was targeted to the basolateral membrane, whereas the localization of basolateral E-cadherin was unaffected. In some cells of the layer, the lateral cell membranes had changed their appearance. (Bar = 20 μm.)
DISCUSSION
We have in this paper demonstrated an important role for VIP17/MAL in the transport of apical proteins from the TGN to the apical plasma membrane in MDCK cells. This point was addressed by two different strategies aimed at modulating the level of VIP17. One strategy was to use a cell line engineered to allow inducible expression of VIP17 or antisense RNA from the lac promoter (29). The other strategy was to use recombinant adenoviruses that effectively infect MDCK cells (31). Both of these strategies led to the same phenotype in MDCK cells, i.e., impaired apical transport from the Golgi complex. Several different apical marker proteins were used in this study. The apical delivery of all of these proteins was impaired after antisense RNA expression, whereas no effect of the basolateral distribution of E-cadherin could be observed. The list of apical proteins tested include gp80, which is a glycosylated secretory protein (37); a transcytosing protein, gp114, which at steady state is preferentially localized apically (38); influenza virus HA, a palmitoylated transmembrane protein (42); and YFP-GL-GPI, a GPI-anchored protein.
There was a difference in protein transport depending on the cell clones after the induction of antisense RNA against VIP17 in MDCK(lac) cells (Fig. 2). In Anti(lac) clone A1C4, the polarity of the cell was lost, and HA was mistargeted to the basolateral membrane (Fig. 2C). There was little Golgi accumulation of gp114 in this clone (data not shown). In Anti(lac) clone A5A3, surface transport of HA was reduced to both the apical and the basolateral membrane (Fig. 2C), and gp114 was highly accumulated in the Golgi complex (Fig. 3). This might be because of different repression levels of endogenous VIP17 synthesis by antisense expression. We also saw differences in how gp114 and gp80 were handled in MDCKII and MDCK(lac) cells after antisense expression; gp114 was distributed to the basolateral surface in MDCKII cells while it accumulated in the Golgi complex in MDCK Anti(lac) cells.
Previous studies have demonstrated that HA and GPI-anchored placental alkaline phosphatase associate with lipid rafts and that this association is abolished after cholesterol depletion (43, 44). Moreover, cholesterol depletion of MDCK cells led to impaired delivery of HA from the TGN to the apical cell surface (32). The gp114 protein, on the other hand, is solubilized by Triton X-100 extraction at 4°C and is not included in DIGs (P. Verkade and K.S., unpublished data). Nevertheless, immunoelectron microscopic data show that this protein coclusters with PLAP when the latter protein is cross-linked by antibodies (P.Verkade, F. Lafont, and K.S., unpublished data). Thus, the gp114 protein also associates with lipid rafts under these condition (45). The secretory protein gp80 depends on its N-glycans as sorting signals for apical delivery. The putative lectin involved as an apical sorting protein for segregating apical proteins into apical transport carriers has not yet been identified. On the basis of impaired delivery of gp80 after lowering the VIP17/MAL levels by antisense mRNA expression, we predict that this lectin also becomes raft-associated during the formation of the apical transport containers in the TGN. The present data suggest that all of these apical proteins (HA, YFP-GL-GPI, gp114, and gp80) use the same mechanism for apical delivery and that VIP17/MAL is a component of the protein machinery responsible for their apical transport.
Of interest, overexpression of VIP17/MAL cDNA in MDCK cells led to increased apical delivery of newly synthesized HA and of YFP-GL-GPI in MDCK(lac) cells. Thus, VIP17/MAL may be a rate-limiting component of the delivery machinery. It is actually interesting in this respect that when VIP17 levels are low, the proteins are not exported from Golgi (Fig. 3). We also observed that polarized MDCK cell layers changed their morphology by VIP17/MAL overexpression (Fig. 4B and Fig. 5). The apical plasma membrane domain seemed to increase in size. However, these preliminary immunofluorescence data have to be confirmed by careful electron microscopy.
How could VIP17/MAL function in apical protein sorting and delivery? One possibility is that this tetraspan protein is involved in associating individual rafts into larger rafts, which then form the apical transport carriers. Puertollano and Alonso (46) have identified a short peptide sequence, LIRW, located close to the C terminus of the protein, presumably at the cytoplasmic interface of the lipid bilayer. When this peptide sequence is deleted from VIP17/MAL, the protein is no longer included in DIGs (46). Whether this VIP17/MAL mutant is still targeted to apical transport containers and delivered to the apical cell surface is not yet known. The LIRW-peptide motif could be involved in protein–lipid interactions leading to raft association. Alternatively, other protein components of the apical sorting machinery could link VIP17/MAL to lipid rafts.
Other proteins involved in apical sorting and delivery are VIP21/caveolin-1 and annexin XIIIb. VIP21/caveolin-1 has a hydrophobic peptide loop in the lipid bilayer with its N- and C-terminal regions on the cytoplasmic side of the membrane (10). This protein binds cholesterol (12) and forms large homooligomers when incorporated into apical transport carriers (13, 47). In MDCK cells, VIP21/caveolin-1 also forms heterooligomers with caveolin-2 that are routed to the basolateral cell surface where they form caveolae (13). How the VIP21/caveolin-1 homooligomers and the caveolin-1–caveolin-2 heterooligomers become segregated in the TGN into apical and basolateral transport carriers is not known. Obviously, interactions with other apical and basolateral proteins must be responsible. Annexin XIIIb is also involved in the formation of apical transport carriers in the TGN, and this protein is specifically routed to the apical membrane (14). Annexin XIIIb is a myristoylated peripheral membrane protein that associates with the cytoplasmic side of lipid rafts (15).
The list of proteins involved in apical delivery also include microtubule motors that carry the containers from the TGN to the apical surface (16, 17). Recent work hasalso shown that members of proteins of the SNARE family of proteins, syntaxin 3 (18), and TI-VAMP (19), are present in apical transport carriers and that both of these proteins can associate with rafts as judged by the DIG criterion (48). Our recent results also document a role of SNAREs for apical membrane trafficking (48).
All of these putative protein components of the apical sorting machinery must somehow interact to carry out their functions in the formation of apical carriers in the TGN and/or in their release and delivery to the apical membrane. The delivery of apical cargo is known to be regulated by heterotrimeric G proteins. Stimulation of trimeric GαS increases apical transport (49). How this multisubunit machine interacts with lipid rafts and with apical cargo proteins are questions that will have to be elucidated by future research. An interesting feature of this machinery is that several of the known components are integral membrane proteins and must associate with lipids to carry out their functions. This contrasts with the mechanisms operating in coated vesicle formation in which protein–protein interactions regulate their biogenesis (50). For instance, for clathrin-coated vesicles, a peripheral protein scaffold is assembled around the membrane, with clathrin binding to protein adaptors that in turn bind to the cargo proteins that are sorted into the clathrin-coated vesicles (50).
Sphingolipid–cholesterol rafts have to be considered ubiquitous features of the organization of the Golgi and post-Golgi membrane systems (2, 6). Not only epithelial cells, but also neurons (51) and fibroblasts (52) use rafts as platforms for delivery of rafts-associated cargo proteins to the cell surface (53). Most of the protein components of the apical sorting machinery that have been identified in MDCK cells have tissue-specific distributions. Therefore, other proteins will have to perform similar functions in cells lacking these proteins. For instance, VIP17/MAL is a member of an extended family of proteins including the BENE protein and plasmolipin (25, 54). Only further work will tell whether the other family members will be involved in regulating raft traffic as we have shown to be the case for VIP17/MAL.
Acknowledgments
We thank K. Ekroos for cell culture and C. Koch-Brand for antibodies against gp80. We especially thank P. Keller and D. Toomre for their support. The Simons lab helped with stimulating discussions throughout the work. We also thank M. Zerial for critical reading of the manuscript. K.H.C. was supported by the Korea Science And Engineering Foundation (1997 KOSEF) fellowship and is supported by the Alexander von Humboldt fellowship. D.Z. was supported by a Human Capital and Mobility Eueopean Community grant and an Italian Telethon fellowship. This work was supported by a Training and Mobility of Researchers (TMR) network grant of the European Community and by SFB 352 of the Deutsche Forschungsgemeinschaft.
ABBREVIATIONS
- HA
hemagglutinin
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
- DIG
detergent-insoluble glycosphingolipid complex
- IPTG
isopropylthio-β-d-galactoside
- MDCK
Madin-Darby canine kidney
- SNAP23
synaptosomal-associated 23-kDa protein
- SNARE
SNAP receptor
- TI-VAMP
tetanus toxin-insensitive vesicle-associated membrane protein
- VIP
vesicular integral protein
- TGN
trans-Golgi network
- GFP
green fluorescent protein
- GPI
glycophosphatidylinositol
References
- 1.Rodriguez-Boulan E, Powell S K. Annu Rev Cell Biol. 1992;8:395–427. doi: 10.1146/annurev.cb.08.110192.002143. [DOI] [PubMed] [Google Scholar]
- 2.Simons K, Ikonen E. Nature (London) 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 3.Matter K, Mellman I. Curr Opin Cell Biol. 1994;6:545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 4.Ikonen E, Simons K. Semin Cell Dev Biol. 1998;9:503–509. doi: 10.1006/scdb.1998.0258. [DOI] [PubMed] [Google Scholar]
- 5.Wandinger-Ness A, Bennett M K, Antony C, Simons K. J Cell Biol. 1990;111:987–1000. doi: 10.1083/jcb.111.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown D A, London E. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 7.Rietveld A, Simons K. Biochim Biophys Acta. 1998;1376:467–479. doi: 10.1016/s0304-4157(98)00019-7. [DOI] [PubMed] [Google Scholar]
- 8.Friedrichson T, Kurzchalia T V. Nature (London) 1998;394:802–805. doi: 10.1038/29570. [DOI] [PubMed] [Google Scholar]
- 9.Varma R, Mayor S. Nature (London) 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- 10.Dupree P, Parton R G, Raposo G, Kurzchalia T V, Simons K. EMBO J. 1993;12:1597–1605. doi: 10.1002/j.1460-2075.1993.tb05804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurzchalia T V, Dupree P, Parton R G, Kellner R, Virta H, Lehnert M, Simons K. J Cell Biol. 1992;118:1003–1014. doi: 10.1083/jcb.118.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia T V, Simons K. Proc Natl Acad Sci USA. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheiffele P, Verkade P, Fra A M, Virta H, Simons K, Ikonen E. J Cell Biol. 1998;140:795–806. doi: 10.1083/jcb.140.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiedler K, Lafont F, Parton R G, Simons K. J Cell Biol. 1995;128:1043–1053. doi: 10.1083/jcb.128.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lafont F, Lecat S, Verkade P, Simons K. J Cell Biol. 1998;142:1413–1427. doi: 10.1083/jcb.142.6.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lafont F, Burkhardt J K, Simons K. Nature (London) 1994;372:801–803. doi: 10.1038/372801a0. [DOI] [PubMed] [Google Scholar]
- 17.Fath K R, Burgess D R. J Cell Biol. 1993;120:117–127. doi: 10.1083/jcb.120.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Low S H, Chapin S J, Wimmer C, Whiteheart S W, Komuves L G, Mostov K E, Weimbs T. J Cell Biol. 1998;141:1503–1513. doi: 10.1083/jcb.141.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galli T, Zahraoui A, Vaidyanathan V V, Raposo G, Tian J M, Karin M, Niemann H, Louvard D. Mol Biol Cell. 1998;9:1437–1448. doi: 10.1091/mbc.9.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delgrossi M H, Breuza L, Mirre C, Chavrier P, Le Bivic A. J Cell Sci. 1997;110:2207–2214. doi: 10.1242/jcs.110.18.2207. [DOI] [PubMed] [Google Scholar]
- 21.Zacchetti D, Peranen J, Murata M, Fiedler K, Simons K. FEBS Lett. 1995;377:465–469. doi: 10.1016/0014-5793(95)01396-2. [DOI] [PubMed] [Google Scholar]
- 22.Alonso M A, Weissman S M. Proc Natl Acad Sci USA. 1987;84:1997–2001. doi: 10.1073/pnas.84.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim T, Fiedler K, Madison D L, Krueger W H, Pfeiffer S E. J Neurosci Res. 1995;42:413–422. doi: 10.1002/jnr.490420316. [DOI] [PubMed] [Google Scholar]
- 24.Schaeren-Wiemers N, Valenzuela D M, Frank M, Schwab M E. J Neurosci. 1995;15:5753–5764. doi: 10.1523/JNEUROSCI.15-08-05753.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magyar J P, Ebensperger C, Schaeren-Wiemers N, Suter U. Gene. 1997;189:269–275. doi: 10.1016/s0378-1119(96)00861-x. [DOI] [PubMed] [Google Scholar]
- 26.Martin-Belmonte F, Kremer L, Albar J P, Marazuela M, Alonso M A. Endocrinology. 1998;139:2077–2084. doi: 10.1210/endo.139.4.5875. [DOI] [PubMed] [Google Scholar]
- 27.Millan J, Puertollano R, Fan L, Rancano C, Alonso M A. Biochem J. 1997;321:247–252. doi: 10.1042/bj3210247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frank M, van der Haar M E, Schaeren-Wiemers N, Schwab M E. J Neurosci. 1998;18:4901–4913. doi: 10.1523/JNEUROSCI.18-13-04901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarthy K M, Skare I B, Stankewich M C, Furuse M, Tsukita S, Rogers R A, Lynch R D, Schneeberger E E. J Cell Sci. 1996;109:2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- 30.Pimplikar S W, Ikonen E, Simons K. J Cell Biol. 1994;125:1025–1035. doi: 10.1083/jcb.125.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He T C, Zhou S, da Costa L T, Yu J, Kinzler K W, Vogelstein B. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keller P, Simons K. J Cell Biol. 1998;140:1357–1367. doi: 10.1083/jcb.140.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lafont F, Ikonen E, Simons K. Cell Biology: A Laboratory Handbook. Vol. 2. New York: Academic; 1998. pp. 229–238. [Google Scholar]
- 34.Field J, Nikawa J, Broek D, MacDonald B, Rodgers L, Wilson I A, Lerner R A, Wigler M. Mol Cell Biol. 1988;8:2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shevchenko A, Keller P, Scheiffele P, Mann M, Simons K. Electrophoresis. 1997;18:2591–2600. doi: 10.1002/elps.1150181415. [DOI] [PubMed] [Google Scholar]
- 36.Gumbiner B, Simons K. J Cell Biol. 1986;102:457–268. doi: 10.1083/jcb.102.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urban J, Parczyk K, Leutz A, Kayne M, Kondor-Koch C. J Cell Biol. 1987;105:2735–2743. doi: 10.1083/jcb.105.6.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brandli A W, Parton R G, Simons K. J Cell Biol. 1990;111:2909–2921. doi: 10.1083/jcb.111.6.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graichen R, Losch A, Appel D, Koch-Brandt C. J Biol Chem. 1996;271:15854–15857. doi: 10.1074/jbc.271.27.15854. [DOI] [PubMed] [Google Scholar]
- 40.Lisanti M P, Rodriguez-Boulan E. Cell Biol Int Rep. 1991;15:1023–1049. doi: 10.1016/0309-1651(91)90054-m. [DOI] [PubMed] [Google Scholar]
- 41.Brown D A, Rose J K. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 42.Naim H Y, Amarneh B, Ktistakis N T, Roth M G. J Virol. 1992;66:7585–7588. doi: 10.1128/jvi.66.12.7585-7588.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cerneus D P, Ueffing E, Posthuma G, Strous G J, van der Ende A. J Biol Chem. 1993;268:3150–3155. [PubMed] [Google Scholar]
- 44.Scheiffele P, Roth M G, Simons K. EMBO J. 1997;16:5501–5508. doi: 10.1093/emboj/16.18.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harder T, Scheiffele P, Verkade P, Simons K. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puertollano R, Alonso M A. J Biol Chem. 1998;273:12740–12745. doi: 10.1074/jbc.273.21.12740. [DOI] [PubMed] [Google Scholar]
- 47.Monier S, Dietzen D J, Hastings W R, Lublin D M, Kurzchalia T V. FEBS Lett. 1996;388:143–149. doi: 10.1016/0014-5793(96)00519-4. [DOI] [PubMed] [Google Scholar]
- 48.Lafont F, Verkade P, Galli T, Wimmer C, Louvard D, Simons K. Proc Natl Acad Sci USA. 1999;96:3734–3738. doi: 10.1073/pnas.96.7.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pimplikar S W, Simons K. Nature (London) 1993;362:456–458. doi: 10.1038/362456a0. [DOI] [PubMed] [Google Scholar]
- 50.Kirchhausen T, Bonifacino J S, Riezman H. Curr Opin Cell Biol. 1997;9:488–495. doi: 10.1016/s0955-0674(97)80024-5. [DOI] [PubMed] [Google Scholar]
- 51.Ledesma M D, Simons K, Dotti C G. Proc Natl Acad Sci USA. 1998;95:3966–3971. doi: 10.1073/pnas.95.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshimori T, Keller P, Roth M G, Simons K. J Cell Biol. 1996;133:247–256. doi: 10.1083/jcb.133.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keller P, Simons K. J Cell Sci. 1997;110:3001–3009. doi: 10.1242/jcs.110.24.3001. [DOI] [PubMed] [Google Scholar]
- 54.Fischer I, Sapirstein V S. J Biol Chem. 1994;269:24912–24919. [PubMed] [Google Scholar]