Abstract
Hyperlipidemia/hypercholesteremia are major risk factors for atherosclerosis and cardiovascular diseases. Root of Asparagus racemosus (AR) is widely used in Ayurvedic system of medicine in India and is known for its steroidal saponin content. This study was designed to investigate the hypocholesteremic and antioxidant potential of AR root in both normo- and hypercholesteremic animals. Normal and hypercholesteremic male albino rats were administered with root powder of AR (5 and 10 g% dose levels) along with normal and hypercholesteremic diets, respectively, for a duration of 4 weeks. Plasma and hepatic lipid profiles, fecal sterol, bile acid excretion and hepatic antioxidant activity were assessed. Inclusion of AR root powder in diet, resulted in a dose-dependant reduction in plasma and hepatic lipid profiles, increased fecal excretion of cholesterol, neutral sterol and bile acid along with increases in hepatic HMG-CoA reductase activity and bile acid content in hypercholesteremic rats. Further, AR root also improved the hepatic antioxidant status (catalase, SOD and ascorbic acid levels). No significant changes in lipid and antioxidant profiles occurred in the normocholesteremic rats administered with AR root powder. AR root appeared to be useful as a dietary supplement that offers a protection against hyperlipidemia/hypercholesteremia in hypercholesteremic animals. The results of the present study indicate that the potent therapeutic phyto-components present in AR root i.e. phytosterols, saponins, polyphenols, flavonoids and ascorbic acid, could be responsible for increased bile acid production, elimination of excess cholesterol and elevation of hepatic antioxidant status in hypercholesteremic conditions.
Keywords: antioxidant, Asparagus racemosus, cholesterol metabolism, hypercholesteremia
Introduction
Atherosclerotic cardiovascular disease is rapidly becoming a major cause of death in many societies throughout the world due to changed dietary habits and occupational stress. The hallmark of atherosclerosis is the accumulation of cells containing excessive lipids (i.e. foam cells) within the arterial wall. The major risk factors for the development of atherosclerosis are hypercholesteremia and elevated low-density lipoprotein cholesterol (LDL-C) concentration (1). Persistent hypercholesteremia results from prolonged circulation of lipid-rich lipoproteins that increase oxidative stress leading to oxidative modification of LDL to oxy-LDL (2,3). A number of plants with potent therapeutic components such as fibers, sterols, saponins, polyphenols, flavonoids, etc., have been investigated for their antihyperlipidemic, antioxidant and antiatherosclerotic properties. These compounds are reported to be beneficial with great variation in magnitude and mechanism of action and hence have a potential therapeutic value in combating multifactorial atherosclerotic disorders (4–9). Research in such plant-based diet therapy therefore, has a major role in reducing the risk of atherosclerosis, which can lead to development of effective and better management of hyperlipidemia thereby reducing the risk of cardiovascular diseases.
Asparagus racemosus (AR) willd. (Family: Liliaceae), commonly known as shatavari, satawar or satmuli, is found at low altitudes throughout India. The dried root of the plant is used in Ayurveda as a tonic, galactogogue, aphrodisiac, rejuvenator, antispasmodic, antiulcerous and antiinflammatory and the medicinal/pharmacological value of AR root is attributed to the presence of steroidal saponins and sapogenins (10,11). The root of AR is also used in the treatment of nervous disorders, dyspepsia, diarrhea, dysentery, tumors, hyperdipsia, neuropathy and hepatopathy (11). This plant is reported to have immunostimulant, antihepatotoxic and antioxytocic activities (11,12). Recent reports on AR indicate that the root extracts have antioxidant and antidiarrheal activities in laboratory animals (13,14). The present study was undertaken to investigate the potential of Asparagus root (powder) as a dietary supplement in normalizing the hyperlipidemic/hypercholesteremic and oxidative stress conditions induced by chronic intake of lipid-rich diets with regard to plasma and hepatic lipid profiles, cholesterol metabolism and antioxidant status. Additionally, the effect of Asparagus root was assessed on normocholesteremic animals with a view to investigate and to compare its influence on lipid metabolism in both normal and hyperlipidemic rats.
Methods
Plant Material
Roots of AR were collected from university botanical garden, Sardar Patel University, Vallabh Vidyanagar, Gujrat, India. The roots were washed thoroughly with tap water and dried at 37°C in an incubator, diced into small pieces, powdered in a mixer grinder and used for the experiments. Phytosterol and saponin content of the root were estimated using ferric chloride—sulphuric acid and vanillin-sulphuric acid methods, respectively (15,16). The polyphenol and flavonoid content of the root were analyzed using Folin–Ciocalteu and vanillin-sulphuric acid reagents, respectively (17,18). The total ascorbic acid (TAA) content was estimated using 2,4-dinitrophenyl hydrazine reagent (19) (Table 1).
Table 1.
Phytoanalysis of AR root (Triplicate values: mean ± standard deviation)
| Phytoconstituents mg/g dry tissue | AR root |
|---|---|
| Phytosterols | 7.90 ± 0.90 (0.79%) |
| Saponins | 88.33 ± 1.61 (8.833%) |
| Polyphenols | 16.92 ± 1.10 (1.692%) |
| Flavonoids | 4.76 ± 0.32 (0.476%) |
| Total ascorbic acid | 7.62 ± 0.06 (0.762%) |
Albino Rats
Male albino rats (Charles Foster) weighing 150–200 g were used for the experiment. Rats were housed individually with ad libitum access to water in a well-ventilated animal unit (26 ± 2°C, humidity 62%, 12 h light/dark cycle). Rats were fed standard chow (Pranav Agro-Industries Ltd., Baroda, India). The care and procedures adopted for the present investigation were in accordance with the approval of Institutional Animal Ethics Committee.
Experimental Design
After a 10-day adaptation period, 48 rats were divided into 6 groups of 8 rats each (NC-normal control; NAR-I- normal animals administered with 5 g% AR root powder; NAR-II- normal animals administered with 10 g% AR root powder; HC- hypercholesteremic animals; HAR-I- hypercholesteremic animals administered with 5 g% AR root powder; HAR-II- hypercholesteremic animals administered with 10 g% AR root powder). Hypercholesteremia was induced by addition of 0.5 g% cholesterol and 1.0 g% sodium taurocholate to the basal diet. The basal diet contained carbohydrates (56 g%), proteins (22 g%), fat (4 g%), fiber (4 g%) and mineral mixture (6 g%).
Their body weight and food intake were recorded every third day during the experimental period (4 weeks). Fecal samples of the last 24 h of the experimental period were collected from individual animals from each group (5 g/rat), dried, powdered and extracted for fecal sterols. At end of experiment, animals were deprived of food overnight, sacrificed under light anesthesia and blood was collected immediately by cardiac puncture; plasma was separated by centrifugation. Liver was excised and, both plasma and liver were kept frozen until analyzed.
Biochemical Analysis
Plasma and Hepatic Lipid Profiles
Plasma total lipid (TL) content was estimated by sulphophosphovanillin method (20). Total cholesterol (TC) and triglycerides (TG) were estimated by ferric perchlorate-sulphuric acid and L-α-glycerophosphate oxidase (GPO) methods, respectively (21,22). HDL-cholesterol (HDL-C) was extracted using phosphotungstate-magnesium chloride reagent from plasma (23) and estimated (21). LDL-C, very low-density lipoprotein cholesterol (VLDL-C) and Atherogenic Index (AI) was calculated (24). The liver TL was extracted in chloroform: methanol (2 : 1) (25) and estimated by gravimetric analysis. TC and TG were extracted (25) and estimated (21,22).
Hepatic HMG-CoA Reductase and Bile Acid Profile
Hepatic HMG-CoA reductase (EC 1.1.1.34) activity was measured in terms of the ratio of HMG-CoA to mevalonate (26). Colorimetric assays were carried out for both HMG-CoA and mevalonate using hydroxylamine reagent at alkaline and acidic PH, and the ratio of HMG-CoA to mevalonate determined. It is inversely proportional to the enzyme activity i.e. the increase in ratio corresponds to a decrease in enzyme activity. The alkaline-ethanolic extract of hepatic bile acid was acidified and estimated using vanillin-phosphoric acid reagent (27).
Fecal Cholesterol, Neutral Sterol and Bile Acid Content
The fecal cholesterol, neutral sterols and bile acid were extracted using alkaline methanol medium (28) for fecal cholesterol and neutral sterols estimation (21,29). A portion of the extract was acidified and used for bile acid estimation (27).
Hepatic Antioxidant Profile
Catalase (EC 1.11.1.6) was assayed spectrophotometrically as decomposition of H2O2 at 240 nm (30) and superoxide dismutase (SOD; EC 1.15.1.1) activity was measured using nitrobluetetrazolium reduction method (31). TAA contents were estimated (19).
Statistical Evaluation
Results are expressed as means ± SEM. Significant differences among the groups were determined by one-way ANOVA using 10th version of SPSS with Duncan's test as post hoc analysis. Differences were considered significant if P < 0.05.
Results
Body Weight, Food Intake and Liver Weight
There were no significant differences in final body weights; food intake or liver weights in all AR-treated groups except HAR-II group. This group registered a significant decline in liver weight (P < 0.05, 22%) as compared to HC group (data not presented).
Plasma and Hepatic Lipids
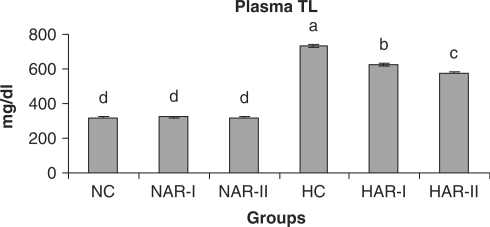
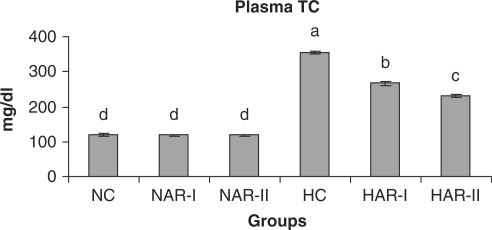
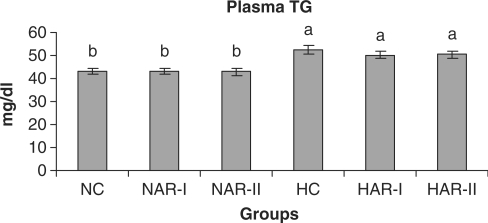
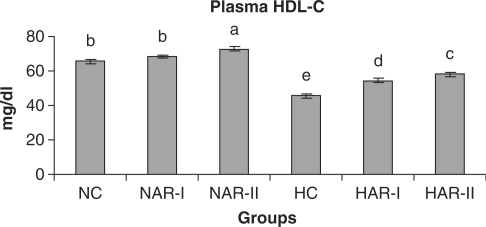
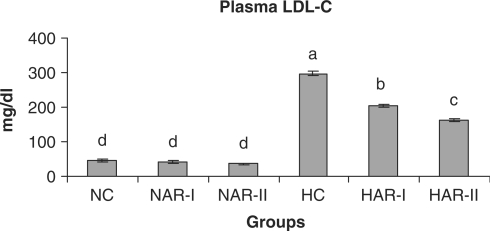
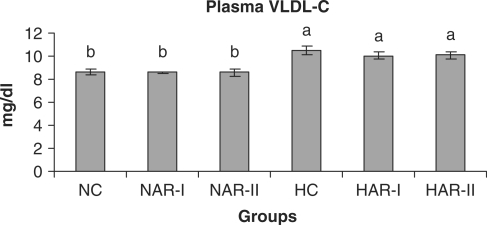
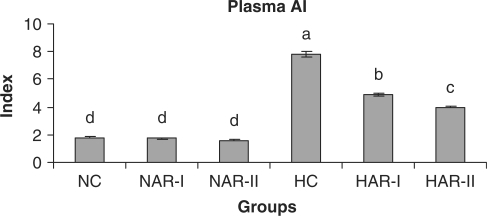
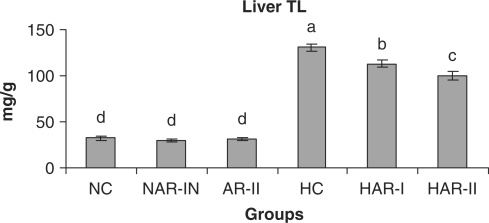
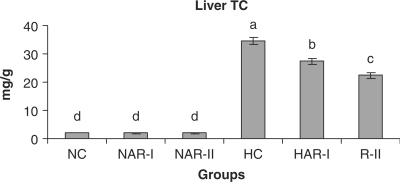
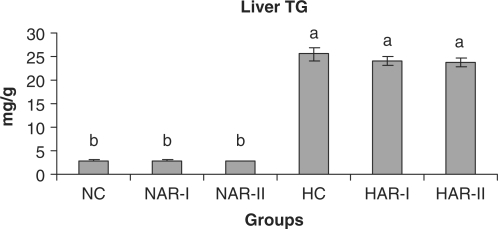
When normal animals administered with AR root powder at 5 and 10 g% dosages, the plasma and hepatic lipid profiles showed no significant differences although NAR-II animals registered a significant increase in plasma HDL-C fraction (P < 0.05, 11%) as compared to NC animals. However, AR administered hypercholesteremic groups (HAR-I and HAR-II) revealed significant decreases (P < 0.05) in TL (14%, 21%), TC (25%, 35%), LDL-C (32%, 45%) and AI (37%, 49%) with increase in HDL-C (P < 0.05, 19%, 27%) as compared to HC group. The TG and VLDL-C content however, remained unaffected in these groups (Figs. 1–7). The HAR-I and HAR-II groups also registered a significant reduction (P < 0.05) in hepatic TL (13%, 24%) and TC (21%, 36%) while the TG level showed no significant change (Figs. 8–10).
Figure 1.
Effect of AR (root powder) feeding on plasma TL levels. Results are mean ± SEM, n = 8. Values with different letters are significantly different from each other (P < 0.05).
Figure 2.
Effect of AR feeding on plasma total cholesterol levels. Results are mean ± SEM, n = 8. Values with different letters are significantly different from each other (P < 0.05).
Figure 3.
Effect of AR feeding on plasma TG levels. Results are mean ± SEM, n = 8. Values with different letters are significantly different from each other (P < 0.05).
Figure 4.
Effect of AR feeding on plasma HDL-C levels. Results are mean ± SEM, n = 8. Values with different letters are significantly different from each other (P < 0.05).
Figure 5.
Effect of AR feeding on plasma LDL-C levels. Results are mean ± SEM, n = 8. Values with different letters are significantly different from each other (P < 0.05).
Figure 6.
Effect of AR feeding on plasma VLDL-C levels. Results are mean ± SEM, n = 8. Values with different letters are significantly different from each other (P < 0.05).
Figure 7.
Effect of AR feeding on plasma AI. Results are mean ± SEM, n = 8. Values with different letters are significantly different from each other (P < 0.05).
Figure 8.
Effect of AR feeding on liver TL levels. Results are mean ± SEM, n = 8. Values with different letters are significantly different from each other (P < 0.05).
Figure 9.
Effect of AR feeding on liver total cholesterol levels. Results are mean ± SEM, n = 8. Values with different letters are significantly different from each other (P < 0.05).
Figure 10.
Effect of AR feeding on liver TG levels. Results are mean ± SEM, n = 8. Values with different letters are significantly different from each other (P < 0.05).
Cholesterol Metabolism
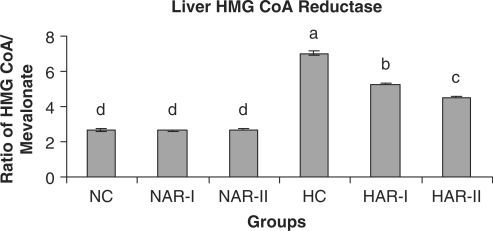
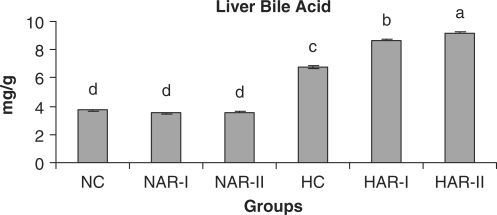
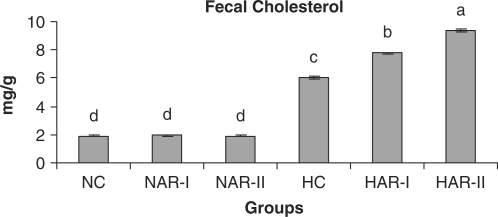
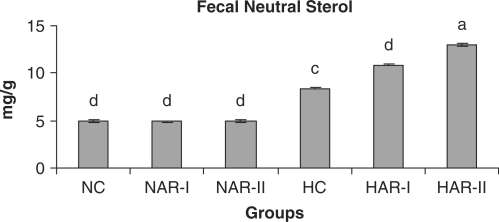
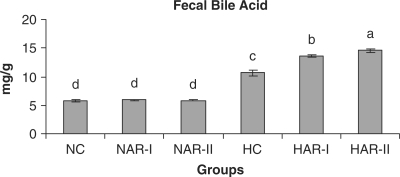
The NAR-I and NAR-II groups revealed no significant changes in hepatic HMG-CoA reductase activity and bile acid production when compared to those of NC animals. The HAR-I and HAR-II groups however, exhibited a significant rise (P < 0.05) in HMG-CoA reductase activity (25%, 35%) and bile acid production (28%, 36%) as compared to HC group (Figs. 11 and 12). There was no significant variation in fecal cholesterol, neutral sterol and bile acid content of NAR-I and NAR-II groups as compared to NC group. However, both HAR-I and HAR-II groups revealed significantly higher (P < 0.05) amounts of cholesterol (29%, 56%), neutral sterol (30%, 55%) and bile acid (28%, 37%) in fecal matter when compared to HC group (Figs. 13–15).
Figure 11.
Effect of AR feeding on liver HMG-CoA reductase activity. Results are mean ± SEM, n = 8. Values with different letters are significantly different from each other (P < 0.05).
Figure 12.
Effect of AR feeding on liver bile acid levels. Results are mean ± SEM, n = 8. Values with different letters are significantly different from each other (P < 0.05).
Figure 13.
Effect of AR feeding on fecal cholesterol levels. Results are mean ± SEM, n = 8. Values with different letters are significantly different from each other (P < 0.05).
Figure 14.
Effect of AR feeding on fecal neutral sterol levels. Results are mean ± SEM, n = 8. Values with different letters are significantly different from each other (P < 0.05).
Figure 15.
Effect of AR feeding on fecal bile acid levels. Results are mean ± SEM, n = 8. Values with different letters are significantly different from each other (P < 0.05).
Antioxidant Status
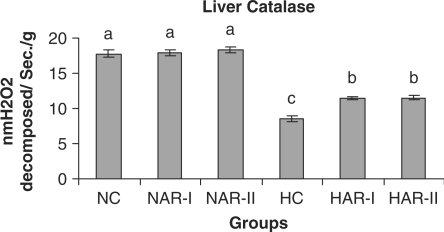
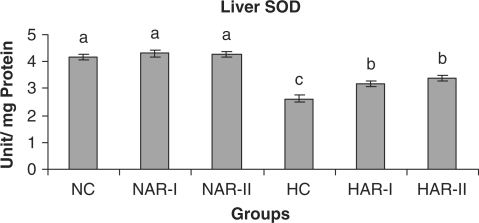
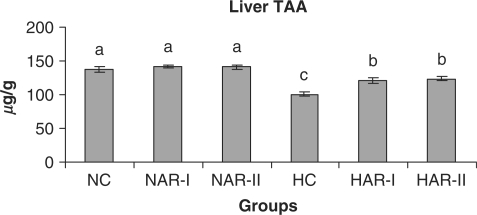
The antioxidant status (activities of catalase, SOD and ascorbic acid content) did not vary significantly in NAR-I and NAR-II groups from that of NC. However, HAR-I and HAR-II groups registered a significant increase (P < 0.05) in catalase (33%, 35%) and SOD (21%, 29%) activities and TAA content (20%, 23%) as compared to HC group (Figs. 16–18).
Figure 16.
Effect of AR feeding on liver catalase activity. Results are mean ± SEM, n = 8. Values with different letters are significantly different from each other (P < 0.05).
Figure 17.
Effect of AR feeding on liver SOD activity. Results are mean ± SEM, n = 8. Values with different letters are significantly different from each other (P < 0.05).
Figure 18.
Effect of AR feeding on liver TAA levels. Results are mean ± SEM, n = 8. Values with different letters are significantly different from each other (P < 0.05).
Discussion
The lipid-lowering effects of AR root in hypercholesteremic rats demonstrated in the present investigation are related primarily to an increased excretion of cholesterol, neutral sterols, bile acid and an increase in hepatic bile acid content. In this context, the phytosterol (0.79 g%) and saponin (8.83 g%) contents of AR root (besides polyphenols, 1.69 g%; flavonoids, 0.47 g%; ascorbic acid, 0.76 g%) could be responsible for increased fecal sterol excretion and decreased cholesterol levels in the hypercholesteremic rats, as on one hand phytosterols are reported to compete and displace cholesterol from the intestinal bile acid micelles and decrease the cholesterol circulation (7,32) and on the other hand, saponins precipitate cholesterol from micelles and interfere with enterohepatic circulation of bile acids making it unavailable for intestinal absorption of cholesterol leading to a reduction in plasma cholesterol levels (8,33).
Although the main component of the Asparagus root is a steroidal saponin, the root also contains alkaloids, flavonoids, sterols, terpenes, tannins, phenolics and mucilage (34). The ethanol extract of the root was shown to contain several steroidal saponins—Shatavarins (sarsasapogenins) of which Shatavarin-I is the major glycosidal component, which was characterized as (25S, 22R)-3-O-β-D-glucopyranosyal- (1→4)-[α-L-rhamnopyranosyal- (1→2)]-β-D-glucopyrano-side-26-O-β-D-glucopyranoside-5β-furostane-3β, 22,26-triol (35). Shatavarin-IV of the root was identified as β-D-glucose- (1→4)-β-D-glucose- (1→)-α-L-rhamnose- (1→2) sarsasapogenin (12). However, no reports are available on biological effects of Shatavarins (sarsasapogenins) with reference to lipid metabolism. Earlier reports on saponins such as steroid glycosides digitonin (36), tomatine (37), sarsasaponin (38), steroid saponins and sapogenins of fenugreek fractions (39) as well as triterpenoid glycosides of alfalfa (40), soya, quillaja and saponaria (38) indicated that they inhibit intestinal cholesterol absorption and reduce plasma cholesterol concentrations in diet-induced hypercholesteremic animal models.
Whereas steroid glycosides such as digitonin and tomatine bind to cholesterol to induce its precipitation in vitro (36,37) and inhibit cholesterol absorption without affecting bile acid absorption in vivo (37), many of the triterpenoid saponins interfere with micelle size and structure (41) and alter bile acid absorption in addition to inhibiting cholesterol absorption (36,41). Besides, saponins also lower the plasma LDL-C levels through an increased turnover of LDL-C to hepatic tissue, which is then converted to bile acid (8). Although saponins are reported to lower TG by inhibiting pancreatic lipase activity (42) and the subsequent decline in VLDL-C levels could be directly correlated to a decline in TG levels (43), the present study does not demonstrate any significant changes in both TG and VLDL-C levels in any groups, the mechanism for which is unclear.
An increased HMG-CoA reductase activity in both HAR-I and HAR-II groups compared to that of HC group appears to constitute a metabolic alteration occurring in hepatic tissue as a response to dietary saponin; such a compensatory increase in hepatic cholesterol synthesis occurs when intestinal cholesterol absorption is impaired or when bile acid synthesis is stimulated (8). However, the normocholesteremic animals (NC) under AR treatment, exhibited no significant variations either in excretion of cholesterol, neutral sterols and bile acid or in hepatic cholesterol and bile acid content. This observation indicates that the effect of AR root on body cholesterol metabolism is strongly influenced by hyperlipidemia and hypercholesteremia. It is well documented that while a low level of HDL-C is indicative of high risk for cardiovascular disease, an increase in HDL-C level could potentially contribute to antiatherogenicity, inhibit LDL oxidation and protect endothelial cells from the cytotoxic effects of oxidized LDL (44,45). In the present context, a significant increase in plasma HDL-C levels with a concurrent decline in plasma cholesterol level and an improvement in AI of HAR-I and HAR-II animals clearly indicate the beneficial role of AR root administration to hypercholesteremic animals.
An increased production of superoxide anions and metabolites and/or a reduced bioavailability of antioxidants causes oxidative damage to cells and tissues. An imbalance between pro-oxidant and antioxidant levels in the body gives rise to cellular oxidative stress that plays an important role in the genesis of cardiovascular diseases (46). Cholesterol- rich diets are known to increase both LDL-C levels and oxidative stress and result in increased oxidized LDL levels leading to atherosclerotic plaque formation (3). Several studies suggest that naturally occurring antioxidants such as polyphenols, flavonoids and vitamin C in diet may a play role as antiatherogenic agents (3,9,47,48). The polyphenols and flavonoids are known to scavenge-free radicals, including hydroxyl and superoxide anions, inhibit lipid peroxidation and improve lipid profiles (47–50). Both these are also known to stimulate catalase and SOD gene transcription and decrease malondialdehyde concentration (51,52). Several lines of evidence suggest that ascorbic acid is a powerful antioxidant in biological systems as an electron donor, readily scavenging the reactive oxygen and nitrogen species to prevent cellular oxidative damage (53) and to reduce genotoxic effects of lipid peroxidation products (54). Further, ascorbic acid can also stimulate SOD activity (55). Wiboonpun et al. (56) identified that roots of AR possess an in vitro antioxidant property against DPPH and the antioxidant compound was identified as racemofuran along with two known compounds—asparagamine A and racemosol. Furan fatty acids possess a radical-scavenging ability and are generated in large quantities in both plants and algae, and when animals consume foods containing these furan fatty acids, they contribute to the body's overall antioxidant activity owing to their affinity to react with peroxyl radicals (57). Additionally, Asparagus root extract possesses in vivo antioxidant activity in rats although the principles responsible for this antioxidant activity were not reported (13). Presently noted higher levels of hepatic catalase and SOD activities in HAR-I and HAR-II groups indicate the possible role of polyphenols and flavonoids of AR root, in modulating the expression of both catalase and SOD activities. While the hypercholesteremia reduced the hepatic ascorbic acid content in HC group, both HAR-I and HAR-II groups registered significantly higher amounts of ascorbic acid in hepatic tissues. Taken together, these observations indicate that AR root powder administration to HC animals can reduce lipid levels in both blood and liver, increase bile acid production and modulate antioxidant enzyme activities. We conclude that AR root administration could ameliorate hyperlipidemic/hypercholesteremic and oxidative stress in hypercholesteremic conditions.
Acknowledgement
The financial assistance in the form of a Research Grant (A.V.R.L.N) and Project Fellow (N.P.V.) from U.G.C., New Delhi, India is gratefully acknowledged.
References
- 1.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg D. Low density lipoprotein oxidation and its pathological significance. J Biol Chem. 1997;272:20963–6. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- 3.Tiwari AK. Natural product antioxidants and their therapeutic potential in mitigating peroxidative modification of lipoproteins and atherosclerosis: recent development. J Med Arom Plant Sci. 1999;21:730–41. [Google Scholar]
- 4.Lindequist U, Niedermeyer THJ, Julich W-D. The pharmacological potential of mushrooms. Evid Based Complement Alternat Med. 2005;2:285–99. doi: 10.1093/ecam/neh107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nozaki K, Hikiami H, Goto H, Nakagawa T, Shibahara N, Shimada Y. Keishibukuryogan (Gui-Zhi-Fu-Ling-Wan), a kampo formula, decreases disease activity and soluble vascular adhesion molecule-1 in patients with rheumatoid arthritis. Evid Based Complement Alternat Med. 2006;3:359–64. doi: 10.1093/ecam/nel025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkatesan N, Devaraj SN, Devaraj H. Increased binding of LDL and VLDL to apo B, E, receptors of hepatic plasma membrane of rats treated with fibernat. Eur J Nutr. 2003;42:262–71. doi: 10.1007/s00394-003-0420-8. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda I, Sugano M. inhibition of cholesterol absorption by plant sterols for mass intervention. Curr Opin Lipidol. 1998;9:527–31. doi: 10.1097/00041433-199812000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Harwood HJ, Jr, Chandler CE, Pellarin LD, Bangerter FW, Wilkins RW, Long CA, et al. Pharmacologic consequences of cholesterol absorption inhibition: alteration in cholesterol metabolism and reduction in plasma cholesterol concentration induced by the synthetic saponin β-tigogenin cellobioside (CP-88818; tiqueside) J Lipid Res. 1993;34:377–95. [PubMed] [Google Scholar]
- 9.Vinson JA, Hu S-J, Jung S, Stanski AM. A citrus extract plus ascorbic acid decreases lipids, lipid peroxidation, lipoprotein oxidation susceptibility, and atherosclerosis in hypercholesterolemic hamsters. J Agric Food Chem. 1998;46:1453–9. [Google Scholar]
- 10.Kapoor LD. Handbook of Ayurvedic Medicinal Plants. New York: Herbal Reference Library Edition, CRC Press; 2001. [Google Scholar]
- 11.Goyal RK, Singh J, Lal H. Asparagus racemosus - an update. Indian J Med Res. 2003;57:408–14. [PubMed] [Google Scholar]
- 12.Ravikumar PR, Soman R, Chetty GL, Pandey RC. Sukh Dev. Chemistry of Ayurvedic crude drugs: part VI–(Shatavari-I). Structure of Shatavarin –IV. Indian J Chem. 1987;26((B)):1012–7. [Google Scholar]
- 13.Bhatnagar M, Sisodiya SS, Bhatnagar R. Antiulcer and antioxidant activity of Asparagus racemosus WILLD and Withania somnifera DUNAL in rats. Ann NY Acad Sci. 2005;1056:261–78. doi: 10.1196/annals.1352.027. [DOI] [PubMed] [Google Scholar]
- 14.Venkatesan N, Thiyagarajan V, Narayanan S, Arul A, Raja S, Vijaya Kumar SG, et al. Anti-diarrhoeal potential of Asparagus racemosus wild root extracts in laboratory animals. J Pharm Pharm Sci. 2005;8:39–45. [PubMed] [Google Scholar]
- 15.Goad LJ, Akihisa T. Analysis of Sterols. 1st. London: Blackie Academic and Professional, an imprint of Chapman and Hall; 1997. [Google Scholar]
- 16.Ebrahimzadeh H, Niknam V. A revised spectrophotometric method for determination of triterpenoid saponins. Indian Drugs. 1998;35:379–81. [Google Scholar]
- 17.Yen G, Hsieh C. Antioxidant activity of extracts from Du-zhong (Eucommia ulmoides) toward various lipid peroxidation models in vitro. J Agric Food Chem. 1998;46:3952–7. [Google Scholar]
- 18.Thimmaiah SK. Standard Methods of Biochemical Analysis. New Delhi: Kalyani Publishers; 1999. [Google Scholar]
- 19.Schaffert RR, Kingsley GR. A rapid, simple method for the determination of reduced, dehydro-, and total ascorbic acid in biological material. J Biol Chem. 1955;212:59–68. [PubMed] [Google Scholar]
- 20.Frings CS, Fendley T, Dunn RT, Owen CA. Improved determination of total serum lipids by sulphosphovanillin reaction. Clin Chem. 1972;18:673–4. [PubMed] [Google Scholar]
- 21.Wybenga DR, Pileggi VJ, Dirstine PH, Di Glorgio J. Direct manual determination of serum total cholesterol with a single stable reagent. Clin Chem. 1970;16:980–4. [PubMed] [Google Scholar]
- 22.Mc Gowan MW, Artiss JD, Strandbergh DR, Zak B. A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin Chem. 1983;29:538–42. [PubMed] [Google Scholar]
- 23.Burstein M, Scholnic HR, Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanion. J Lipid Res. 1970;11:583–95. [PubMed] [Google Scholar]
- 24.Friedewald WT, Levy RT, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 25.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 26.Rao AV, Ramakrishnan S. Indirect assessment of hydroxy-methylglutaryl-CoA reductase (NADPH) activity in liver tissue. Clin Chem. 1975;21:1523–5. [PubMed] [Google Scholar]
- 27.Snell FD, Snell CT. Colorimetric Methods of Analysis. 3rd. Vol. 3. New York: D. Van nostrand company, Inc.; 1953. [Google Scholar]
- 28.Kaiek H-D, Stellaard F, Kruis W, Paumgartner G. Detection of increase bile acid excretion by determination of bile acid content in simple stool samples. Clin Chim Acta. 1984;140:85–90. doi: 10.1016/0009-8981(84)90154-2. [DOI] [PubMed] [Google Scholar]
- 29.Snell FD, Snell CT. Colorimetric Methods of Analysis. 3rd. Vol. 4. New York: D. Van nostrand company, Inc.; 1954. [Google Scholar]
- 30.Aebi HCatalase. Methods of Enzymatic Analysis In: Bergmeyer (ed). 2nd. Vol. 2. New York: Academic Press; 1974. pp. 673–84. [Google Scholar]
- 31.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 32.Quilez J, Garcia-Lorda P, Salas-Salvado J. Potential uses and benefits of phytosterols in diet: present situation and future directions. Clin Nutr. 2003;22:343–51. doi: 10.1016/s0261-5614(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 33.Oakenfull DG, Sidhu GS. Could saponins be a useful treatment for hypercholesterolaemia? Eur J Clin Nutr. 1990;44:79–88. [PubMed] [Google Scholar]
- 34.Gupta AK, Tandon N. Reviews on Indian Medicinal Plants. Vol. 3. New Delhi: Indian Council of Medical Research; 2004. [Google Scholar]
- 35.Joshi J. Sukh Dev. Chemistry of ayurvedic crude drugs: part VIII – Shatavari-2: structure elucidation of bioactive shatavarin-I and other glycosides. Indian J Chem. 1988;27((B)):12–6. [Google Scholar]
- 36.Malinow MR, McLaughlin P, Stafford C. Prevention of hypercholesterolemia in monkeys (Macaca facicularis) by digitonin. Am J Clin Nutr. 1978;31:814–8. doi: 10.1093/ajcn/31.5.814. [DOI] [PubMed] [Google Scholar]
- 37.Cayen MN. Effect of dietary tomatine on cholesterol metabolism in the rat. J Lipid Res. 1971;12:482–90. [PubMed] [Google Scholar]
- 38.Price KR, Johnson IT, Fenwick GR. The chemistry and biological significance of saponins in foods and feedstuffs. CRC Crit Rev Food Sci Nutr. 1987;26:27–135. doi: 10.1080/10408398709527461. [DOI] [PubMed] [Google Scholar]
- 39.Sauvaire Y, Ribes G, Baccou JC, Loubatieeres-Mariani MM. Implication of steroid saponins and sapogenins in the hypochoelsterolemic effect of the fenugreek. Lipids. 1991;26:191–7. doi: 10.1007/BF02543970. [DOI] [PubMed] [Google Scholar]
- 40.Malinow MR. Triterpenoid saponins in mammals: effects on cholesterol metabolism and atherosclerosis. In: Nes WD, Fuller G, Tsai LS, editors. Isopentenoids in Plants, Biochemistry and Function. New York: Marcel Dekker Inc.;; 1984. pp. 229–46. [Google Scholar]
- 41.Sidhu GS, Oakenfull DG. A mechanism for the hypocholesterolaemic activity of saponins. Br J Nutr. 1986;55:643–9. doi: 10.1079/bjn19860070. [DOI] [PubMed] [Google Scholar]
- 42.Han L-K, Zheng Y-N, Xu B-J, Okuda H, Kimura Y. Saponins from Platycodi radix ameliorate high diet-induced obesity in mice. J Nutr. 2002;132:2241–5. doi: 10.1093/jn/132.8.2241. [DOI] [PubMed] [Google Scholar]
- 43.Howell TJ, MacDougall DE, Jones PJH. Phytosterols partially explain differences in cholesterol metabolism caused by corn or olive oil feeding. J Lipid Res. 1998;39:892–900. [PubMed] [Google Scholar]
- 44.Wilson PW, Abbott RD, Castelli WP. High-density lipoprotein cholesterol and mortality. The Framingham heart study. Arterioscler Thromb Vasc Biol. 1988;8:737–41. doi: 10.1161/01.atv.8.6.737. [DOI] [PubMed] [Google Scholar]
- 45.Assmann G, Nofer J. Atheroprotective effects of high-density lipoproteins. Annu Rev Med. 2003;54:321–41. doi: 10.1146/annurev.med.54.101601.152409. [DOI] [PubMed] [Google Scholar]
- 46.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655–73. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 47.Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB. The relative antioxidant activity of plant-derived polyphenolic flavonoids. Free Radical Res. 1995;22:375–83. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 48.Tripathi YB, Singh BK, Pandey RS, Kumar M. BHUx: a patent polyherbal formulation to prevent atherosclerosis. Evid Based Complement Alternat Med. 2005;2:217–21. doi: 10.1093/ecam/neh095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ljubuncic P, Dakwar S, Portnaya I, Cogan U, Azaizeh H, Bomzon A. Aqueous extracts of Teucrium polium possess remarkable antioxidant activity in vitro. Evid Based Complement Alternat Med. 2006;3:329–38. doi: 10.1093/ecam/nel028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Punitha ISR, Rajendran K, Shirwaikar A, Shirwaikar A. Alcoholic stem extract of Coscinium fenestratum regulates carbohydrate metabolism and improves antioxidant status in streptozotocin–nicotinamide induced diabetic rats. Evid Based Complement Alternat Med. 2005;2:375–81. doi: 10.1093/ecam/neh099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toyokuni S, Tanaka T, Kawaguchi W, Fang NR, Ozeki M, Akatsuka S, et al. Effects of the phenolic contents of Mauritian endemic plant extract on promoter activities of antioxidant enzymes. Free Radical Res. 2003;37:1215–24. doi: 10.1080/10715760310001598150. [DOI] [PubMed] [Google Scholar]
- 52.Ranaivo HR, Rakotoarison O, Tesse A, Schott C, Randriantsoa A, Lobstein A, et al. Cedrelopsis grevei induced hypotension and improved endothelial vasodilatation through an increase of Cu/Zn SOD protein expression. Am J Physiol Heart Circ Physiol. 2004;286:H775–81. doi: 10.1152/ajpheart.00584.2003. [DOI] [PubMed] [Google Scholar]
- 53.Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr. 1999;69:1086–107. doi: 10.1093/ajcn/69.6.1086. [DOI] [PubMed] [Google Scholar]
- 54.Sowell J, Frei B, Stevens JF. Vitamin C conjugates of genotoxic lipid peroxidation products: structural characterization and detection in human plasma. Proc Natl Acad Sci USA. 2004;101:17964–9. doi: 10.1073/pnas.0408433102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Chen X, Touyz RM, Park JB, Schiffrin EL. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension. 2001;38:606–11. doi: 10.1161/hy09t1.094005. [DOI] [PubMed] [Google Scholar]
- 56.Wiboonpun N, Phuwapraisirisan P, Tip-pyang S. Identification of antioxidant compound from Asparagus racemosus. Phytother Res. 2004;18:771–3. doi: 10.1002/ptr.1526. [DOI] [PubMed] [Google Scholar]
- 57.Spiteller G. Furan fatty acid: occurrence, synthesis, and reaction. Are furan fatty acids responsible for the cardioprotective effects of a fish diet? Lipids. 2005;40:755–71. doi: 10.1007/s11745-005-1438-5. [DOI] [PubMed] [Google Scholar]