Abstract
The purpose of this study was to investigate the efficacy of four different Japanese and Chinese herbal prescriptions, Ren-Shen-Yang-Rong-Tang (Ninjin’yoeito, NYT), Chai-Hu-Gui-Zhi-Gan-Jiang-Tang (Saikokeishikankyoto, SKKT), Si-Jun-Zi-Tang (Shikunshito, SKT) and Si-Wu-Tang (Shimotsuto, SMT), which are traditionally used for anemia and fatigue, against hematotoxicity in mice treated with 5-fluorouracil (5-FU). NYT 1–100 mg kg–1 day–1 injected orally for 7 consecutive days before and after 5-FU injection significantly suppressed reductions in red blood cell, white blood cell and platelet counts in peripheral blood, and accelerated their recovery. Administration of SKKT also produced a slight but significant improvement in 5-FU-induced erythrocytopenia, whereas SMT and SKT could not prevent anemia. Oral injection of NYT also inhibited 5-FU-induced decreases in peripheral reticulocyte and bone marrow cell counts on day 10, and markedly hastened their recovery on day 20, in a dose-dependent manner. Erythroid progenitor colonies, such as colony forming units-erythroid and burst forming units-erythroid, formed by marrow cells from mice treated with 5-FU were significantly increased by oral administration of NYT. These findings suggest that NYT has the potential to protect against hematotoxicity, and also has hematopoietic activity, through stimulation of immature erythroid progenitor cell differentiation.
Keywords: anemia, erythroid progenitor cells, 5-fluorouracil, Kampo medicines, Ninjin’yoeito
Introduction
Severe anemia that is resistant to medical treatment is often observed in patients with malignancies who are undergoing chemotherapy, and pathogenesis of this anemia is multifactorial (1). A low level of circulating hematopoietic growth factor such as erythropoietin (EPO), shortened survival time of circulating red blood cells (RBCs), and a decrease in the number of immature erythroid cells in bone marrow, probably due to chemotherapy or chronic inflammation, have been demonstrated as causes of anemia (2–5). To cope with chronic anemia, blood transfusion, as well as EPO, have been used clinically in various situations. Clinical trials of EPO in patients undergoing cancer chemotherapy have shown prevention of the development of anemia, although complete recovery has not been found (6). Clibon et al. (7) have reported that EPO cannot sufficiently overcome the reduced hematopoiesis induced by inflammatory cytokine tumor necrosis factor (TNF)-α. Besides hematopoietic growth factors and transfusion, Japanese herbal medicines, such as Juzen-taiho-to, have been used clinically to ameliorate the erythrocytopenia, fatigue or anorexia of patients who are undergoing cancer chemotherapy (8,9). In one clinical trial, Juzen-taiho-to has been reported to enhance peripheral blood counts in cancer patients receiving radiation therapy (8), and the active constituents have been identified as oleic and linoleic acids (9). Recently, an anti-cancer polysaccharide, lentinan, has been shown to stimulate proliferation of immature erythroid progenitors, burst forming units-erythroid (BFU-E), and to improve 5-fluorouracil (5-FU)-induced reduction of the number of BFU-E in mice (10). We have also reported that hot-water extracts from Angelica acutiloba Kitagawa, which is used as herbal medicine for postmenstrual blood loss and EPO-resistant anemia in chronic renal failure, and its main active polysaccharide constituent increase peripheral RBC and reticulocyte counts, as well as BFU-E mix and colony forming unit-erythroids (CFU-E) in cultured marrow cells from mice with 5-FU-induced anemia, and these mechanisms are, in part, due to inhibition of inflammatory cytokine production without EPO expression (11). These results indicate that herbal medicines and plant metabolites that stimulate the proliferation of erythroid progenitors, and that have the potential to recover erythrocytopenia in animal models of anemia, may be useful for ameliorating anemia in clinical trials.
In this experiment, we chose four different kinds of Chinese and Japanese herbal medicines: Ren-Shen-Yang-Rong-Tang (‘Ninjin’yoeito’ in Japanese, NYT), Chai-Hu-Gui-Zhi-Gan-Jiang-Tang (Saikokeishikankyoto, SKKT), Si-Jun-Zi-Tang (Shikunshito, SKT) and Si-Wu-Tang (Shimotsuto, SMT), which are used clinically for postmenstrual blood loss and EPO-resistant anemia, and investigated whether their crude extracts recovered anemia induced by 5-FU in mice. 5-FU exerts its cytotoxic effect mainly by inhibiting thymidylate synthase activity, and this agent has often been used in chemotherapeutic combinations for malignancy. Using a well-established experimental model of 5-FU-induced anemia (10–13), we attempted to compare the therapeutic efficacy of NYT, SMT, SKT and SKKT, and then investigated the pharmacological mechanisms of the effective extracts leading to erythropoiesis.
Methods
Preparation of Kampo Medicines and Hot-Water Extracts
Chopped crude drugs, which were standardized by the Japanese Pharmacopoeia (JP) XV, were purchased from Nakai-kohshindo (Kobe, Japan). Four different Kampo formulas were prepared according to prescriptions for a 1-day dose (14), and are described later (see also Table 1). Each prescription (1-day dose) was decocted in a beaker with 600 ml water by boiling for 30 min over an electric heater (600 W), and filtered through absorbent cotton, followed by concentration in vacuo, and freeze-drying. The yields for individual extracts are described subsequently. NYT (yield, 8.04 g): Rehmannia root (Rehmannia glutinosa, from China) 4.0 g, Japanese Angelica root (Angelica acutiloba, from Japan) 4.0 g, Atractylodes rhizome (Atractylodes ovata, from China) 4.0 g, Poria sclerotium (Poria cocos, from China) 4.0 g, Cinnamon bark (Cinnamomum cassia, from China) 2.5 g, Peony root (Paeonia lactiflora, from Japan) 2.0 g, Polygala root (Polygala tenuifolia, from China) 2.0 g, Citrus unshiu peel (Citrus unshiu, from Japan) 2.0 g, Astragalus root (Astragalus membranaceus, from China) 2.0 g, Ginseng (Panax ginseng, from China) 3.0 g, Glycyrrhiza (Glycyrrhiza uralensis, or Glycyrrhiza glabra, from China) 1.0 g, Schisandra fruit (Schisandra chinensis, from China) 1.0 g. SKKT (yield, 3.50 g): Bupleurum root (Bupleurum falcatum, from China) 6.0 g, Cinnamon bark (Cinnamomum cassia, from China) 3.0 g, Trichosanthes root (Trichosanthes kirilowii, from China) 3.0 g, Scutellaria root (Scutellaria baicalensis, from China) 3.0 g, Oyster Shell (Ostrea gigas, from Japan) 3.0 g, processed ginger (steamed rhizome of Zingiber officinale, from China) 1.0 g, Glycyrrhiza (G. uralensis, or G. glabra, from China) 2.0 g. SKT (yield, 4.54 g): Ginseng (root of Panax ginseng, from China) 4.0 g, Atractylodes rhizome (Atractylodes ovata, from China) 4.0 g, Poria sclerotium (Poria cocos, from China) 4.0 g, Ginger (rhizome of Zingiber officinale, from China) 1.0 g, Jujube (fruit of Zizyphus jujuba, from China) 2.0 g, Glycyrrhiza (root of G. uralensis, or G. glabra, from China) 2.0 g. SMT (yield, 7.11 g): Japanese Angelica root (Angelica acutiloba, from Japan) 4.0 g, Cnidium Rhizome (Cnidium officinale, from Japan) 4.0 g, Peony root (Paeonia lactiflora, from Japan) 4.0 g, Rehmannia root (Rehmannia glutinosa, from China) 4.0 g.
Table 1.
Ingredients of four Kampo formulas used clinically for anemia
| Kampo prescription | Plant name | Part used | Composition (g) |
|---|---|---|---|
| NYT | Angelica acutiloba Kitagawa | Root | 4.0 |
| Ren-Shen-Yang-Rong-Tang | Astragalus membranaceus Fisch. | Root | 4.0 |
| Atractylodes ovata DC. | Rhizome | 2.0 | |
| Cinnamomum cassia Blume.* | Bark | 2.5 | |
| Citrus unshiu Marc. | Peel | 2.0 | |
| Glycyrrhiza uralensis Fisch.* | Root | 1.0 | |
| Paeonia lactiflora Pall. | Root | 2.0 | |
| Panax ginseng C.A. Meyer | Root | 3.0 | |
| Polygala tenuifolia Willd. | Root | 2.0 | |
| Poria cocos (FR.) Wolf. | Sclerotium | 4.0 | |
| Rehmannia glutinosa Lib. var. purpurea Makino | Root | 4.0 | |
| Schisandra chinensis (Turcz.) Baill | Fruit | 1.0 | |
| SKKT | Bupleurum falcatum L. | Root | 6.0 |
| Chai-Hu-Gui-Zhi-Gan-Jiang- | Cinnamomum cassia Blume.* | Bark | 3.0 |
| Tang | Glycyrrhiza uralensis Fisch.* | Root | 2.0 |
| Ostrea gigas | Shell | 3.0 | |
| Scutellaria baicalensis Georgi. | Root | 3.0 | |
| Trichosanthes kirilowii var. japonica | Root | 3.0 | |
| Zingiber officinale Rocs. | Steamed rhizome | 1.0 | |
| SKT | Atractylodes ovata DC. | Rhizome | 4.0 |
| Si-Jun-Zi-Tang | Glycyrrhiza uralensis Fisch. | Root | 2.0 |
| Panax ginseng C.A. Meyer | Root | 4.0 | |
| Poria cocos (FR.) Wolf. | Sclerotium | 4.0 | |
| Zingiber officinale Rocs. | Rhizome | 1.0 | |
| Zizyphus jujube Miller var. inermis Rehder | Fruit | 2.0 | |
| SMT | Angelica acutiloba Kitagawa | Root | 4.0 |
| Si-Wu-Tang | Cnidium officinale Makino | Rhizome | 4.0 |
| Paeonia lactiflora Pall. | Root | 4.0 | |
| Rehmannia glutinosa Lib. var. purpurea Makino | Root | 4.0 |
Composition of crude drug in each prescription indicated as 1-day dosage. Asterisks indicate similarity of ingredients between NYT and SKKT.
Analysis of Kampo Extracts by Photodiode Array (PDA)–High Performance Liquid Chromatography (HPLC)
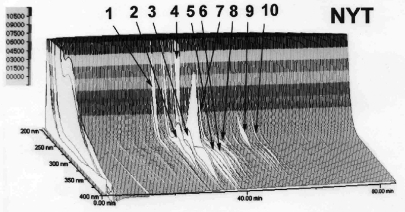
HPLC analysis of the ingredients of NYT extracts was performed using a previously described method (15), with minor modifications. In brief, lyophilized NYT extracts (1.0 g) were individually extracted with 100 ml methanol by ultrasonication for 30 min. Supernatants were removed by centrifugation (450 g) and filtrated through a membrane filter (pore size, 0.45 μm). Then, the filtrate was analyzed by HPLC (Hitachi, Tokyo, Japan) equipped with L-7100 pumps, D-7000 interface, L-7455 PDA detector, L-7200 auto sampler, L-7300 column oven, and Inertsil ODS-2 column (250 × 4.6 mm i.d., 5 μm particle size; GL Sciences, Tokyo, Japan). The solvents used in this analysis were 0.05% (w/w) trifluoroacetic acid and 100% acetonitrile. For detecting ingredients of crude extracts by HPLC, an initial solvent (100% trifluoroacetic acid) was applied for 5 min, and then changed to 100% acetonitrile in a linear gradient manner within 80 min (flow rate, 1.0 ml min–1, column temperature 40°C). Effluents were collected and detected by UV at 200–400 nm. Peak assignment was analyzed using system software, D-7000 HSM (Hitachi). Figure 1 shows the results of 3D-HPLC of NYT.
Figure 1.
PDA–HPLC profile of NYT extracts. Major peaks identified are: 1, paeoniflorin; 2, liquiritin; 3, narirutun; 4, hesperidin; 5, isoliquiritin apioside; 6, isoliquiritin; 7, liquiritigenin; 8, glycyrrhizin; 9, cinnamaldehyde; 10, schizandrin.
Mice
The animals were treated according to the ‘Guiding Principles for the Care and Use of Animals in the Field’ of The Physiological Society of Japan, and the present experiment was approved by the Institute for Experimental Animals, Kanazawa University Advanced Science Research Center, Japan. Female C57BL/6J mice, 8–9 weeks of age, were purchased from Japan SLC (Shizuoka, Japan). Mice were housed in groups of eight in plastic cages with a 12/12 h light/dark cycle and free access to water and mouse chow ad libitum. An adaptation period of at least 1 week for these conditions was allowed before the experiment.
Experimental Protocol
5-FU (Hoffman La Roche–Kyowa, Tokyo, Japan) at 150 mg kg–1 (0.01 ml g–1 body weight) was dissolved in saline and injected intravenously into the tail vein (on day 0) (11). All samples dissolved in distilled water were injected orally at 1–100 mg kg-1 day–1 once a day for 7 consecutive days (days –3 to +3). For the second experiment, the mice treated with 5-FU were subcutaneously injected with 50 ng murine recombinant interleukin-3 [IL-3; dissolved in pyrogen-free PBS containing 0.5% bovine serum albumin (BSA); Genzyme, Boston, MA, USA], simultaneously with 5 U murine recombinant EPO (mrEPO; dissolved in pyrogen-free PBS containing 0.5% BSA; Roche, Mannheim, Germany). The disease control group was treated with vehicle.
Hematological Analysis
Peripheral blood samples were collected on days 0, 5, 10,15 and 20 by the ocular sinus sampling method with an EDTA-coated capillary tube. White blood cells (WBCs), red blood cells (RBCs), platelets and erythrocytic parameters, including hemoglobin (Hb) concentration and hematocrit (Ht), were counted using an automatic hemocytometer (Horiba, MICROS abc, LC-152, Kyoto, Japan) (11,16). Peripheral reticulocytes were enumerated according to a method described by Brecher and Schneiderman (17). That is, freshly prepared methylene blue solution was mixed with an equal volume of peripheral blood and incubated for 15 min at room temperature. Then, blood smears were prepared on glass slides using a cytocentrifuge (Hitachi, Ibaraki, Japan) and stained with Wright solution (Muto Pure Chemicals, Tokyo, Japan). Microscopic observation was performed using an ocular micrometer disk, as described by Brecher and Schneiderman (17).
Marrow Cell Preparation and CFU Assay
Bone marrow was obtained from tibiae and femurs, as described previously (16). Briefly, a cell suspension collected by aspirating the bone with a syringe filled with PBS was washed twice with the same buffer. Marrow cells were counted individually by a hemocytometer, and stored in plastic tubes, on ice, before CFU assays were performed. The marrow cell suspension was then washed twice with α-MEM (Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS), and resuspended in the same medium. CFU-E and BFU-E in femoral bone marrow were analyzed using methylcellulose-based colony assays (11,12,18). Cell suspensions were gently mixed with 0.8% methyl cellulose in α-MEM medium containing 30% FBS, 1% BSA, 200 μM hemin and 100 μM 2-mercapthoethanol. For developing CFU-E (late erythroid progenitors), 1 × 105 cells ml–1 were plated in 35 mm Petri dishes, and stimulated with 2 U mrEPO ml–1 for 2 days. For developing BFU-E mix (myeloid-mixed early erythroid population), the marrow cell suspensions (2 × 105 cells ml–1) were plated in the same dishes, and stimulated with 2 U mrEPO ml–1 and 5 ng IL-3 ml–1 for 9 days. All dishes were incubated at 37°C in a humidified 5% CO2 incubator. After 2 days (for CFU-E) or 9 days (for BFU-E mix), the methylcellulose discs were transferred to glass slides and fixed with glutaraldehyde solution (2% v/v in PBS). Both colonies (BFU-E mix and CFU-E) were enumerated under a microscope. Only colonies consisting of at least 10 cells were counted.
Statistics
Results are expressed as the mean ± SE of at least three independent experiments. Statistical significance was determined by Dunnett's multiple test after one-way analysis of variance (ANOVA), by comparison with a physiologically normal or a disease control group, and P < 0.05 was considered significant.
Results
PDA–HPLC Profile of NYT
As depicted in Fig. 1, PDA–HPLC analysis revealed that broad peaks of chemical constituents from NYT appeared at various retention times. UV and LC-MS/MS analyses of reference compounds indicated the presence of the following known constituents of NYT: paeoniflorin (peak 1) from Peony root, liquiritin (peak 2), isoliquiritin apioside (peak 5), isoliquiritin (peak 6), liquiritigenin (peak 7) and glycyrrhizin (peak 8) from Glycyrrhiza, narirutun (peak 3) and hesperidin (peak 4) from Citrus Unshiu peel, cinnamaldehyde (peak 9) from Cinnamon bark, and schizandrin (peak 10) from Schisandra fruit.
Oral Administration of NYT and SKKT Improved Erythrocytopenia in Mice Treated with 5-FU
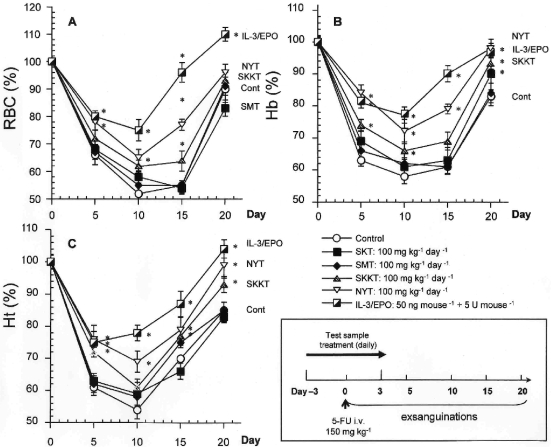
We first examined the kinetics of peripheral RBCs after a higher dose of 5-FU. The experimental protocol is shown in Fig. 2. Peripheral anemia appeared 10 days after intravenous injection of 5-FU 150 mg kg–1. The recovery of RBCs, Ht and Hb began on day 10 and was almost completed by day 20 (Fig. 2). Oral NYT 100 mg kg–1 day–1 for 7 consecutive days significantly improved erythrocytopenia (Fig. 2A) and the reduction in Ht and Hb levels on days 10 and 20, whereas SKT and SMT did not improve anemia in mice treated with 5-FU (Fig. 2B and C). SKKT 100 mg kg–1 day–1 produced a slight improvement in anemia, although to a lesser extent than NYT treatment. Since IL-3 is known to stimulate differentiation of hematopoietic progenitor cells (19,20), and mrIL-3 has been used as a positive control in previous works (16,18), we tested the efficacy of mrIL-3 (50 ng per mouse) in combination with mrEPO (5 U per mouse) on 5-FU-induced anemia. On day 10, the 5-FU-induced anemia decreases in RBCs, Ht; and Hb were strongly suppressed by subcutaneous injection of mrIL-3/mrEPO for 7 consecutive days (Fig. 2). Furthermore, mrIL-3/mrEPO hastened recovery of these erythrocyte parameters on day 20 (Fig. 2). Peripheral RBCs, Ht, and Hb levels in physiological control mice were 9.3 ± 0.2 (×106 cells ml–1), 41.5 ± 0.9%, and 11.6 ± 0.2 g dl–1, respectively. None of the treatments showed any toxic effects in normal mice, as demonstrated by the fact that body and liver weights did not change (data not shown), and the activity of plasma hepatic marker enzyme, alanine aminotransferase (ALT, EC 2.6.1.2), was not elevated (12.1 ± 1.0 U l–1, vehicle once a day for 7 consecutive days, n = 3; 18.0 ± 1.5 U l–1, 100 mg kg–1 day–1 NYT once a day for 7 days, n = 5; 19.9 ± 2.0 U l–1, 100 mg kg–1 day–1 SKKT once a day for 7 days, 16.6 ± 0.2 U l–1, 100 mg kg–1 day–1 SKT once a day for 7 days, n = 5; 15.1 ± 1.1 U l–1, 100 mg kg–1 day–1 SMT once a day for 7 days, n = 5).
Figure 2.
Time course kinetics of RBCs, Hb, and Ht in peripheral blood after 5-FU injection. An experimental protocol is also shown. 5-FU (150 mg kg–1) was injected intravenously into mice on day 0. Peripheral blood samples were collected at the indicated times, and then erythrocyte parameters were measured using an automatic hemocytometer. All tested Kampo samples were orally administered to mice once daily from days –3 to +3 after 5-FU treatment. One group of 5-FU-treated mice was treated subcutaneously with mrIL-3 (50 ng per mouse) and mrEPO (5 U per mouse). Values are presented as the mean ± SE (n = 8–11). *P < 0.05 compared to 5-FU-treated mice.
NYT Suppressed Leukocytopenia and Thrombocytopenia as Well as Reduction of Peripheral Reticulocyte Counts Induced by 5-FU
Since oral NYT was more effective than the other Kampo extracts for improving erythrocytopenia, we next examined the dose-response of the protective activity of NYT against hematotoxicity in anemic mice. 5-FU administered to normal mice reduced not only the erythrocytopenia by 38.1%, but also leukocytopenia and thrombocytopenia by 50.0 and 62.7% on day 10, respectively (Table 2). These reductions were recovered on day 20, and rebound reactions were observed in the case of platelet and leukocyte counts at this time (Table 2). NYT suppressed 5-FU-induced anemia and accelerated the recovery of erythrocytopenia at doses of 1–100 mg kg–1 day–1 in a dose-dependent manner (Table 2). Furthermore, oral NYT also suppressed leukocytopenia in a dose-dependent manner, but did not affect thrombocytopenia at any dose (Table 2). Subcutaneous injection of mrIL-3/mrEPO, as a positive control, markedly improved anemia and thrombocytopenia induced by 5-FU.
Table 2.
Effects of NYT and IL-3 plus EPO on WBC, RBC and platelet counts and hematologic parameters in peripheral blood after 5-FU administration
| Day | Treatment | Dose | WBC | RBC | Ht | Hb | PLT |
|---|---|---|---|---|---|---|---|
| (mg kg–1 per day) | (×103µl−1) | (×106 µl–1) | (%) | (g dl–1) | (×103 µl–1) | ||
| 0 | Normal | – | 3.8 ± 0.1 | 9.7 ± 0.3 | 44.2 ± 0.5 | 14.0 ± 0.1 | 338 ± 6 |
| Control | – | 3.3 ± 0.2 | 9.5 ± 0.2 | 43.8 ± 1.0 | 14.9 ± 0.3 | 407 ± 6 | |
| NYT | 1 | 4.1 ± 0.3 | 9.9 ± 0.1 | 47.1 ± 0.2 | 15.5 ± 0.1 | 467 ± 14 | |
| NYT | 10 | 5.3 ± 0.1* | 10.2 ± 0.2 | 47.1 ± 0.9 | 15.7 ± 0.2 | 520 ± 12 | |
| NYT | 100 | 5.2 ± 0.1 | 10.1 ± 0.1 | 46.7 ± 0.4 | 15.3 ± 0.1 | 432 ± 15 | |
| IL-3/EPO | 50 ng + 5 U | 4.8 ± 0.3# | 10.2 ± 1.3 | 48.7 ± 1.0# | 15.0 ± 0.7 | 555 ± 12# | |
| 10 | Control | – | 1.9 ± 0.2# | 6.0 ± 0.3# | 27.3 ± 1.2# | 9.5 ± 0.1# | 126 ± 9# |
| NYT | 1 | 2.4 ± 0.2 | 6.9 ± 0.2# | 30.3 ± 0.3# | 10.6 ± 0.3# | 109 ± 1 | |
| NYT | 10 | 4.2 ± 0.9# | 7.6 ± 0.3# | 34.0 ± 1.1# | 11.5 ± 0.4# | 137 ± 8# | |
| NYT | 100 | 4.2 ± 0.4# | 8.3 ± 0.1# | 40.4 ± 0.1# | 12.6 ± 0.2# | 146 ± 8# | |
| IL-3/EPO | 50 ng + 5 U | 2.8 ± 0.4# | 8.1 ± 0.2# | 41.1 ± 0.2# | 13.0 ± 0.1# | 163 ± 2# | |
| 20 | Control | – | 14.1 ± 1.7# | 7.3 ± 0.2 | 41.4 ± 0.7 | 13.2 ± 0.5 | 1432 ± 165# |
| NYT | 1 | 12.9 ± 1.2# | 8.8 ± 0.1# | 40.0 ± 0.7 | 12.9 ± 0.1 | 1226 ± 103# | |
| NYT | 10 | 16.9 ± 1.5# | 8.5 ± 0.2# | 40.2 ± 1.0 | 13.2 ± 0.4 | 1386 ± 180 | |
| NYT | 100 | 16.2 ± 1.7# | 9.0 ± 0.3# | 43.5 ± 0.7 | 13.5 ± 0.2 | 1493 ± 188 | |
| IL-3/EPO | 50 ng + 5 U | 13.1 ± 1.6 | 10.1 ± 1.0# | 48.2 ± 0.1# | 15.2 ± 0.1# | 1512 ± 90# |
Mice were injected orally with NYT or subcutaneously with IL-3 (50 ng per mouse) and EPO (5 U per mouse) once daily for 7 consecutive days from 3 days before to 3 days after 5-FU injection. Peripheral blood samples were collected on days 0, 10 and 15, and hematologic parameters were measured by hemocytometer. Data are expressed as the mean±SE of three independent experiments.
#P < 0.05 compared to normal mice. *P < 0.05 compared to 5-FU-treated mice.
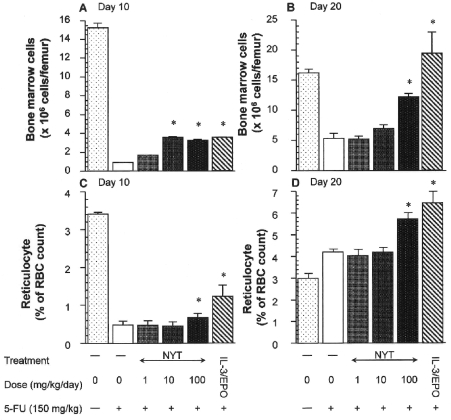
Because peripheral reticulocyte count reflects erythropoiesis (21), we enumerated peripheral reticulocytes and femoral marrow cells in mice treated with NYT after 5-FU injection. The numbers of myelocytes in bone marrow and reticulocytes in peripheral blood were dramatically reduced 10 days after 5-FU injection (Fig. 3A and C). On day 20, the number of marrow cell was still reduced (Fig. 3B), whereas reticulocyte count had recovered (Fig. 3D). Bone marrow seems to be more sensitive than peripheral reticulocytes to 5-FU (Fig. 3). NYT 100 mg kg–1 day–1 significantly protected against the 5-FU-induced cytotoxicity of reticulocytes and myelocytes, and hastened recovery of the number of these hematopoietic cells (Fig. 3B and D). These effects of NYT were more pronounced on day 20 than on day 10. Protective effects against myelotoxicity and hematotoxicity were also observed in mice treated with subcutaneous mrIL-3 and mrEPO (Fig. 3).
Figure 3.
Effect of NYT on the reduced number of bone marrow cells and reticulocytes in mice treated with 5-FU. The number of peripheral reticulocytes and femur marrow cells were determined at 10 and 15 days after 5-FU injection. All tested Kampo samples were administered orally to mice once daily from days –3 to +3 after 5-FU treatment. One group of 5-FU-treated mice was treated subcutaneously with mrIL-3 (50 ng per mouse) and mrEPO (5 U per mouse). Values are presented as the mean ± SE (n = 8–11). *P < 0.05 compared to 5-FU-treated mice.
NYT-Stimulated Immature Erythroid Colony-Formation in Cultured Marrow Cells
Since NYT showed the greatest anti-anemic activity, the effect of oral NYT on the expression of immature erythroid progenitor cells obtained from anemic mice was examined. Table 3 shows the results of the colony-forming assay for IL-3/EPO-induced BFU-E mix and EPO-induced CFU-E. The developing colonies of BFU-E mix and CFU-E in cultured bone marrow cells from normal mice were ∼3200 and 44 000 per femur, respectively. Intravenous 5-FU dramatically reduced their formation by up to 90% on day 5, and only CFU-E formation was completely recovered on day 10 (Table 3). The reduced BFU-E mix was recovered by up to 60% on day 15. NYT 1–100 mg kg–1 day–1 increased development of both colonies in a dose-dependent manner, and recovery of BFU-E mix and CFU-E formation was greater in the NYT-treated mice compared with that in the disease control and normal groups (Table 3). A tendency for their colonies to increase was observed in mice treated with NYT, even at a low dose (1 mg kg–1 day–1, Table 3). Moreover, subcutaneous mrIL-3 (50 ng per mouse) and mrEPO (5 U per mouse) markedly stimulated colony formation of BFU-E mix and CFU-E (Table 3). Oral administration of NYT (Table 3) and other Kampo extracts (data not shown) did not affect marrow cell viabilities at any time points. Although no data are shown, increased BFU-E mix and CFU-E colony formation in mice-treated with NYT (100 mg kg–1 day–1) or mrIL-3/EPO on day 15 (Table 3) reverted to normal levels on day 30.
Table 3.
Effects of NYT and IL-3 plus EPO on formation of CFU-E and BFU-E colonies in cultured marrow cells from mice treated with 5-FU
| Day | Treatment | 5-FU | Dose | Cell viability | CFU-E | BFU-E mix | n |
|---|---|---|---|---|---|---|---|
| (150 mg kg–1) | (mg kg–1 day–1) | (%) | (×103 per femur) | (×102 per femur) | |||
| 5 | Normal | – | – | 95.7 ± 0.6 | 43.6 ± 1.0 | 32.0 ± 0.6 | 5 |
| Control | + | – | 94.8 ± 0.1 | 1.6 ± 0.3# | 0.9 ± 0.1# | 9 | |
| NYT | + | 1 | 93.0 ± 0.1 | 2.2 ± 0.5 | 2.0 ± 0.9* | 10 | |
| NYT | + | 10 | 94.5 ± 0.4 | 3.3 ± 0.5# | 9.6 ± 1.2# | 8 | |
| NYT | + | 100 | 97.6 ± 0.1 | 9.0 ± 0.7# | 11.1 ± 0.5# | 8 | |
| IL-3/EPO | + | 50 ng + 5 U | 96.6 ± 0.3 | 18.6 ± 1.9# | 15.5 ± 2.0# | 8 | |
| 10 | Control | + | – | 93.7 ± 0.8 | 39.0 ± 2.1 | 8.0 ± 0.4# | 7 |
| NYT | + | 1 | 92.2 ± 1.0 | 37.9 ± 4.0 | 8.2 ± 0.1 | 8 | |
| NYT | + | 10 | 96.2 ± 0.4 | 45.2 ± 1.8# | 8.8 ± 0.1 | 8 | |
| NYT | + | 100 | 94.4 ± 0.4 | 57.9 ± 3.3# | 10.0 ± 0.3# | 7 | |
| IL-3/EPO | + | 50 ng + 5 U | 94.9 ± 0.6 | 81.9 ± 9.7# | 13.8 ± 0.3# | 7 | |
| 15 | Control | + | – | 97.4 ± 0.2 | 29.2 ± 1.1# | 20.0 ± 1.4# | 8 |
| NYT | + | 1 | 97.5 ± 1.0 | 32.2 ± 3.0 | 31.2 ± 0.6# | 8 | |
| NYT | + | 10 | 94.5 ± 0.7 | 40.0 ± 3.5# | 36.8 ± 0.3# | 9 | |
| NYT | + | 100 | 95.4 ± 0.6 | 57.9 ± 2.4# | 51.7 ± 2.4# | 8 | |
| IL-3/EPO | + | 50 ng + 5 U | 95.1 ± 0.1 | 77.1 ± 4.7# | 69.1 ± 1.3# | 8 |
Mice were injected orally with NYT or subcutaneously with IL-3 (50 ng per mouse) and EPO (5 U per mouse) for 7 consecutive days before and after 5-FU injection. Marrow cells were collected at 5, 10 and 15 days after 5-FU injection, and cultured with 2 U ml–1 EPO with (for BFU-E mix) or without 5 ng ml–1 IL-3 (for CFU-E). The developing colonies were enumerated 2 (CFU-E) or 9 (BFU-E mix) days after cultivation. Data are expressed as the mean ± SE.
#P < 0.05 compared to normal mice. *P < 0.05 compared to 5-FU-treated mice.
Discussion
To elucidate therapeutic evidences for anti-anemic effect of Kampo medicine, we evaluated the therapeutic effects of four Kampo extracts, traditionally used for anemia and fatigue, on 5-FU-induced anemia. Fujii et al. (22), have previously reported that intraperitoneal injection of NYT 625 μg into BALB/c mice significantly accelerates recovery of erythrocyte count after transplantation of bone marrow cells from syngeneic mice, and this regimen also enhances the total number of CFU-E, BFU-E, and CFU-macrophages in bone marrow and spleen after lethal total body irradiation and syngeneic bone marrow transplantation. However, it is well known that intraperitoneal or intravenous injection of test substances contaminated with endotoxin interferes with experimental results in vivo, because endotoxin is widely distributed in natural materials, and causes non-specific immune responses through monocyte activation (23). Indeed, endotoxin reportedly induces hematopoietic growth factors such as granulocyte–macrophage colony stimulating factor (GM-CSF), G-CSF and M-CSF in monocytes (24,25). Therefore, the net efficacy of NYT in anemia is still unknown. In order to avoid the interference of endotoxin and evaluate the net effects of Kampo extracts on anemia, we used endotoxin-non-responder C57BL/6J mice and oral injection in this study.
Of the Kampo extracts tested in this experiment, oral injection of NYT into C57BL/6J mice protected against myelotoxicity and hastened recovery of anemia (Fig. 2, Table 2). We found that SKKT also significantly prevented erythrocytopenia caused by 5-FU, but its effect was weaker than that of NYT (Fig. 2). These results therefore strongly suggest that NYT and SKKT have net anti-anemic activity. Furthermore, the timing of NYT and SKKT administration with anemic patients is clinically different, which may explain why the protective effect of SKKT is weaker than that of NYT (Fig. 2).
We have recently reported that orally administered hot-water extract of Angelica roots (Angelica acutiloba Kitagawa) and an extract fraction rich in polysaccharide protect against erythrocytopenia, and significantly improve recovery from 5-FU-induced anemia (11). Unexpectedly, SMT, which is mainly composed of Angelica roots (Table 1), could not ameliorate 5-FU-induced anemia, even at a higher dose (500 mg kg–1 day–1, data not shown), in contrast to the effect of NYT (also composed of Angelica roots) (Fig. 2). Although it is unclear why SMT could not prevent erythrocytopenia in this experiment, it could be discussed that the other crude drug in the SMT preparation affects the anti-anemic effect of Angelica root (11). Kiyohara et al. (26) recently reported that combined decoction of all essential herbs in Kampo prescription is important to elicit the combination of ingredients required for expression of the specific activity. Hence, we are now planning to investigate the influence of combination of crude Angelica root-containing drugs on anti-anemic activity in the same model, and to isolate further active constituents from hot-water extracts of NYT. Indeed, Juzentaihoto (Shi-Quan-Da-Bu-Tang in Chinese), whose active substances have been identified as oleic and linoleic acids, is well known to have anti-anemic activity, and to stimulate proliferation of hematopoietic stem cells in vitro and in vivo (9).
NYT has been reported to protect against 5-FU- and cyclophosphamide-induced leukocytopenia at a dose of 1000 mg kg–1 day–1 (for 4 consecutive days), when co-administered with G-CSF (27). In this study, identical results were obtained in 5-FU-treated mice treated with oral NYT at 1–100 mg kg–1 day–1; these doses of NYT were sufficient for recovery from 5-FU-induced leukocytopenia, without co-administration of any other hematopoietic growth factor such as GM-CSF (Table 2). Hence, it is clear that NYT itself has the potential to induce recovery from erythrocytopenia and leukocytopenia in anemic mice.
5-FU has been shown not to damage hematopoietic stem cells directly (28,29), because stem cells rapidly begin proliferation after 5-FU chemotherapy and become sensitive to lineage-specific hematopoietic growth factors (30). Intravenous 5-FU caused a decrease in the RBC, Hb and Ht levels in mice during the experimental period (20 days), with the maximum decrease noted on day 10 (Fig. 2). Estimation of the immature erythroid cells in peripheral blood and bone marrow demonstrated that there was an increase in the number of those cells on days 10 and 20 (Fig. 3). Since CFU-E is less mature than peripheral reticulocytes, the maximum recovery of CFU-E was on day 10 (Table 2) and the recovery of reticulocytes was on day 20 (Fig. 3D). Furthermore, as BFU-E is known to be more immature than CFU-E, BFU-E recovery was expected to occur earlier than that of CFU-E. However, the maximum recovery of BFU-E mix, which contains immature myeloid cells and megakaryocytes, was on day 15 in a CFU assay (Table 3). Our results are in agreement with those of Rich (12), who demonstrated erythropoiesis in mice after 5-FU injection. We found that oral NYT increased the number of marrow cells and reticulocytes, especially on day 20 (Fig. 3B and D). Furthermore, NYT significantly increased the numbers of both CFU-E and BFU-E mix colonies on days 10 and 15 (Table 3). These results suggest that NYT stimulates early differentiation of erythroid lineage cells in bone marrow after 5-FU toxicity, thereby promoting hematopoiesis.
We have recently reported that the detailed mechanisms by which hot-water extracts of Angelica roots increase recovery of erythrocytopenia, and stimulate differentiation of erythroid progenitors without promoting EPO synthesis, may be partly due to inhibiting the secretion of inflammatory cytokines such as TNF-α and interferon (IFN)-γ (11). Besides the therapeutic efficacy of NYT in anemia, it has been reported that NYT suppresses production of TNF-α (31) in cultured rabbit alveolar macrophages, and intraperitoneal NYT in mice also inhibits IFN-γ production in splenocytes co-stimulated with anti-CD3 monoclonal antibody (32). Although we did not measure the expression of EPO mRNA in liver and kidney after NYT treatment, it can be assumed that NYT treatment promotes erythropoiesis, possibly in part, due to inflammatory cytokine production (TNF-α and IFN-γ), the presence of which may suppress the differentiation of erythroid progenitor cells. To clarify the detailed anti-anemic mechanism of NYT (and SKKT), further investigations of the effect of NYT on EPO expression and cytokine production in 5-FU-induced anemia are required.
In summary, the present study demonstrates that oral NYT can ameliorate 5-FU-induced anemia and hasten recovery of erythrocytopenia, by stimulating the expression of early erythroid progenitor cells. Therefore, NYT (and SKKT) has therapeutic and prophylactic potential for erythrocytopenia and leukocytopenia, especially when caused by anti-cancer agents.
Acknowledgement
The authors would like to thank Ms Cui Ming-Yue (MC) of the Graduate School of Natural Science and Technology, Kanazawa University, Japan, for her generous advice.
References
- 1.Griggs JJ. Reducing the toxicity of anticancer therapy: new strategies. Leuk Res. 1998;22(Suppl 1):S27–33. doi: 10.1016/s0145-2126(98)00036-8. [DOI] [PubMed] [Google Scholar]
- 2.DeGowin RL, Gibson DP. Erythropoietin and the anemia of mice bearing extramedullary tumor. J Lab Clin Med. 1979;94:303–11. [PubMed] [Google Scholar]
- 3.Zucker S, Lysik RM, Friedman S. Diminished bone marrow responsiveness to erythropoietin in myelophthisic anemia. Cancer. 1976;37:1308–15. doi: 10.1002/1097-0142(197603)37:3<1308::aid-cncr2820370311>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 4.Casadevall N. Cellular mechanism of resistance to erythropoietin. Nephrol Dial Transplant. 1995;10(Suppl 6):27–30. doi: 10.1093/ndt/10.supp6.27. [DOI] [PubMed] [Google Scholar]
- 5.Means RT, Jr, Dessypris EN, Krantz SB. Inhibition of human erythroid colony-forming units by interleukin-1 is mediated by gamma interferon. J Cell Physiol. 1992;150:59–64. doi: 10.1002/jcp.1041500109. [DOI] [PubMed] [Google Scholar]
- 6.Lundholm K, Daneryd P, Bosaeus I, Korner U, Lindholm E. Palliative nutritional intervention in addition to cyclooxygenase and erythropoietin treatment for patients with malignant disease: effects on survival, metabolism, and function. Cancer. 2004;100:1967–77. doi: 10.1002/cncr.20160. [DOI] [PubMed] [Google Scholar]
- 7.Clibon U, Bonewald L, Caro J, Roodman GD. Erythropoietin fails to reverse the anemia in mice continuously exposed to tumor necrosis factor-alpha in vivo. Exp Hematol. 1990;18:438–41. [PubMed] [Google Scholar]
- 8.Nabeya K, Ri S. Effects of oriental medical herbs on the restoration of the human body before and after operation. Proceedings of the Symposium of Wakan-Yaku. 1983;16:201–8. [Google Scholar]
- 9.Hisha H, Yamada H, Sakurai MH, Kiyohara H, Li Y, Yu C, et al. Isolation and identification of hematopoietic stem cell-stimulating substances from Kampo (Japanese herbal) medicine, Juzen-taiho-to. Blood. 1997;90:1022–30. [PubMed] [Google Scholar]
- 10.Takatsuki F, Miyasaka Y, Kikuchi T, Suzuki M, Hamuro J. Improvement of erythroid toxicity by lentinan and erythropoietin in mice treated with chemotherapeutic agents. Exp Hematol. 1996;24:416–22. [PubMed] [Google Scholar]
- 11.Hatano R, Takano F, Fushiya S, Michimata M, Tanaka T, Kazama I, et al. Water-soluble extracts from Angelica acutiloba Kitagawa enhance hematopoiesis by activating immature erythroid cells in mice with 5-fluorouracil-induced anemia. Exp Hematol. 2004;32:918–24. doi: 10.1016/j.exphem.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Rich IN. The effect of 5-fluorouracil on erythropoiesis. Blood. 1991;77:1164–70. [PubMed] [Google Scholar]
- 13.Weiterova L, Hofer M, Pospisil M, Znojil V, Vacha J, Vacek A, et al. Influence of the joint treatment with granulocyte colony-stimulating factor and drugs elevating extracellular adenosine on erythropoietic recovery following 5-fluorouracil-induced haematotoxicity in mice. Eur J Haematol. 2000;65:310–6. doi: 10.1034/j.1600-0609.2000.065005310.x. [DOI] [PubMed] [Google Scholar]
- 14.Otsuka K, Yakazu D. 1973. “Keiken Kampo-shohoh-bunryo-shu,”. ed. by under supervision of Idohno-nihon-sha, Yokosuka, Japan. [Google Scholar]
- 15.Kobayashi J, Seiwa C, Sakai T, Gotoh M, Komatsu Y, Yamamoto M, et al. Effect of a traditional Chinese herbal medicine, Ren-Shen-Yang-Rong-Tang (Japanese name: Ninjin-Youei-To), on oligodendrocyte precursor cells from aged-rat brain. Int Immunopharmacol. 2003;3:1027–39. doi: 10.1016/S1567-5769(03)00101-2. [DOI] [PubMed] [Google Scholar]
- 16.Takano F, Tanaka T, Aoi J, Yahagi N, Fushiya S. Protective effect of (+)-catechin against 5-fluorouracil-induced myelosuppression in mice. Toxicology. 2004;201:133–42. doi: 10.1016/j.tox.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Brecher G, Schneiderman M. A time-saving device for the counting of reticulocytes. Am J Clin Pathol. 1950;20:1079–83. doi: 10.1093/ajcp/20.11_ts.1079. [DOI] [PubMed] [Google Scholar]
- 18.Takano F, Tanaka T, Tsukamoto E, Yahagi N, Fushiya S. Isolation of (+)-catechin and (-)-epicatechin from Actinidia arguta as bone marrow cell proliferation promoting compounds. Planta Med. 2003;69:321–6. doi: 10.1055/s-2003-38886. [DOI] [PubMed] [Google Scholar]
- 19.Clark SC, Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987;236:1229–37. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- 20.Mangi MH, Newland AC. Interleukin-3 in hematology and oncology: current state of knowledge and future directions. Cytokines Cell Mol Ther. 1999;5:87–95. [PubMed] [Google Scholar]
- 21.Kakiuchi S, Kobayshi M, Satomi Y, Miura D, Kasahara Y, Kondo S. Flow cytometric analysis of erythropoietic abnormality: changes in the cell maturity index of reticulocytes and retic distribution index are useful as indicators of erythropoietic toxicity in non-clinical studies. J Toxicol Sci. 2006;31:111–22. doi: 10.2131/jts.31.111. [DOI] [PubMed] [Google Scholar]
- 22.Fujii Y, Imamura M, Han M, Hashino S, Zhu X, Kobayashi H, et al. Recipient-mediated effect of a traditional Chinese herbal medicine, Ren-shen-yang-rong-tang (Japanese name: Ninjin-youei-to), on hematopoietic recovery following lethal irradiation and syngeneic bone marrow transplantation. Int J Immunopharmacol. 1994;16:615–22. doi: 10.1016/0192-0561(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 23.Chong KT, Huston M. Implications of endotoxin contamination in the evaluation of antibodies to lipopolysaccharides in a murine model of gram-negative sepsis. J Infect Dis. 1987;156:713–9. doi: 10.1093/infdis/156.5.713. [DOI] [PubMed] [Google Scholar]
- 24.Sieff CA, Niemeyer CM, Faller DV. Human colony-stimulating factors and stromal cell function. Soc Gen Physiol Ser. 1988;43:47–55. [PubMed] [Google Scholar]
- 25.Burgess AW, Metcalf D. The nature and action of granulocyte-macrophage colony stimulating factors. Blood. 1980;56:947–58. [PubMed] [Google Scholar]
- 26.Kiyohara H, Matsumoto T, Yamada H. Combination effect of herbs in a multi-herbal formula: expression of Juzen-taiho-to's immuno-modulatory activity on the intestinal immune system. Evid Based Complement Alternat Med. 2004;1:83–91. doi: 10.1093/ecam/neh004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miura S, Takimoto H, Yoshikai Y, Kumazawa Y, Yamada A, Nomoto K. Protective effect of ren-shen-yang-rong-tang (Ninjin-youei-to) in mice with drug-induced leukopenia against Pseudomonas aeruginosa infection. Int J Immunopharmacol. 1992;14:1249–57. doi: 10.1016/0192-0561(92)90061-o. [DOI] [PubMed] [Google Scholar]
- 28.Lerner C, Harrison DE. 5-Fluorouracil spares hemopoietic stem cells responsible for long-term repopulation. Exp Hematol. 1990;18:114–8. [PubMed] [Google Scholar]
- 29.Down JD, Ploemacher RE. Transient and permanent engraftment potential of murine hematopoietic stem cell subsets: differential effects of host conditioning with gamma radiation and cytotoxic drugs. Exp Hematol. 1993;21:913–21. [PubMed] [Google Scholar]
- 30.Harrison DE, Lerner CP. Most primitive hematopoietic stem cells are stimulated to cycle rapidly after treatment with 5-fluorouracil. Blood. 1991;78:1237–40. [PubMed] [Google Scholar]
- 31.Aoki T, Kojima T, Kameda N, Yoshijima S, Ono A, Kobayashi Y. Anti-inflammatory effect of a traditional Chinese medicine, ren-shen-yang-rong-tang (Japanese name: ninjin-youei-to), on alveolar macrophages stimulated by RANTES or TNF-alpha. Arerugi. 1994;43:663–7. [PubMed] [Google Scholar]
- 32.Nakada T, Watanabe K, Jin GB, Triizuk K, Hanawa T. Effect of ninjin-youei-to on Th1/Th2 type cytokine production in different mouse strains. Am J Chin Med. 2002;30:215–23. doi: 10.1142/S0192415X0200034X. [DOI] [PubMed] [Google Scholar]