Abstract
Traditional Chinese Medicine practitioners consider that chronic fatigue reflects a disharmony and depletion in the supply of qi in the body. Qigong is one of the traditional complementary interventions used to strengthen qi through self-practice, and to manage the state of qi to prevent and cure disease. The aim of this study is to assess whether qigong could be used to manage the symptoms of chronic fatigue. Eighteen Caucasian, British female participants were recruited, taught a qigong routine during weekly classes over 6 months, and asked to practice it daily for 15 min. Participants completed the core set of the RAND Medical Outcomes Study questionnaire (RAND MOS) and a sleep diary during the 2-week baseline control period, and at 3 and 6 months following the start of the trial. The qigong intervention resulted in significant changes in sleep rate score and in the following subscales of the RAND MOS: SF36 Vitality, Sleep Problems, Social Activity, Social Activity Limitation due to Health, Health Distress, Mental Health Index and Psychological Well-being. Qigong seems to improve factors related to chronic fatigue such as sleep, pain, mental attitude and general mobility after 3 and 6 months. Qigong's positive effects indicate that it represents a potentially safe method of treatment for chronic fatigued patients. However, we cannot completely discount the possible influence of placebo effects, and more objective clinical measures are needed to reproduce our findings with long-term follow-up in a randomized, controlled study involving a larger number of subjects.
Keywords: chronic fatigue, sleep disturbance, qigong, quality of life
Introduction
Chronic fatigue syndrome (CFS) consists of a range of symptoms including physical and mental fatigue, headaches, sleep disturbances, concentration difficulties and muscle pain (1). In 2002 it was estimated that 240 000 people in the UK (∼0.4%) were suffering from CFS, with the associated lost working hours, medication costs and medical support costs amounting to around £3.5 billion annually (2).
Common pharmacological treatments include the use of reduced nicotinamide, adenine dinucleotide (NADH) (3), sulbutiamine (4), fludrocortisone (5,6) and antidepressants (7,8), as well as certain pain and sleep medications. Because of the potential for adverse reactions to pharmacologic therapy in CFS patients, complementary and alternative interventions including massage (9), cognitive behavioral therapy (10–12) and osteopathy (13) are used to manage their symptoms. Traditional Chinese medicine considers chronic fatigue to reflect a disharmony and depletion in the supply of qi, with blockage, stagnation, imbalance or change in the pattern or organization of qi resulting in disease (14,15). Disruption to qi manifests in symptoms such as pain, fatigue and mood disturbances. Qigong is one of the traditional complementary interventions used to manage the deregulated state of qi to prevent and cure disease, to improve health and to strengthen qi by self-practice or by receiving qi from a practitioner.
Although neither qi itself nor the mechanism of its effects is understandable or explicable within any paradigm of modern medical science, its effects on the human body are apparent and some studies have been tried to find the underlying mechanism with laboratory experiments (16). Recent in vitro studies show that qi protected mitochondria from oxidative stress and calcium ions may play an important role in this protection (17). Another study suggest the possibility that qi may be beneficial for cancer patients because it suppresses cancer cell growth, and at the same time, it stimulates the patients immune functions (18,19).
Previous clinical research shows that qigong training significantly affects symptoms of asthma (20) and measurable parameters of bodily physiological processes, including levels of superoxide and growth hormones in elderly men (21), reductions in blood pressure and blood lipids in middle-aged adults with hypertension (22), improvement of immunological blood parameters (23) and cellular antigen immunity (24). Considering these effects, qigong can be applied to supportive care for symptoms of CFS such as vitality, sleep problems, social activity limitations due to health problems and psychological well-being. However, few clinical studies have examined the effects of qigong in chronically fatigued patients. Hence, this study aimed to determine whether qigong could help patients with chronic fatigue.
Participants and Methods
Study Design
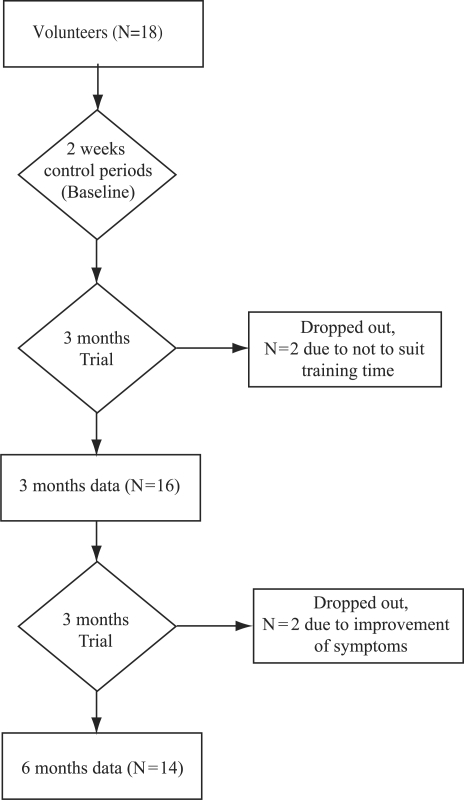
An uncontrolled 6-month trial with three measurement periods (baseline, 3 and 6 months) was conducted (Fig. 1).
Figure 1.
Diagram of study design showing the flow of participants.
Participants
Recruitment of volunteers was from patients that general practitioners (GPs) or practice nurses had identified and referred as suffering from chronic fatigue. Participants were included if they had demonstrated symptoms of fatigue (tiredness with an inability to sleep, inability to concentrate, bodily pain, irritability and emotional problems) over a period of 3 months and could stand unaided for at least 10 min. No attempt was made to analyze the reason for their fatigue. All the participants were visiting a GP regularly and continued to do so for the trial's duration. The study was carried out within a nurse-led GP in Derby, UK between January and October 2004. This practice is unusual in being a (female only) nurse-led practice, and its nursing staff provided all recruitment, support and collected all data. Most patients were female and thus the volunteers recruited were also female. Their ages ranged from 25 to 55. No enquiry was made about whether they were pre- or post-menopausal or about any other factors such as social class, income or marital status because there was an insufficient number of participants to render any meaningful statistical analysis. The Southern Derbyshire Local Research Ethics Committee granted ethical approval (LREC REF: 0309/724). All participants agreed to participate and signed an informed consent form provided by this ethics committee.
Intervention Protocol
Over an initial 2-week period (Fig. 1), participants recorded pre-intervention outcome measures (i.e. sleep diaries and RAND MOS responses) so as to establish a stable baseline prior to the qigong intervention. During this period (and for the duration of the trial) they continued to attend the GP practice and to take their prescribed medication.
Participants were invited to attend a weekly qigong session held at their local GP, when they were taught a 10-min qigong routine (Tai Yi Shen Gong) and were then asked to sit quietly for 5 min with their eyes closed while resting their hands on their knees. A certified qigong master who had trained in qigong for about 20 years led the classes. He had studied qigong and Tai Chi in China at Anhui and Beijing TCM Universities between 1992 and 2006. Tai Yi Shen Gong is a qigong routine comprising sequential arm movements performed slowly in time with steady breathing, such as reaching downward, forward and above the head. Participants repeated the routine at weekly classes over a 3-month period. Following this initial phase, they then practiced the qigong routine daily at home in addition to attending the weekly qigong class for a further 3-month period.
Adverse Events
The instructor monitored participants before, during and after each qigong class for any sign of adverse events.
Outcome Measures
RAND Medical Outcomes Study Questionnaire
Participants completed the core set of the RAND Medical Outcomes Study questionnaire (RAND MOS) (25) during the 2-week control period, and 3 and 6 months following the start of the trial. They completed every question on the questionnaire, and every index therein was calculated. The core set of the RAND MOS is comprised of 116 questions that measured participants’ functioning and well-being in 37 categories of concepts related to the following (25): Physical health (e.g. physical functioning, satisfaction with physical ability, mobility, pain effect, pain severity and role limitations due to physical health), Mental health (e.g. psychological distress/anxiety, depression, psychological well-being, positive effect, feeling of belonging, cognitive functioning and role limitations due to emotional problem), and General health (e.g. energy/fatigue, sleep problems, psychophysiological symptoms, social functioning, role functioning- unable to work, role functioning—unable to do housework, current health perceptions and health distress). The instrument's subscales were scored as summated rating scales on a 0–100 scale, where higher scores indicated better health. The validity and reliability of RAND MOS were examined in detail in a previous study (26).
Sleep Diary
Participants were also asked to keep a subjective sleep diary, which is generally used at UK National Health Service hospitals (27). The following variables were computed from the diary entries: (i) interruptions to sleep, defined as number of times waking up at night; (ii) total sleep time, defined as number of hours actually slept out of the number in bed, and quality of sleep, anchored by: 1—very disrupted, 2—restless, 3—average, 4—sound and 5—very sound (subjectively rated by participants). The diaries were completed for an initial 2 weeks before the trial commenced and then for 1 week each month for the duration of the trial. Participants were given detailed instructions about how to complete the various measures in the diary.
Statistical Analysis
Data from the RAND MOS were converted into RAND indices using recommended procedures, and sleep-diary data were converted into means for each week. Data were analyzed using the intent-to-treat principle. All outcomes were compared using a non-parametric Friedman's test across treatment time (control, 3 and 6 months) for each subscale. Follow-up tests were performed with multiple comparisons of pairs of times (control versus 3 months, control versus 6 months and 3 months versus 6 months) using a Wilcoxon-singed rank test. The SPSS version 11.0 (SPSS, Inc., Chicago, IL, USA) software package was used for all statistical calculations.
Results
Attrition and Compliance
Details of attrition are shown in Fig. 1. Eighteen patients were approached and all agreed to participate. The trial commenced with 18 female volunteers (mean age ± SD, 40.7 ± 9.5 years). Two of them withdrew after the first qigong session because the study's timing did not suit their personal circumstances. The remaining 16 completed the first 3-month phase of the study. In the second 3-month phase, two more participants stopped attending because of improvement in their condition (although they continued to practice qigong at home); hence 14 participants completed the study. After the initial period when two participants stopped attending, all of the remaining participants continued practicing qigong for the 6-month period, and no negative or adverse events were reported. The total attendance rate was 91.88% at the weekly sessions.
Outcome Measures: Qigong Improves Factors Related to Chronic Fatigue
Figure 2 shows the mean ± SD values for subscales of the sleep parameters from the RAND MOS (Fig. 2A) and the sleep diary (Fig. 2B). The qigong intervention resulted in significant changes in the following subscales of the RAND MOS: SF36 Vitality, Sleep Problems, Social Activity, Social Activity Limitation due to Health, Health Distress, Mental Health Index, Psychological Well-being and Psychological Well-being II; whereas Effects of Pain and SF-36 Pain did not change significantly. Significant improvement was also noted in the Sleep Rate score (P < 0.005), but not in the Total Sleep or Total Interruptions scores.
Figure 2.
Mean changes of subscales of (A) RAND Medical Outcomes Study questionnaire (The subscales of the instrument were scored as summated rating scales on a 0–100 scale where higher scores indicated better health. All subscales were statistically significant (P < 0.05) by Friedman's test. *P < 0.05 by Wilcoxon signed rank test compared with control period) and (B) Sleep diary. All results are presented as means ± SD. The numbers of subject were 18, 16 and 14 for baseline, 3 months and 6 months, respectively (Quality of sleep were scored on a 0–5 scores where higher score indicate a good sleep. P < 0.05 by Friedman's test).
The participants differed significantly from the control period after 3 months in SF36 Vitality, Sleep Problems, Social Activity, Social Activity Limitation due to Health, Health Distress, Mental Health Index, Psychological Well-being I, Psychological Well-being II, Pain and SF-36 Pain (all P < 0.05).
Significant differences compared with the control period were also noted after 6 months in SF36 Vitality, Sleep Problems, Social Activity Limitation due to Health, Mental Health Index, Psychological Well-being I, Psychological Well-being II and Pain (P4) (P < 0.05). However, there were no significant differences in sleep parameters compared with the control period.
Discussion
Epidemiological evidence suggests that both a single session of qigong (acute effects) and prolonged qigong over several months (chronic effects) can produce significant and positive changes in psychological characteristics and in the neuroendocrine and immune systems (23,28–31). Our pilot study suggests that regular qigong training reduces pain and improves sleep, vitality and physical functioning in patients with chronic fatigue. This finding is consistent with results from previous studies. Astin et al. (32) reported that 8 weeks of qigong combined with mind–body intervention reduced multiple sclerosis patients’ pain level. Another study also reported qigong's beneficial effects on general health measured with SF-36 in patients with muscular dystrophy (33).
Assuming that qigong is a potentially useful treatment option for chronic fatigue related symptoms, its possible mechanism of action may be of interest. Possible mechanisms include increases in oxygen and decreases in carbon dioxide concentrations in the blood, which may enhance the removal of pain-inducing substances (such as metabolic waste products) from the tissues (34). Qigong may also enhance the circulation of pain-killing substances such as endorphins and other agents that control pain (35). In general, qigong, which consists of steady breathing, slow bodily movements and sitting quietly, affects the muscular systems, resulting in muscular adaptation, and ultimately leads to increased muscle strength and relaxation, if performed regularly. These effects also improve physical and psychological functioning, and therefore beneficially affect fatigue, sleep disturbances, concentration difficulties and muscle pain.
However, this study has several possible limitations. One potential limitation is its use of a small sample and the absence of an active control group. Moreover, other objective outcome measures that are clinically related to symptoms, including the level of muscle strength, other physical activities and instrument-related fatigue and sleep disturbances should be used in addition to the measurements employed in this study. Furthermore, because all the participants were women, we cannot assume that the results are representative across the whole population.
In summary, the present results suggest that qigong can improve factors related to CFS such as sleep, pain, mental attitude and general mobility after 3 and 6 months. Qigong's positive effects indicate that it represents a potentially safe method of treating chronic fatigued patients. However, we cannot completely discount the possibility that the placebo effect during the intervention caused improvements in symptoms related to chronic fatigue. The reproducibility of our findings should also be tested using objective measurements and long-term follow-up in a randomized, controlled study involving a larger number of subjects.
References
- 1.Barnse MP, Ward AB. Oxford Handbook of Rehabilitation Medicine. New York: Oxford University Press; 2005. [Google Scholar]
- 2.Whiting P, Bagnall AM, Sowden AJ, Cornell JE, Mulrow CD, Ramirez G. Interventions for the treatment and management of chronic fatigue syndrome: a systematic review. JAMA. 2001;286:1360–8. doi: 10.1001/jama.286.11.1360. [DOI] [PubMed] [Google Scholar]
- 3.Forsyth LM, Preuss HG, MacDowell AL, Chiazze L, Jr., Birkmayer GD, Bellanti JA. Therapeutic effects of oral NADH on the symptoms of patients with chronic fatigue syndrome. Ann Allergy Asthma Immunol. 1999;82:185–91. doi: 10.1016/S1081-1206(10)62595-1. [DOI] [PubMed] [Google Scholar]
- 4.Tiev KP, Cabane J, Imbert JC. Treatment of chronic postinfectious fatigue: randomized double-blind study of two doses of sulbutiamine (400–600 mg/day) versus placebo. Rev Med Interne. 1999;20:912–8. doi: 10.1016/s0248-8663(00)80096-x. [DOI] [PubMed] [Google Scholar]
- 5.Peterson PK, Pheley A, Schroeppel J, Schenck C, Marshall P, Kind A, et al. A preliminary placebo-controlled crossover trial of fludrocortisone for chronic fatigue syndrome. Arch Intern Med. 1998;158:908–14. doi: 10.1001/archinte.158.8.908. [DOI] [PubMed] [Google Scholar]
- 6.Rowe PC, Calkins H, DeBusk K, McKenzie R, Anand R, Sharma G, et al. Fludrocortisone acetate to treat neurally mediated hypotension in chronic fatigue syndrome: a randomized controlled trial. JAMA. 2001;285:52–9. doi: 10.1001/jama.285.1.52. [DOI] [PubMed] [Google Scholar]
- 7.Natelson BH, Cheu J, Pareja J, Ellis SP, Policastro T, Findley TW. Randomized, double blind, controlled placebo-phase in trial of low dose phenelzine in the chronic fatigue syndrome. Psychopharmacology (Berl) 1996;124:226–30. doi: 10.1007/BF02246661. [DOI] [PubMed] [Google Scholar]
- 8.Vercoulen JH, Swanink CM, Zitman FG, Vreden SG, Hoofs MP, Fennis JF, et al. Randomised, double-blind, placebo-controlled study of fluoxetine in chronic fatigue syndrome. Lancet. 1996;347:858–61. doi: 10.1016/s0140-6736(96)91345-8. [DOI] [PubMed] [Google Scholar]
- 9.Field T, Subshine W, Hernandez-Reif M, Quintino O, Schanberg S, Kuhn C, et al. Massage therapy effects on depression and somatic symptoms in chronic fatigue syndrome. J Chronic Fatigue Syndrome. 1997;3:43–51. [Google Scholar]
- 10.Fulcher KY, White PD. Randomised controlled trial of graded exercise in patients with the chronic fatigue syndrome. Br Med J. 1997;314:1647–52. doi: 10.1136/bmj.314.7095.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powell P, Bentall RP, Nye FJ, Edwards RH. Randomised controlled trial of patient education to encourage graded exercise in chronic fatigue syndrome. Br Med J. 2001;322:387–90. doi: 10.1136/bmj.322.7283.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wearden AJ, Morriss RK, Mullis R, Strickland PL, Pearson DJ, Appleby L, et al. Randomised, double-blind, placebo-controlled treatment trial of fluoxetine and graded exercise for chronic fatigue syndrome. Br J Psychiatry. 1998;172:485–90. doi: 10.1192/bjp.172.6.485. [DOI] [PubMed] [Google Scholar]
- 13.Perrin RN, Edwards J, Hartley P. An evaluation of the effectiveness of osteopathic treatment on symptoms associated with myalgic encephalomyelitis. A preliminary report. J Med Eng Technol. 1998;22:1–13. doi: 10.3109/03091909809009993. [DOI] [PubMed] [Google Scholar]
- 14.Shin MS. Brief view of chi and alternative therapy. Orient Pharm Exp Med. 2002;2:1–16. [Google Scholar]
- 15.Xing C. Chinese Acupuncture and Moxibustion. Beijing, China: Foreign Languages Press; 1987. [Google Scholar]
- 16.Ohnishi ST. Evid Based Complement Alternat Med. 2007. Ki: a key to transform the century of death to the century of life. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohnishi ST, Ohnishi T, Nishino K. Ki-energy (life-energy) protects isolated rat liver mitochondria from oxidative injury. Evid Based Complement Alternat Med. 2006;3:475–82. doi: 10.1093/ecam/nel032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee MS, Huh HJ, Jang HS, Han CS, Ryu H, Chung HT. Effects of emitted Qi on in vitro natural killer cell cytotoxic activity. Am J Chin Med. 2001;29:17–22. doi: 10.1142/S0192415X01000034. [DOI] [PubMed] [Google Scholar]
- 19.Ohnishi ST, Ohnishi T, Nishino K, Tsurusaki Y, Yamaguchi M. Growth inhibition of cultured human liver carcinoma cells by Ki-energy (life-energy): scientific evidence for Ki-effects on cancer cells. Evid Based Complement Alternat Med. 2005;2:387–93. doi: 10.1093/ecam/neh116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reuther I, Aldridge D. Qigong Yangsheng as a complementary therapy in the management of asthma: a single-case appraisal. J Altern Complement Med. 1998;4:173–83. doi: 10.1089/acm.1998.4.173. [DOI] [PubMed] [Google Scholar]
- 21.Lee MS, Ryu H. Qi-training enhances neutrophil function by increasing growth hormone levels in elderly men. Int J Neurosci. 2004;114:1313–22. doi: 10.1080/00207450490476084. [DOI] [PubMed] [Google Scholar]
- 22.Lee MS, Lee MS, Kim HJ, Choi ES. Effects of qigong on blood pressure, high-density lipoprotein cholesterol and other lipid levels in essential hypertension patients. Int J Neurosci. 2004;114:777–86. doi: 10.1080/00207450490441028. [DOI] [PubMed] [Google Scholar]
- 23.Ryu H, Jun CD, Lee BS, Choi BM, Kim HM, Chung HT. Effect of qigong training on proportions of T lymphocyte subsets in human peripheral blood. Am J Chin Med. 1995;23:27–36. doi: 10.1142/S0192415X95000055. [DOI] [PubMed] [Google Scholar]
- 24.Ryu H, Mo HY, Mo GD, Choi BM, Jun CD, Seo CM, et al. Delayed cutaneous hypersensitivity reactions in Qigong (chun do sun bup) trainees by multitest cell mediated immunity. Am J Chin Med. 1995;23:139–44. doi: 10.1142/S0192415X95000183. [DOI] [PubMed] [Google Scholar]
- 25.Hays RD, Sherbourne CD, Mazel RM. RAND Corporation; 1995. User's Manual for the Medical Outcomes Study (MOS) Core Measures of Health-Related Quality of Life. Available at http://www.rand.org/health/surveys_tools/mos/index.html. [Google Scholar]
- 26.McHorney CA, Ware JE, Jr., Rogers W, Raczek AE, Lu JF. The validity and relative precision of MOS short- and long-form health status scales and Dartmouth COOP charts. Results from the Medical Outcomes Study. Med Care. 1992;30:MS253–65. doi: 10.1097/00005650-199205001-00025. [DOI] [PubMed] [Google Scholar]
- 27.East Sussex NHS Trust (Sleep Studies Unit) Sleep diary. Available at: www.esht.nhs.uk/sleep/pdf/sleep_diary.pdf.
- 28.Lee MS, Hong SS, Lim HJ, Kim HJ, Woo WH, Moon SR. Retrospective survey on therapeutic efficacy of Qigong in Korea. Am J Chin Med. 2003;31:809–15. doi: 10.1142/S0192415X03001442. [DOI] [PubMed] [Google Scholar]
- 29.Lee MS, Jeong SM, Oh SW, Ryu H, Chung HT. Effects of chundosunbup Qi-training on psychological adjustments: a cross-sectional study. Am J Chin Med. 1998;26:223–30. doi: 10.1142/S0192415X98000270. [DOI] [PubMed] [Google Scholar]
- 30.Lee MS, Ryu H, Chung HT. Stress management by psychosomatic training: effects of ChunDoSunBup Qi-training on symptoms of stress - cross-sectional study. Stress Med. 2000;16:161–66. [Google Scholar]
- 31.Sancier KM. Medical applications of qigong. Altern Ther Health Med. 1996;2:40–6. [PubMed] [Google Scholar]
- 32.Astin JA, Berman BM, Bausell B, Lee WL, Hochberg M, Forys KL. The efficacy of mindfulness meditation plus Qigong movement therapy in the treatment of fibromyalgia: a randomized controlled trial. J Rheumatol. 2003;30:2257–62. [PubMed] [Google Scholar]
- 33.Wenneberg S, Gunnarsson LG, Ahlstrom G. Using a novel exercise programme for patients with muscular dystrophy. Part II: a quantitative study. Disabil Rehabil. 2004;26:595–602. doi: 10.1080/09638280410001696665. [DOI] [PubMed] [Google Scholar]
- 34.Lee MS, Ryu H, Song J, Moon SR. Effects of Qi-training (Qigong) on forearm blood gas concentrations. Int J Neurosci. 2004;114:1503–10. [PubMed] [Google Scholar]
- 35.Sancier KM, Hole LC. Qigong and neurologic illness. In: Weintraub MI, editor. Alternative and Complementary Treatment in Neurologic Illness. Pennsylvania: Churchill Livingstone; 2001. pp. 197–220. [Google Scholar]