Abstract
Centella asiatica (CeA) is a creeping herb, growing in moist places in India and other Asian Countries. Ayurvedic system of medicine, an alternate system of medicine in India, uses leaves of CeA for memory enhancement. Here, we have investigated the role of CeA fresh leaf juice treatment during growth spurt period of rats on dendritic morphology of amygdaloid neurons, one of the regions concerned with learning and memory. The present study was conducted on neonatal rat pups. The rat pups (7-days-old) were fed with 2, 4 and 6 ml/kg body of fresh leaf juice of CeA for 2, 4 and 6 weeks. After the treatment period, the rats were killed, brains removed and amygdaloid neurons impregnated with Silver nitrate (Golgi staining). Amygdaloid neurons were traced using camera lucida and dendritic branching points (a measure of dendritic arborization) and intersections (a measure dendritic length) quantified. These data were compared with those of age-matched control rats. The results showed a significant increase in dendritic length (intersections) and dendritic branching points along the length of dendrites of the amygdaloid neurons of rats treated with 4 and 6 ml/kg body weight/day of CeA for longer periods of time (i.e. 4 and 6 weeks). We conclude that constituents/active principles present in CeA fresh leaf juice has neuronal dendritic growth stimulating property; hence it can be used for enhancing neuronal dendrites in stress and other neurodegenerative and memory disorders.
Keywords: amygdaloid neurons, dendritic branches, dendritic intersections, neonatal rat pups, camera lucida
Introduction
Ayurveda, an alternate system of medicine in India, uses a number of plants either individually or in combination with other plants for treatment of a variety of diseases. One such combination of herbal medicines is called ‘Medhya rasayana’, which is known to act on the nervous system and is claimed to improve mental ability. The Medhya rasayana contains mainly the extracts from plants like Centella asiatica (CeA), Acorus calamus, Jatamansi, Bacopa monnieri, etc (1).
CeA is an herb growing in damp places throughout India. It is being used in Ayurvedic preparations either as a whole plant, or leaves in the fresh or in the extract form (1). CeA is useful in improving learning and memory (2–4). It is also used as a brain tonic for promoting brain growth and improving memory. The plant is also used in mentally retarded children to improve general mental ability and in people suffering from cognitive disorders (3,5–7). Though the fresh leaf juice (extract) of CeA has been claimed to improve learning and memory in different clinical studies (2,3,5,6), there is no evidence to show the effect of this plant extract on brain regions involved in learning and memory, namely hippocampus (8–10), limbic cortex and especially amygdala (11–14).
We hypothesize that treatment with fresh leaf juice of CeA will bring about the structural changes in the amygdaloid neurons in young growing rats. The objective of the present study was to investigate the effect of fresh leaf juice of CeA on the dendritic arborization of amygdaloid neurons in rats which are in their growth spurt period.
Methods
Extraction and Administration of Centella asiatica Leaf Juice
The plant, CeA was identified by Prof P. Venugopal Tantry, Department of Botany, Vijaya College, Mulky, Karnataka, India. A voucher specimen number ‘525PP’ has been given and entered in the registry in the Department of Pharmocognosy, Manipal College of Pharmaceutical Sciences, Manipal, India. For the present experiment, we have cultivated the plant in a uniform soil condition in order to maintain the same source of plant throughout the experiment. Fresh, 15–20 days mature leaves of CeA were collected in the morning between 6.30 and 7.00 am. Fresh leaf juice was extracted from these leaves after washing, air-drying and homogenizing in a glass vessel and finally filtered through a sterile gauge cloth. This is to keep consistency with the explanations given in the classic texts of Ayurveda (1), i.e. to conduct the experiment using the fresh leaf juice (extraction) without going for standard (aqueous or alcoholic) extraction. Leaves are extracted maximally, so that from a given weight of leaves (5.0 g), a known volume of juice was extracted (1.63 ± 0.15 ml, n = 6 samples). Since soil water was uniformly maintained, we could extract the same volume of juice from a given weight of leaves on different days. Further, we have established that, the dry weight of a given volume (1 ml) of juice prepared on different days is same (0.0.079 ± 0.01 g, n = 6 samples). The fresh leaf juice so obtained was fed to the rat pups as such, through a gastric tube, a capillary tube attached to a 1 ml hypodermic syringe. Since the volume of juice to be fed to an individual rat pup is very little, its dose was blended with an appropriate volume of saline for convenient feeding. Control rats remained undisturbed in their home cage, and saline control rats were fed with a volume of saline equivalent to volume of extract that their age matched experimental rats received on each day.
Since standard extraction procedures, which involve boiling in water, ethyl alcohol or other organic solvents, may alter the structure of bioactive principles, we have avoided standard extraction protocols. Though there may be minor variation in daily preparations, it will be minimal as leaves of equal maturation are collected from the same place on all days. This minor daily variation will be compensated by a long period (2, 4 or 6 weeks) of treatment. It has been shown in recent literature that a CeA plant extract obtained from ethanol extraction, is different from water extraction in its biological activity (15).
Rats
Neonatal Wistar rat pups (7-days-old) of both sexes, bred and maintained in our central research animal facility are used in this experiment. Since active growth of the brain occurs during growth spurt (preweaning) period (16), the present study was carried out on neonatal rats. The rats were fed with food and water ad libitum and maintained in 12 : 12 h dark and light cycle. Room temperature was kept constant at 25°C. All experiments were carried out with prior approval from the institutional animal ethical committee. In each group, only eight rats were used and handled in a humane way.
Experimental Groups
Rat pups were assigned into 2-, 4- and 6-week treatment groups. Pups in each of these groups were divided into 2, 4 and 6 ml/kg body weight dose group (n = 8 for each dose group). Each rat pup, in the given dosage group, was fed through gastric intubation with a given amount of fresh leaf juice of CeA daily for 2, 4 or 6 weeks. Along with these experimental groups, normal control group (n = 8) and vehicle group (saline, n = 8)) were also maintained.
Rapid Golgi-Staining Procedure (17)
At the end of extract treatment period (2, 4 or 6 weeks), the rats were deeply anesthetized with anesthetics ether and sacrificed; brains were removed rapidly and fixed in a rapid Golgi fixative. Tissue was processed for rapid Golgi staining as detailed earlier (18). In brief, tissues were fixed for 5 days in Golgi fixative, and impregnated with 0.75% aqueous silver nitrate for 48 h. Sledge microtome sections of 120 µ thickness were taken, dehydrated, cleared and mounted with DPX mounting media.
Camera Lucida Tracing
Slides were coded prior to camera lucida tracing and quantification to avoid experimenter's bias. Decoding was done after completion of data collection and analysis. From each rat, 8–10 amygdaloid neurons were traced using camera lucida and their dendritic branching points and dendritic intersections were quantified. Those neurons with minimal overlap of dendrites, heavily impregnated with silver nitrate and without truncate dendrites were selected for tracing.
Quantification of Dendritic Branching Points and Dendritic Intersections (A Measure of Dendritic Length)
Concentric circle method of Sholl (19) was used for dendritic quantification. On a transparent sheet, concentric circles were drawn. The radial distance between two adjacent concentric circles was 20 microns. For dendritic quantification, the sheet with concentric circles was placed on the camera lucida trace of the neuron in such a way that the center of the cell body of the neuron coincided with the center of the concentric circles. The number of branching points between the two successive concentric circles i.e. within each successive 20 μ radial spheres was counted. The dendritic intersection is the point where a dendrite intersects the given concentric circle. Both branching points and intersections were counted up to a radial distance of 100 μ from the center of the soma. Mean number of dendritic branching points in each concentric zone (CZ)/neuron and number of dendritic intersections at each concentric circle/neuron were calculated.
Data Analysis
Data was analyzed using analysis of variance (ANOVA) followed by Bonferroni's post-test using Graph Pad in Stat (GPIS) software, version 1.13. P-values less than or equivalent to 0.05 were considered as significant.
Results
The rats treated with all doses of CeA remained healthy throughout the treatment period. They gained better body weight than that of control and saline-treated rats (Table 1). Moreover, the rats fed with CeA leaf juice were more active than control rats and their learning and memory was significantly better in rats treated with higher doses for longer periods (20).
Table 1.
Showing the initial and final body weight (in grams) of the rats treated with 2, 4 and 6 ml of CeA for 2, 4 and 6 weeks and of age matched control and saline-treated rats
| Body weight(g) |
|||||||
|---|---|---|---|---|---|---|---|
| 2 weeks |
4 weeks |
6 weeks |
|||||
| Groups | n | Initial weight | Final weight | Initial weight | Final weight | Initial weight | Final weight |
| Normal control | 8 | 12.15 ± 1.83 | 25.49±2.68 | 11.92 ± 2.01 | 67.17 ± 6.38 | 13.15 ± 1.18 | 79.72 ± 5.72 |
| Saline control | 8 | 12.53 ± 1.15 | 27.19 ± 5.76 | 12.12 ± 2.05 | 70.2 ± 8.67 | 12.57 ± 1.42 | 82.00 ± 10.16 |
| CeA-2 ml | 8 | 11.98 ± 2.63 | 27.78 ± 6.25 | 12.99 ± 1.03 | 79.54 ± 4.55 | 12.81 ± 1.22 | 98.81 ± 6.4 |
| CeA- 4 ml | 8 | 13.01 ± 0.89 | 46.22 ± 1.66 | 12.56 ± 1.11 | 85.14 ± 5.63 | 11.99 ± 2.03 | 102.11 ± 13.89 |
| CeA- 6 ml | 8 | 12.95 ± 1.73 | 50.55 ± 1.96 | 12.09 ± 1.14 | 97.66 ± 2.56 | 11.98 ± 1.38 | 109.23 ± 8.48 |
Each value represents Mean ± standard deviation. CeA- Centella asiatica, n- number of rats
Amygdaloid Neuronal Dendritic Quantification
The amygdaloid neuronal dendritic analysis showed significant increase in dendritic length and branching in CeA-treated groups, particularly in higher doses (4 and 6 ml/kg CeA) for longer duration (4 and 6 weeks). Figures 1 and 2 illustrate the dendritic arborization of amygdaloid neurons in control and 4 and 6 weeks CeA leaf juice treated rats. There was no difference in dendritic length and branching pattern between the control and saline-treated rats, suggesting that daily handling of the rats (handling stress and vehicle) itself did not alter the dendritic pattern. Since there was no significant difference in the dendritic length and branching, only comparisons between control and experimental groups were subsequently detailed, and in all figures ignoring the vehicle control group data.
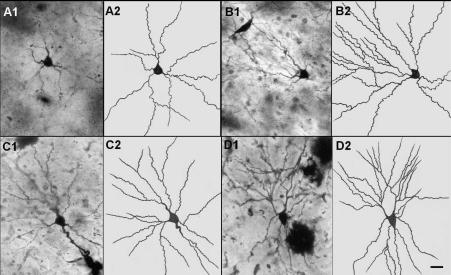
Figure 1.
Representative photomicrographs (A1,B1,C1,D1) and camera lucida tracings(A2,B2,C2,D2) of amygdaloid neurons from control (A1, A2) and treated with 2 ml/kg CeA (B1, B2); 4 ml/kg CeA (C1, C2) and 6 ml/kg CeA (D1, D2) for 6 weeks. Note the significant increase in dendritic length and branches in rats treated with 2, 4 and 6 ml/kg CeA for 6 weeks. Scale bar = 20 µ.
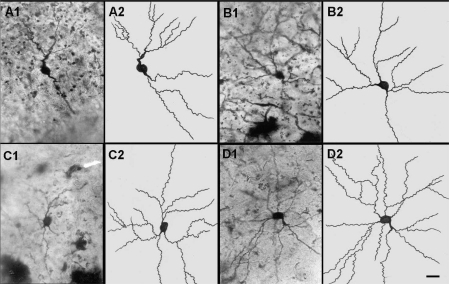
Figure 2.
Representative photomicrographs (A1, B1, C1, D1) and camera lucida tracings (A2, B2, C2, D2) of amygdaloid neurons from control (A1, A2) and 2 ml/kg CeA (B1, B2); 4 ml/kg CeA (C1, C2) and 6 ml/kg CeA (D1, D2) treated for 4 weeks. Note the significant increase in dendritic length and branches in rats treated with 4 and 6 ml/kg CeA for 4 weeks. Scale bar = 20 µ.
Six-Weeks Treatment Group
A significant increase in dendritic intersections and branching points, at different radial distance from soma was observed in rats treated with CeA leaf juice for 6 weeks. Figure 1 illustrates photomicrographs and camera lucida tracings of normal control (A1, A2) and 2, 4,and 6 ml/kg body weight of CeA leaf juice treated (B1, B2, C1, C2, D1, D2, respectively) for 6 weeks. Note that these Golgi-stained (Silver nitrate impregnated) neurons have significantly increased number of dendritic branching points and dendritic length in 2, 4 and 6 ml/kg CeA fresh leaf juice treated rats compared to normal control rats.
Dendritic Intersections
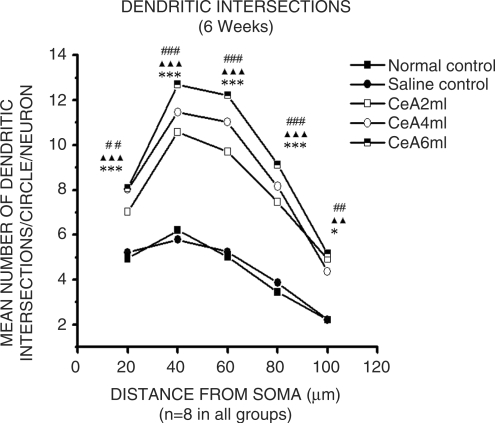
CeA 2, 4 and 6 ml/kg for 6 weeks showed a significant increase in the dendritic intersections at 20 µ (42.94, 62.34 and 63.36% increase), 40 µ (70.48, 84.83 and 104.67% increase), 60 µ (93.22, 119.52 and 143.22% increase), 80 µ (116.86, 137.2 and 165.11% increase) and 100 µ (122.62, 97.28 and 133.48% increase) concentric circles, Fig. 3. Twenty microns concentric circle: 4.94 ± 1.06 in normal control group versus 7.02 ± 0.82 in CeA 2 ml group, P < 0.01; 8.02 ± 0.8 in CeA 4 ml group, P < 0.001 and 8.07 ± 1.02 in CeA 6 ml group, P < 0.001. Forty microns concentric circle: 6.2 ± 1.68 in normal control group versus 10.57 ± 1.02 in CeA 2 ml group, P < 0.001; 11.46 ± 1.09 in CeA 4 ml group, P < 0.001 and 12.69 ± 1.92 in CeA 6 ml group, P < 0.001. Sixty microns concentric circle: 5.02 ± 1.18 in normal control group versus 9.7 ± 1.05 in CeA 2ml group, P < 0.001; 11.02 ± 1.12 in CeA 4 ml group, P < 0.001 and 12.21 ± 2.19 in CeA 6 ml group, P < 0.001. Eighty microns concentric circle: 3.44 ± 0.77 in normal control group versus 7.46 ± 1.55 in CeA 2 ml group, P < 0.001; 8.16 ± 1.42 in CeA 4 ml group, P < 0.001 and 9.12 ± 2.18 in CeA 6 ml group, P < 0.001. Hundred microns concentric circle: 2.21 ± 0.58 in normal control group versus 4.92 ± 1.61 in CeA 2 ml group, P < 0.01; 4.36 ± 1.63 in CeA 4 ml, P < 0.05 and 5.16 ± 1.79 in CeA 6 ml group, P < 0.01.
Figure 3.
Dendritic intersections of amygdaloid neurons in rats treated with 2, 4 and 6 ml/kg body weight of CeA for 6 weeks, and age matched control and saline-treated rats. Each point represents mean of 8–10 neurons from each rat (standard deviation not shown). F-value: 21.3, 43.28, 50.57, 25.75 and 10.98 at 20, 40, 60, 80, 100 µ distance from the soma, respectively. Note there is a significant increase in the dendritic intersections in rats treated with 2, 4 and 6 ml/kg CeA. NC versus 2 ml/kg CeA: ##P < 0.01, ###P < 0.001; NC versus 4 ml/kg CeA: *P < 0.05, ***P < 0.001; NC versus 6 ml/kg CeA: ▴▴P < 0.01, ▴▴▴P < 0.001 (One way ANOVA, Bonferroni's test).
Dendritic Branching Points
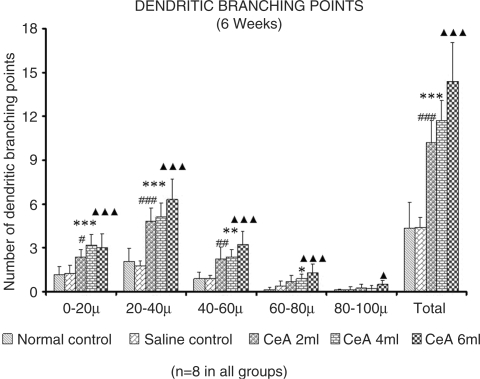
CeA 2, 4 and 6 ml/kg for 6 weeks, showed a significant increase in dendritic branching points Fig. 4. First (0–20 µ) zone: 1.16 ± 0.57 in normal control group versus 2.38 ± 0.5 in CeA 2 ml group (105.17% increase), P < 0.05; 3.19 ± 0.74 in CeA 4 ml group (175% increase), P < 0.001 and 3.02 ± 0.94 in CeA 6ml group (160.34% increase), P < 0.001. Second (20–40 µ) zone: 2.05 ± 0.93 in normal control group versus 4.81 ± 0.91 in CeA 2 ml group (134.63% increase), P < 0.001; 5.09 ± 0.98 in CeA 4 ml group (148.29% increase), P < 0.001 and 6.31 ± 1.41 in CeA 6ml group (207.8% increase), P < 0.001. Third (40–60 µ) zone: (0.89 ± 0.45 in normal control group versus 2.25 ± 0.81 in CeA 2 ml group (152.8% increase), P < 0.05; 2.36 ± 0.51 in CeA 4 ml group (165.16% increase), P < 0.01 and 3.22 ± 0.91 in CeA 6ml group (261.79% increase), P < 0.001. Fourth (60—80 µ) zone: 0.15 ± 0.13 in normal control group versus 0.88 ± 0.32 in CeA 4 ml group (486.66% increase), P < 0.05 and 1.28 ± 0.6 in CeA 6 ml group (753.33% increase), P < 0.01. Fifth (80–100 µ) zone: CeA 6 ml group only showed significantly increased number of dendritic branching points [0.09 ± 0.08 in normal control group versus 0.51 ± 0.27 in CeA 6 ml group (466.66% increase), P < 0.05.
Figure 4.
Dendritic branching points in the amygdaloid neurons of rats treated with 2, 4 and 6 ml/kg body weight of CeA for 6 weeks and age matched control and saline-treated rats at CZ and total number of branching points. Each value represents mean + standard deviation of 8–10 neurons from each rat. F-value: 15.9, 33.39, 20.52, 9.45, 4.96 at 0–20, 20–40, 40–60, 60–80, 80–100 µ CZs, respectively and 53.56 for total number of branching points. Note the significant increase in dendritic branching points in 2, 4 and 6 ml/kg CeA-treated rats compared to control rats. NC versus 2 ml/kg CeA: #P < 0.05, ##P < 0.01, ###P < 0.001; NC versus 4 ml CeA/kg: *P < 0.05, **P < 0.01, ***P < 0.001; NC versus 6 ml CeA/kg: ▴P < 0.05, ▴▴▴P < 0.001 (One way ANOVA, Bonferroni's test).
Four-Weeks Treatment Group
There was a significant increase in the dendritic intersections and branching points in rats treated with CeA for 4 weeks, at different radial distance from soma. Figure 2 illustrates photomicrographs and camera lucida tracings of normal control (A1, A2) and 2, 4 and 6 ml/kg of CeA leaf juice treated (B1, B2; C1, C2; D1, D2, respectively) for 4 weeks. Note that these Golgi-stained (Silver nitrate impregnated) neurons have significantly increased dendritic length and number of dendritic branching points in 4 ml/kg (C1,C2) and 6 ml/kg (D1,D2) CeA fresh leaf juice treated rats.
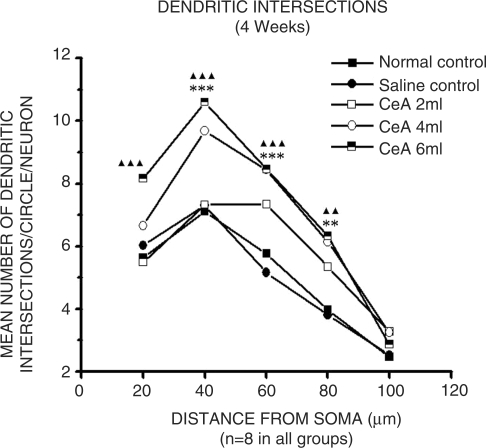
Dendritic Intersections
CeA 4 and 6 ml/kg groups showed significant increase in dendritic intersections at 40 µ (36.33 and 49.43% increase), 60 µ (46.52% and 46.87% increase) and 80 µ (131.48 and 59.44% increase) concentric circles Figure 5 (40 µ concentric circle: 7.1 ± 1.24 in normal control group versus 9.68 ± 1.06 in CeA 4 ml group, P < 0.001 and 10.61 ± 0.52 in CeA 6 ml group, P < 0.001. Sixty microns concentric circle: 5.76 ± 1.21 in normal control group versus 8.44 ± 0.92 in CeA 4 ml group, P < 0.001 and 8.46 ± 0.83 in CeA 6 ml group, P < 0.001. Eighty microns concentric circle: 3.97 ± 1.43 in normal control group versus 6.14 ± 0.92 in CeA 4 ml group, P < 0.01 and 6.33 ± 0.9 in CeA 6 ml group, P < 0.01. In addition, CeA 6 ml group showed increased number of dendritic intersections in 20 µ (44.64% increase) concentric circle (20 µ concentric circle: 5.64 ± 1.43 in normal control group versus 8.16 ± 1.03 in CeA 6 ml group, P < 0.001). There were no significant changes in dendritic intersections at any of the concentric circles in CeA 2 ml group when compared to the normal control group.
Figure 5.
Dendritic intersections of amygdaloid neurons in rats treated with 2, 4 and 6 ml/kg body weight of CeA for 4 weeks, and age matched control and saline-treated rats. Each point represents mean of 8–10 neurons from each rat (standard deviation not shown). F-value: 11.51, 29.46, 24.58, 14.24 and 1.24 at 20, 40, 60, 80, 100 µ distance from the soma, respectively. Note there is a significant increase in the dendritic intersections in rats treated with 4 and 6 ml/kg CeA. NC versus 4 ml/kg CeA: **P < 0.01, ***P < 0.001; NC versus 6 ml/kg CeA: ▴▴P < 0.01, ▴▴▴P < 0.001 (One way ANOVA, Bonferroni's test).
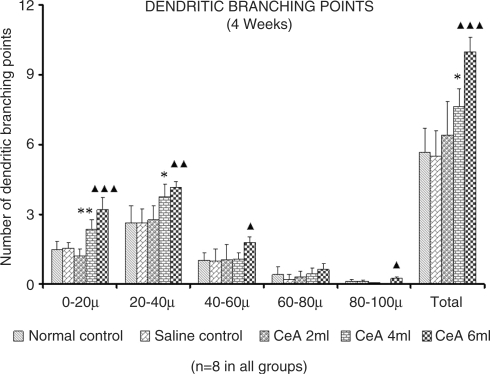
Dendritic Branching Points
CeA 4 and 6 ml/kg showed a significant increase in dendritic branching points (Fig. 6).First (0–20 µ) zone: 1.47 ± 0.38 in normal control group versus 2.36 ± 0.41 in CeA 4 ml group (60.54% increase), P < 0.01 and 3.19 ± 0.53 in CeA 6 ml group (117% increase) P < 0.001. Second (20–40 µ) zone: 2.63 ± 0.73 in normal control group versus 3.74 v 0.57 in CeA 4 ml group (42.2% increase), P < 0.05 and 4.15 ± 0.26 in CeA 6ml group (57.79% increase), P < 0.01. In addition, 6 ml group showed a significant increase in dendritic branching points in the 3rd and 5th zones; 3rd (40–60 µ) zone [1.02 ± 0.33 in normal control group versus 1.79 ± 0.24 in CeA 6ml group (75.49% increase), P < 0.05] and in the 5th (80–100 µ) zone [0.11 ± 0.08 in normal control group versus 0.23 ± 0.09 in CeA 6ml group (109.09% increase), P < 0.05]. There were no significant changes in dendritic branching points at any of the CZs in CeA 2 ml group when compared to the normal control group.
Figure 6.
Dendritic branching points in the amygdaloid neurons of rats treated with 2, 4 and 6 ml/kg body weight of CeA for 4 weeks and age matched control and saline-treated rats at CZs and total number of branching points. Each value represents mean + standard deviation of 8–10 neurons from each rat. F-value: 35.87, 12.25, 4.81, 2.91, 11.43 at 0–20, 20–40, 40–60, 60–80, 80–100 µ CZs, respectively and 25.63 for total number of branching points. Note the significant increase in dendritic branching points in 4 and 6 ml/kg CeA-treated rats compared to control rats. NC versus 4 ml CeA/kg: *P < 0.05, **P < 0.01; NC versus 6 ml CeA/kg: ▴P < 0.05, ▴▴P < 0.01, ▴▴▴P < 0.001 (One way ANOVA, Bonferroni's test).
Results of 2-Weeks Treatment Group
There was no significant change in dendritic intersections as well as branching points of amygdaloid neurons in rats treated with CeA for 2 weeks in any (2, 4 and 6 ml/kg) of the dose groups (data not illustrated).
Discussion
Centella asiatica Fresh Leaf Juice Induced Increase in the Amygaloid Neuronal Dendritic Length and Branching
CeA fresh leaf juice, 2 ml/kg body weight for 6 weeks showed 49–122% increase in the dendritic intersections at different concentric circles. Dendritic arborization enhancing effect of 4 ml/kg body weight of CeA is even higher. It showed 36–137% increase in dendritic intersections at different concentric circles in both 4- and 6-weeks treatment groups. The increase in the dendritic intersections, in rats treated with 6 ml/kg body weight for 4 and 6 weeks is still higher (44–165%).c Accordingly, there is a dose- and duration-dependent increase in the dendritic branching points. Two milliliters CeA fresh leaf juice treatment for 6 weeks showed total of 133.71% increase, 4 ml CeA fresh leaf juice treatment for 4 and 6 weeks showed total of 35.22 and 168.34% increase, respectively and 6ml CeA fresh leaf juice treatment for 4 and 6 weeks showed total of 76.63 and 229.58% increase, respectively.
Dendritic Arborization and Learning and Memory
The dendritic branching leads to enhancement in learning and memory. Enhancement of hippocampal CA3 neuronal dendritic arborization by CeA fresh leaf juice treatment in neonatal rats has been related to improved learning and memory (18). It is not only the plant extracts but repeated exposure to the enriched environments that has been shown to increase the spine density and dendritic complexity in certain brain structures (21).
The memory-enhancing property of fresh leaf juice of CeA in neonatal rats has been reported (18). Nalini et al. (22) have reported the memory-enhancing effect of aqueous extract of CeA in adult rats.
A few other herbal extracts are proved to act on the central nervous system, thereby enhancing learning and memory. A recent study has shown that B. monnieri improves memory in humans (23–27). Clitorea ternatea and Jatamansi have also been reported to be excellent memory enhancers (25,28). C. ternatea has been shown to enhance acetylcholine content in the rat hippocampus (29).
Amygaloid Neuronal Connections
The dendrites of the neurons of amygdala receive inputs from various parts of cerebral cortex, sub cortical areas and nuclei of the brain stem. Significant number of these afferents is from the parts of limbic system like hippocampus and hypothalamus which are concerned with learning and memory (30,31). Alterations such as increase/decrease in the dendritic length, increase/decrease in the dendritic branches in these neurons may result in alterations in the learning and memory behavior.
Increased dendritic intersections and branches of amygdaloid neurons in animals treated with 2, 4 and 6 ml of CeA fresh leaf juice for 4 and 6 weeks in the present study, suggests that these doses of plant juice were adequate to induce structural changes in these neurons. Naturally, such changes will have profound effect on the behavior because of the additional dendrites which are available on these neurons for the formation of new synapses. It can be noted from the results that a significant number additional dendrites are formed closer to the soma of neurons that is within 60 µ zones. This means that, these new synapses are formed closer to the soma of the neurons resulting in a more rapid and effective conduction of impulses, which probably is one of the reasons for enhanced learning and memory in these animals reported earlier (18). Such an increase in dendrites during neonatal period is shown to be more effective in enhancing learning and memory (32).
Thus, from the present study administration of fresh leaf juice of CeA during growth spurt period (neonatal) facilitates and increases dendritic length and branches of neurons of amygdala. Such enhanced dendritic arborization probably is the neuronal basis for improved learning and memory observed in these rats.
Prolonged immobilization stress increases the dendritic length and spine density in the basolateral amygdaloid neurons. This morphological change was associated with the anxiety like behavior in the elevated plus-maze (33). Here, we showed increased dendritic length in the amygdaloid neurons. These rats however did not show anxiety-like behavior, rather showed improved learning ability (18). This suggests that, the plant juice used, not only serves as a stimulant for enhancing the dendritic arborization, but also acts as an antianxiolytic drug.
Development of stable LTP (long-term potentiation) in response to high frequency stimulation requires new gene expression and protein synthesis (synaptic consolidation). Several lines of evidence have implicated endogenous BDNF-TrkB signaling in synaptic consolidation. The immediate early gene Arc (activity-regulated cytoskeleton-associated protein) is strongly induced and transported to dendritic processes after LTP induction in the dentate gyrus in live rats (34). The process of Arc-dependent synaptic consolidation is activated in response to brief infusion of BDNF (34). The mechanisms underlying the observed increase in the dendritic arborization may be that the plant juice may contain factors similar to BDNF and other neurotrophic factors which will activate the expression of the early genes such as Arc, thereby leading to enhancement of the dendritic arborization as well as improved learning and memory (18).
Various molecular mechanisms have been proposed for the dendritic enhancement in different parts of the brain in vivo and in vitro (35–38). Though we have not tested such a role of CeA, it may possess such a stimulative property.
Dendrites are the major determinants of how neurons integrate and process the incoming information and, thus they play a vital role in the functional properties of the neuronal circuits. The functional organization of the various parts of the brain is dynamic and can change in response to the experimental manipulations. Alterations in the synaptic function, neuronal membrane properties and axonal trajectories are associated with these changes. The plant juice used in this study may play its role in such manipulation leading to dendritic alteration.
To conclude, fresh leaf juice of CeA has shown to enhance the dendritic arborization in the amygdaloid neurons in rats. The mechanism of such alteration in the dendritic arborization is not clear. Probably it may contain neurostimulant factors which activate various pathways of dendritic alteration. The actual mechanism responsible for the dendritic enhancement needs to be explored and understood.
References
- 1.Sharma PV. Dravyaguna Vignana. 13th. New Delhi, India: Chaukhamba Vishwa Bharati Academy; 1992. pp. 3–5. [Google Scholar]
- 2.Sivarajan VV, Indira Balachandran. Ayurvedic Drugs and Their Plant Sources. Vol. 97. New Delhi, India: Oxford and IBH Publishing Co Pvt Ltd; 1994. pp. 289–90. [Google Scholar]
- 3.Dash PK, Mistry IU, Rao AR, Patel KS. Role of Medhya Rasayana in school children. Ayu. 1996;:12–15. [Google Scholar]
- 4.Satyavati GV, Gupta AK, Tandon N. Medicinal plants of India. 1st. New Delhi, India: Indian Council of Medical Research; 1976. pp. 18–21. 216–20. [Google Scholar]
- 5.Shah LP. An open clinical trial of Mentat in hyperkinetic children. Probe. 1992;31:125–9. [Google Scholar]
- 6.Appa Rao MVR, Srinivasan K, Rao KT. The effect of Mandookaparni (Centella asiatica) on the general mental ability (Medhya) of mentally retarded children. J Res Indian Med. 1973;8:9–12. [Google Scholar]
- 7.Joshi H, Parle M. Brahmi rasayana improves learning and memory in Mice. Evid Based Complement Alternat Med. 2006;3:79–85. doi: 10.1093/ecam/nek014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galani R, Weiss I, Cassel JC, Kelche C. Spatial memory habituation and reactions to spatial and nonspatial changes in rats with selective lesions of the hippocampus, the entorhinal cortex or the subiculum. Behav Brain Res. 1998;96:1–12. doi: 10.1016/s0166-4328(97)00197-6. [DOI] [PubMed] [Google Scholar]
- 9.Batch ME, Hawkins RQ, Osman M, Kandel ER, Mayfond M. Impairment of spatial but not contextual memory in CaMKII mutant mice with a selective loss of hippocampal LTP in the range of theta sequency. Cell. 1995;81:905–15. doi: 10.1016/0092-8674(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 10.Kesner RP, Hopkins RO. Short-term memory for duration and distance in humans: role of the hippocampus. Neuropsychology. 2001;15:58–68. doi: 10.1037//0894-4105.15.1.58. [DOI] [PubMed] [Google Scholar]
- 11.Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 1998;21:294–9. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- 12.Cahill L, Hair RJ, Fallon J, Alkire MT, Tang C, Keator D, et al. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proc Natl Acad Sci USA. 1996;93:8016–21. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flood JF, Morley JE, Roberts E. Pregnenolone sulphate enhances post training memory processes when injected in very low doses into limbic system structures: the amygdala is by far the most sensitive. Proc Natl Acad Sci USA. 1995;92:10806–10. doi: 10.1073/pnas.92.23.10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ridley RM, Baker HF. A critical evaluation of monkey models of amnesia and dementia. Brain Res Rev. 1991;16:15–37. doi: 10.1016/0165-0173(91)90018-4. [DOI] [PubMed] [Google Scholar]
- 15.Soumyanath A, Zhong YP, Gold SA, Yu X, Koop DR, Bourdette D, et al. Centella asiatica accelerates nerve regeneration upon oral administration and contains multiple active fractions increasing neurite elongation in-vitro. J Pharm Pharmacol. 2005;57:1221–9. doi: 10.1211/jpp.57.9.0018. [DOI] [PubMed] [Google Scholar]
- 16.Dobbing J, Sands J., Annotation Comparative aspects of the brain growth spurt. Early Human Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 17.Rao BS, Desiraju T, Raju TR. Neuronal plasticity induced by self stimulation rewarding experiences in rats- a study on alteration in dendritic branching in pyramidal neurons of hippocampus and motor cortex. Brain Res. 1993;627:216–24. doi: 10.1016/0006-8993(93)90324-g. [DOI] [PubMed] [Google Scholar]
- 18.Rao Mohandas KG, Rao Muddanna S, Rao Gurumadhva S. Centella asiatica (Linn) leaf extract treatment during growth spurt period enhances Hippocampal CA3 neuronal dendritic arborization in rats. Evid Based Complement Alternat Med. 2006;3:349–57. doi: 10.1093/ecam/nel024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sholl DA. The organization of the cerebral cortex. London: Methuen; 1956. [Google Scholar]
- 20.Rao Mohandas KG, Rao Muddanna S, Rao Gurumadhva S. Centella asiatica (linn) induced behavioural changes during growth spurt period in neonatal rats. Neuroanatomy. 2005;4:18–23. [Google Scholar]
- 21.Moser MB. Making more synapses: a way to store information? Cell Mol Life Sci. 1999;55:593–600. doi: 10.1007/s000180050317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nalini K, Aroor AR, Karanth KS, Rao A. Effect of Centella asiatica fresh leaf aqueous extract on learning and memory and biogenic amine turnover in albino rats. Fitoterapia. 1992;63:232–8. [Google Scholar]
- 23.Roodenrys S, Booth D, Bulzomi S, Phipps A, Micallef C, Smoker J. Chronic effects of Brahmi (Bacopa monnieri) on human memory. Neuropsychopharmacology. 2002;27:279–81. doi: 10.1016/S0893-133X(01)00419-5. [DOI] [PubMed] [Google Scholar]
- 24.Stough C, Lloyd J, Clarke J, Downey LA, Hutchison CW, Rodgers T, et al. The chronic effects of an extract of Bacopa monnieri (Brahmi) on cognitive function in healthy human subjects. Psychopharmacology. 2001;156:481–4. doi: 10.1007/s002130100815. [DOI] [PubMed] [Google Scholar]
- 25.Indurwade NH, Biyani KR. Evaluation of comparative and combined depressive effect of Brahmi, Shankapushpi and Jatamansi in mice. Indian J Med Sci. 2000;54:339–41. [PubMed] [Google Scholar]
- 26.Shukia B, Khanna NK, Godhwani JL. Effect of Brahmi rasayan on central nervous system. J Ethnopharmacol. 1987;21:65–74. doi: 10.1016/0378-8741(87)90095-x. [DOI] [PubMed] [Google Scholar]
- 27.Singh HK, Dhawan BN. Effect of Bacopa monnieri Linn. (Brahmi) extract on avoidance responses in rat. J Ethnopharmacol. 1982;5:205–14. doi: 10.1016/0378-8741(82)90044-7. [DOI] [PubMed] [Google Scholar]
- 28.Rai KS, Murthy KD, Karanth KS, Rao MS. Clitorea ternatea (Linn) root extract treatment during growth spurt period enhances learning and memory in rats. Indian J Physiol Pharmacol. 2001;45:305–13. [PubMed] [Google Scholar]
- 29.Rai KS, Murthy KD, Karanth KS, Nalili K, Rao MS, Srinivasan KK. Clitorea ternatea root extract enhances acetylcholine content in rat hippocampus. Fitoterapia. 2002;73:685–9. doi: 10.1016/s0367-326x(02)00249-6. [DOI] [PubMed] [Google Scholar]
- 30.Luskin MB, Price JL. The topographic organization of associational fibers of olfactory system in the rat, including centrifugal fibers to the olfactory bulb. J Comp Neurol. 1983;216:264–91. doi: 10.1002/cne.902160305. [DOI] [PubMed] [Google Scholar]
- 31.Berk ML, Finkelstein JA. Efferent connections of lateral hypothalamic area of the rat: an autoradigraphic investigation. Brain Res Bull. 1982;8:511–26. doi: 10.1016/0361-9230(82)90009-0. [DOI] [PubMed] [Google Scholar]
- 32.Tang AC. Neonatal exposure to novel environment enhances hippocampal dependent memory function during infancy and adulthood. Learn Mem. 2001;8:257–64. doi: 10.1101/lm.43101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–93. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Soule J, Messaoudi E, Bramham CR. Brain-derived neurotrophic factor and control of synaptic consolidation in the adult brain. Biochem Soc Trans. 2006;34:600–04. doi: 10.1042/BST0340600. [DOI] [PubMed] [Google Scholar]
- 35.Jaworski J, Spangler S, Seeburg DP, Hoogenraad CC, Sheng M. Control of dendritic arborization by the phosphoinositide-3′-kinase-Akt-mammalian target of rapamycin pathway. J Neurosci. 2005;25:11300–12. doi: 10.1523/JNEUROSCI.2270-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zagrebelsky M, Holz A, Dechant G, Barde YA, Bonhoeffer T, Korte M. The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J Neurosci. 2005;25:9989–99. doi: 10.1523/JNEUROSCI.2492-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tolias KF, Bikoff JB, Burette A, Paradis S, Harrar D, Tavazoie S, et al. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron. 2005;45:525–38. doi: 10.1016/j.neuron.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 38.Jugloff DG, Jung BP, Purushotham D, Logan R, Eubanks JH. Increased dendritic complexity and axonal length in cultured mouse cortical neurons overexpressing methyl-CpG-binding protein MeCP2. Neurobiol Dis. 2005;19:18–27. doi: 10.1016/j.nbd.2004.11.002. [DOI] [PubMed] [Google Scholar]