Abstract
The role of abrin, a toxic lectin isolated from seeds of Abrus precatorius Linn in inducing apoptosis in murine Dalton's Lymphoma Ascites (DLA) cells was evaluated. Abrin when incubated at the concentration of 10 ng per million DLA cells could bring about cell death as typical morphological changes with apoptosis. However, necrotic cell death dominated when a higher dose of abrin was used. DNA samples, isolated from DLA cells treated with abrin showed fragmentation. Abrin brought about induction of apoptosis by stimulating the expression of pro-apoptotic Caspase-3, at the same time blocking the expression of Bcl-2, which is an anti apoptotic gene. However, the expression of tumor suppressor gene p53 has not been observed in control and abrin-treated DLA cells. Results suggested that abrin effectively induced apoptotic changes in the tumor cells that led to cellular death.
Keywords: abrin, apoptosis, Bcl-2, Caspase-3, p53
Introduction
Abrus precatorius Linn which is commonly known as Rosary pea/Jequirity bean belonging to family Leguminosea and subfamily Papilionoidea has a toxic glycoprotein lectin known as abrin in its seed. Three toxins, namely abrin I, II and III and two agglutinins, namely APA I and II, in the seeds A. precatorius, was isolated by lactamyl Sepharose affinity chromatography followed by gel filtration and DEAE Sephacel column chromatography (1). Like ricin, from Ricinus communis, abrin is also a type II ribosome inactivating protein that inhibits protein synthesis in eukaryotic cells. The toxins and agglutinins share high degree of sequence similarity however, agglutinins are weaker in their activity. Some of the other documented actions of abrin being galactose specific (2), mitogenic (3), hemagglutinating (4), tumoricidal (5,6) and immunopotentiating (7,8). We have earlier reported the in vivo tumoricidal property of abrin against DLA and EAC (Ehrlich's Ascites Carcinoma)-induced solid cell tumors. It was also found that DLA cells were more sensitive to sub lethal doses of abrin than EAC cell line, while it spares normal host cells (9). The susceptibility of either normal or cancerous cells to cytotoxic insult of abrin is related to the number of galactose specific glycosylated cell membrane receptors (9).
In the last decade, basic cancer research has produced remarkable advances in our understanding of cancer biology and cancer genetics. Among the most important of these advances is the realization that apoptosis and the genes that control it have a profound effect on the malignant phenotype. For example, it is now clear that some oncogenic mutations disrupt apoptosis, leading to tumor initiation, progression or metastasis. Conversely, compelling evidence indicates that other oncogenic changes promote apoptosis, thereby producing selective pressure to override apoptosis during multistage carcinogenesis. The life span of both normal and cancer cells within a living system are regarded to be substantially affected by rate of apoptosis. In addition, apoptosis is a discrete way of cell death, different from necrotic cell death and is regarded as an ideal way of cell elimination. In recent years, many cancer chemotherapeutic agents have been shown to induce apoptosis (10–12) and conversely, several tumor promoters have also been shown to inhibit apoptosis (13). Like abrin, agglutinin-I also induces apoptosis in cells by triggering the intrinsic mitochondrial pathway, though at higher concentrations as compared to abrin (14,15). There are pro-apoptotic genes (e.g. Caspase-3, 8, 9) and anti-apoptotic genes (e.g. Bcl-2, Bcl-xL, Bcl-w) within a cell and the expression of each group of genes are tightly regulated by a complex mechanism. The objective of the present study is to evaluate the role of abrin, a toxic lectin from A. precatorius, in inducing apoptosis in murine DLA cells.
Methods
Drug Preparation
Abrin was isolated from seeds of red variety of A. precatorius using Sepharose 4B affinity column chromatography and purified as per an earlier report (16). The stock solution so obtained, was diluted with Phosphate Buffered Saline (PBS, pH 7.2) to get a concentration of 250 ng ml−1.
Cell Culture
DLA cell line was obtained from Adayar Cancer Institute (Chennai, India) and was propagated as transplantable ascites tumors in female Swiss albino mice. Cells were aspirated freshly from mouse peritoneum, washed with PBS and the concentration determined using hemocytometer. Viability of cells was checked by the dye exclusion method using trypan blue (1%). Five million live cells were added to 15 ml of Dulbeco's Minimum Essential Medium (DMEM) supplemented with 10% fetal calf serum in culture flasks and maintained at 37°C in 5% CO2 atmosphere.
Cytotoxicity of Abrin
Two concentrations of abrin namely 10 and 25 ng per million DLA cells were utilized for culture in triplicate. A control culture of DLA cells alone in triplicate was also maintained. After adding the drug, 48 h incubation was allowed. Following these treatments, cells were washed thrice with PBS and tested for viability by using dye exclusion method in order to test the toxicity of abrin. Cell pellet was aspirated following centrifugation. To study morphological changes, a small portion of the pellet was resuspended in PBS and cell smear was prepared and stained with hematoxylin and eosin. The numbers of normal and apoptotic cells were counted from the stained smears.
Isolation of Genomic DNA and Analysis of DNA Fragmentation
The DLA cells incubated with different drug concentrations for 48 h were washed twice with Tris borate saline (TBS), centrifuged at 300 RPM. for 10 min and the supernatant was discarded. The pellet was washed and resuspended in saline EDTA (SE) buffer. Later 20 μl of proteinase K (20 mg ml−1) and 50 μl of 20% SDS were added and mixed. The digested sample was cooled to room temperature and 100 μl of 5M NaCl was added and mixed. Saturated phenol (pH 7.8) was added in equal volume, mixed and centrifuged at 4000 RPM. for 15 min. The aqueous phase was collected in fresh tube. A mixture of saturated phenol : chloroform : isoamyl alcohol (25 : 24 : 1) was added in equal volume and mixed and centrifuged at 4000 RPM. for 15 min. The aqueous phase was transferred in fresh tube. An equal volume of chloroform: isoamyl alcohol (24 : 1) was mixed and centrifuged. The aqueous phase was then transferred to a sterile 100 ml beaker added one-tenth volume of 3M sodium acetate (pH 5.5) was added and mixed well. Equal volume of isopropanol was added and the precipitated DNA was pooled out on clean micropipette tip, washed with 70% ethanol, air dried and resuspended in TE buffer (pH 8.0). Purity and quantification of the DNA was carried out by monitoring the ODs at 260 and 280 nm. Ten microliter (50 ng) of each samples were resolved at 100 V for 2 h on a 2% agarose gel containing 0.5 μg ml−1 ethidium bromide. A 100 bp ladder DNA sample was used as marker. The resulting DNA fragmentation was visualized under UV transilluminator followed by polaroid photography in gel documentation unit (Vilber Lourmat, France).
Preparation of cDNA
The cDNA was prepared from cell lysate without RNA isolation (17). A total of 1 × 104 DLA cells were suspended in serum free DMEM (250 μl) and incubated in the microtiter plate for 12 h. Abrin, at a concentration of 0.1 ng per well was added and incubated for 4 h. The medium was aspirated and 200 μl ice cold PBS was added. The PBS was aspirated before adding 100 μl ice cold cell lysis buffer. This was then transferred to a water bath at 75°C and incubated for 15 min and the cell lysate transferred to 200 μl PCR vials. Two Microliter DNase-I per 100 μl cell lysis buffer was added and incubated for 15 min at 37°C. The DNase was inactivated by heating to 75°C for 15 min in a water bath. To the 10 μl of cell lysate (RNA) 4 μl of dNTP mixture, 2 μl of Oligo (dT)18 primer and 16 μl of nuclease free water were added, vortexed, centrifuged briefly and heated for 3 min at 70°C in a water bath. Then this was placed on ice for 1 min. Later 1 μl of M-MLV Reverse Transcriptase (RT), 2 μl of 10 × RT buffer and 1 μl of RNase inhibitor were added. The final mixture was incubated at 42°C for 60 min, followed by 95°C for 10 min to inactivate the RT.
Reverse Transcriptase (RT)–Polymerase Chain Reaction (PCR) for the Amplification of Caspase-3, Bcl-2 and p53
The RT–PCR was carried out for p53: tumor suppressor gene (Maxim Biotech. Inc., SF, USA). The pre-mixed primer of 250 µl was added to PCR buffer (750 µl) containing enhancer, stabiliser and dNTPs to make master mixture according to the manufacturer's instruction. Master mixture (39 μl), Taq DNA polymerase (1 μl) and specimen/control cDNA (10 μl) were mixed and PCR reaction was carried out in the minicycler (MJ Research, Germany).
One-tenth of the amplified samples were electrophoresised in an agarose gel containing 0.5 μg ml−1 ethidium bromide. Similarly RT–PCR procedure was carried out for Caspase-3 and Bcl-2 (Maxim Biotech. Inc., SF, USA) and the house keeping gene Glyceraldehyde phosphate dehydrogenase (GAPDH) (Biosource International Inc. USA) as per the manufacturer's instructions.
Agarose Gel Electrophoresis for DNA/PCR Products Separation
To 1 g agarose in 5 ml of 10 × Tris borate EDTA (TBE) buffer (pH 8.0), 50 ml of double distilled water was added and boiled until all the agarose was dissolved. This was allowed to cool to 50°C. Sufficient ethidium bromide, was added to get a final concentration of 0.5 μg ml−1 of gel. The gel was casted and placed in the electrophoresis apparatus containing enough 1 × TBE buffer and 0.5 μg/ml ethidium bromide to cover the gel. Ten microliter of DNA sample per 10 μl of PCR product per 10 μl of DNA molecular weight marker was mixed with 2 μl of 6 × gel loading buffer (0.25% bromphenol blue, 30% glycerol). Each 12 μl of these samples were loaded into wells. Samples were run at 100 V for 2 h or until the dye had migrated three-fourth of the gel. Results were read in a gel documentation unit.
Results
Cytotoxicity of Abrin
Viable cells which remained unstained by trypan blue were counted in a hemocytometer. The number of dead cells encountered with abrin treatment at 10 and 25 ng per million DLA cells were 40 ± 5 and 53 ± 5, whereas control cultures exhibited 22 ± 4 dead cells. This result emphasized abrin's cytotoxic nature, which was dose dependent (Table 1).
Table 1.
Assessment of cytotoxicity of abrin on DLA cells (n = 3)
| Concentration of abrin per 106 DLA cells | % dead cells after 48 h | Number of apoptotic cells per 103 DLA cells | Number of necrotic cells per 103 DLA cells |
|---|---|---|---|
| 0 | 22 ± 4 | 33 ± 4 | 234 ± 27 |
| 10 ng | 40 ± 5 | 570 ± 37 | 280 ± 14 |
| 25 ng | 53 ± 5 | 393 ± 23 | 331 ± 31 |
Values are mean ± SE.
Apoptotic Cells Versus Necrotic Cells
Microscopic examination of abrin-treated and control DLA cells revealed that the cell death could be either with necrotic or with apoptotic characteristics. On studying the morphology of cultured DLA cells, necrotic cells were identified as swollen, disintegrating with more eosinophilic cytoplasm, pale nucleus but conserved chromatin. While apoptotic cells were distinguished as highly condensed/shrunken cell with nuclear elongation, margination, fragmentation and sacculation, cell blebbing and presence of apoptotic bodies. Active normal DLA cells were characterized by less eosinophilic cytoplasm and nucleus with uniform distribution of chromatin material. The proportion of DLA cells with apoptotic changes was approximately double when compared to necrotic cells in cultures containing 10 ng abrin per million DLA cells. However, in the culture having 25 ng abrin, the number of DLA cells with apoptotic and necrotic changes were 393 ± 23 and 331 ± 31 per 103 cells, respectively (Table 1).
DNA Fragmentation
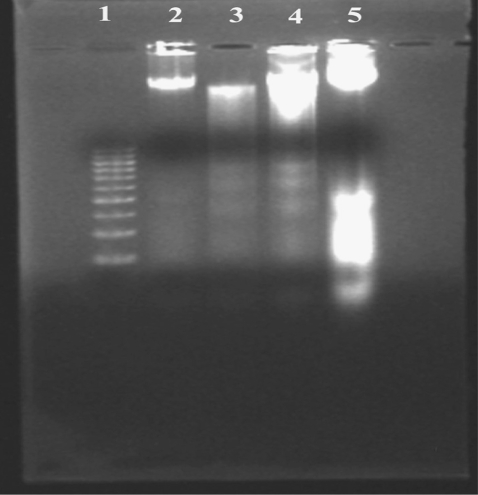
Results of electrophoretic run of DNA samples (Fig. 1) showed that abrin-treated DLA cells exhibited extensive double strand breaks; thereby yielding a ladder appearance (lane 5), while the DNA of control DLA cells exhibited minimum breakage (lane 3). The DNA isolated from fresh DLA cells did not show any double strand breaks (lane 2). The DNA isolated from DLA cells treated with 10 ng abrin showed more fragmentation than those treated with 25 ng abrin (lane 4). This kind of DNA cleavage is one of the characteristics of apoptosis.
Figure 1.
Electrophoretic separation of DNA samples of DLA cells treated with abrin. Lane 1: DNA molecular weight markers, lane 2: DNA isolated from fresh DLA cells, lane 3: DNA isolated from culture of untreated DLA cells, lane 4: DNA isolated from abrin-treated (25 ng per million) DLA cells and lane 5: DNA isolated from abrin-treated (10 ng per million) DLA cells.
Apoptotic Gene Expressions in Abrin-treated DLA Cells
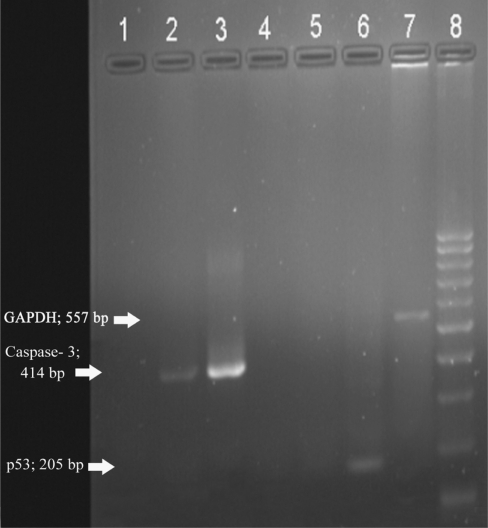
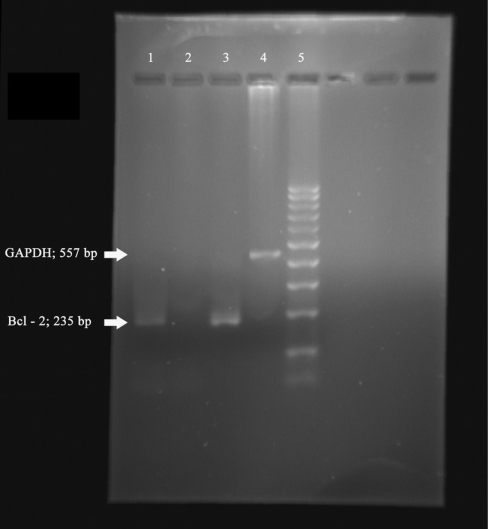
On electrophoresis, bands corresponding to 205 base pairs in size indicated the presence of target sequences in the specimens for p53 (Fig. 2), 414 base pairs for Caspase-3 (Fig. 2), 235 base pairs for Bcl-3 (Fig. 3) and 557 base pairs for house keeping gene GAPDH (Figs 2 and 3). Results of the present study indicated that in vitro treatment with abrin at a concentration of 0.1 ng per 1 × 104 DLA cells resulted in the selective expression of the pro-apoptotic gene Caspase-3 (Fig. 2, lane 2). Control cells did not exhibit the expression of this gene (Fig. 2, lane 1). Treatment with abrin led to the suppression of Bcl-2 expression (Fig. 3, lane 2), which is an anti-apoptotic gene. Control DLA cells showed the expression of Bcl-2 (Fig. 3, lane 1). Though expression of GAPDH could be obtained in all (Fig. 2, lane 7 and Fig. 3, lane 4), the band corresponding to p53 gene expression could not be noticed in any of the cell lines screened.
Figure 2.
Electrophoretic separation of RT–PCR samples. Lane 1: PCR product from control DLA cells for Caspase-3, lane 2: PCR product from abrin-treated DLA cells for Caspase-3, lane 3: PCR product from positive control for Caspase-3, lane 4: PCR product from control DLA cells for p53, lane 5: PCR product from abrin-treated DLA cells for p53, lane 6: PCR product from positive control for p53, lane 7: PCR product from control DLA cells for GAPDH and lane 8: 100 bp molecular weight marker.
Figure 3.
Electrophoretic separation of RT–PCR samples. Lane 1: PCR product from control DLA cells for Bcl-2, lane 2: PCR product from abrin-treated DLA cells for Bcl-2, lane 3: PCR product from positive control for Bcl-2, lane 4: PCR product from control DLA cells for GAPDH and lane 5: 100 bp molecular weight marker.
Discussion
Anti-tumor agents, which can modulate apoptosis, may be able to affect the steady state of cell populations that are helpful in the management and therapy of cancer. Results of the present study suggested that abrin could induce tumor cell death both by physiological or pathological means. Most of the cytotoxic anti-cancer drugs in current use have been shown to induce apoptosis in susceptible cells. It has been established that apoptotic cells display DNA fragmentation at internucleosomal sites followed by morphological changes and loss of membrane integrity (18,19).
The abrin molecule consists of two chains namely toxic A-chain and lectin B-chain, held together by disulfide bonds. The toxic A-chain of abrin is capable of deactivating each and every ribosome in the cell, which culminates in cell death. The lectin part of toxin molecule (B-chain) is liable for recognition of cell surface and binding. Having bound diffusely to the cell membrane, the protein is internalized by way of receptor mediated endocytosis (20). Like abrin, other galactose specific lectins like ricin, is reported to induce apoptosis in target cells by inhibiting protein synthesis (21), and specific lectin from Viscum album L, namely ML I brings about apoptosis in peripheral lymphocytes (22). This study revealed that the potency of abrin to bring about apoptotic changes decreases with dose, as more cell death through necrotic pathway dominated when 25 ng of abrin was present in the culture in place of 10 ng per 1 million cells. Such a kind of dose-dependent cytotoxicity was recently reported on T-lymphocytes by Viscum album agglutinin –I (23).
From this study we establish that nuclear changes, as a part of apoptosis precedes the loss of membrane integrity thereby making trypan blue impermeable. More than 50% of DLA cells incubated with abrin showed intact cell membrane responding as live cells in the dye exclusion test, but DNA fragmentation could be detected at that time. This finding closely agreed with earlier reports (24,25), suggesting that DNA cleaveage of abrin-treated cells occurs before loss of membrane integrity. It was earlier reported that a good positive correlation existed between agglutinating activity of abrin-a and development of apoptosis to DNA fragmentation. The B-chain probably triggers the apoptosis, while the A-chain and breakage of the disulfide bond are responsible for its progress on leukemia cell lines (26).
We reported earlier that abrin when administered in mice at7.5 µg kg−1 body weight (one tenth LD50) i.p., on every alternate day for 10 days, could effectively reduce solid tumor mass development and also increase the life span of ascites tumor bearing mice. The above sublethal dose of abrin caused no significant change in body weight gain or in the hematological profiles like total erythrocyte count, total leukocyte count and hemoglobin concentration in normal mice (9). Moreover, mice that received the safe dose of abrin exhibited no significant change in activities of certain serum enzymes such as alanine amino transferase, aspartate amino transferase and Alkaline phosphatase as well as in the levels of certain serum metabolites such as urea nitrogen, creatinine and bilirubin.
When compared to normal cells, tumor cells possess abundant glycosylated binding sites on the cell surface (27); cells require lesser number of abrin molecules for initiating cell death. Abrin is highly toxic to Epstein Barr Virus (EBV) transformed lymphocytes when compared to normal lymphocytes (28). Such varying responses of different cells to the toxic abrin may be due to several factors including relative number of abrin binding sites on cell surface and the rate of degradation of internalized toxin.
However, sensitivities of cells to abrin do not necessarily correlate with their normal state as exhibited by mouse embryonic fibroblasts (MEF) and untransformed mouse cell line (NIH 3T3) or with their neoplastic state as encountered with two mouse tumor cell lines namely LMTK and S-180 (26). NIH 3T3 and LMTK cells had significantly fewer abrin binding sites than MEF and S-180 cells while, the degradation of internalized abrin was more rapid in NIH 3T3 and S-180 cells than in LMTK and MEF cells. The induction of apoptosis in tumor cells make them render more for host phagocytic clearance without initiating inflammation which could also be attributed for abrin's tumouricidal activity. The possible reasons for abrin causing moderate toxicity to normal healthy cells while bringing about severe cytotoxicity against cancerous cells should be investigated thoroughly.
The p53, also known as TP53 is a gene that codes for a protein that regulates the cell cycle and hence functions as a tumor suppressor. It is very important for a cell in multicellular organisms to suppress cancer.The p53 has conserving stability of the cell by preventing genome mutation (29). In cancerous cells, the expression of p53 is much suppressed and in the present study even control DLA cells did not exhibit its expression. The loss of this protein due to mutation is a primary event in the formation of many types of leukemias, breast cancer, colon, lung and liver. Bcl-2 was first discovered as a translocated locus [t(14;22)] in B-cell leukemias, and is an anti-apoptotic protein that protects cells from programmed cell death by preventing the activation of pro-apoptotic Caspase proteins like Bax, Bak and Bok. Caspase-3 is an enzyme that plays a key role in programmed cell death and is a member of the family of aspartate specific cysteinyl proteases.
Results of this study showed that pre-treatment of DLA cells with abrin at a low sublethal dose brought about induction of apoptosis chiefly by stimulating the expression of Caspase-3, at the same time blocking the expression of Bcl-2. It was earlier reported that release of mitochondrial cytochrome c, and the sequential Caspase-9 and Caspase-3 activations were important events in the signal transduction pathway of abrin-induced apoptotic cell death in the HeLa cell line (30) and their observation closely agreed with our results. Regarding the activation of Caspase-3 for initiation of apoptosis, our results fully agreed with an earlier report (31), which claimed that, the apoptotic pathway induced by abrin was Caspase 3-dependent but Caspase 8-independent. It is generally considered that Caspase 3-dependent apoptosis involves mitochondrial membrane potential damage and reactive oxygen species production. Abrin effectively induceing apoptotic changes in tumor cells leading to cellular death have been scientifically proved through systematic electrophoretic analysis, and the product under study appears to be promising for clinical trials. So abrin as a new potential anti-cancer agent is very promising.
References
- 1.Hegde R, Maiti TK, Podder SK. Purification and characterisation of three toxins and two agglutinins from Abrus precatorius seeds by using lactymyl-sepharose affinity chromatography. Anal Biochem. 1991;194:101–9. doi: 10.1016/0003-2697(91)90156-n. [DOI] [PubMed] [Google Scholar]
- 2.Refsnes K, Olsnes S, Phil A. Toxic proteins abrin and ricin-studies of their binding to and entry into Ehrlich ascites cells. J Biol Chem. 1974;249:3557–62. [PubMed] [Google Scholar]
- 3.Kaufman SJ, Pherson A. Abrin and hurin: two new lymphocyte mitogens. Cell. 1975;4:263–68. doi: 10.1016/0092-8674(75)90174-9. [DOI] [PubMed] [Google Scholar]
- 4.Irvin Liener E. Phytohaemagglutinins (Phytolectins) Annu Rev Plant Physiol. 1976;27:291–319. [Google Scholar]
- 5.Ohba H, Toyokawa T, Yasuda S, Hoshino T, Itoh K, Yamasaki N. Spectroscopic analysis of the cytoagglutinating activity of abrin-b isolated from Abrus precatorius seeds against leukemic cells. Biosci Biotechnol Biochem. 1997;61:737–39. doi: 10.1271/bbb.61.737. [DOI] [PubMed] [Google Scholar]
- 6.Lin JY, Lee TC, Tung TC. Inhibitory effects of four isoabrins on the growth of sarcoma 180 cells. Cancer Res. 1982;42:276–9. [PubMed] [Google Scholar]
- 7.Ramnath V, Kuttan G, Kuttan R. Immunopetiating activity of abrin, a lectin from Abrus precatorius Linn. Indian J Exp Biol. 2002;40:910–3. [PubMed] [Google Scholar]
- 8.Ramnath V, Kuttan G, Kuttan R. Effect of abrin on cell-mediated immune responses in mice. Immunopharmacol Immunotoxicol. 2006;28:259–68. doi: 10.1080/08923970600816764. [DOI] [PubMed] [Google Scholar]
- 9.Ramnath V, Kuttan G, Kuttan R. Antitumour effect of abrin on transplanted tumours in mice. Indian J Physiol Pharmacol. 2002;46:69–77. [PubMed] [Google Scholar]
- 10.John AH. Apoptosis induced by anticancer drugs. Cancer Metast Rev. 1992;11:121–39. doi: 10.1007/BF00048059. [DOI] [PubMed] [Google Scholar]
- 11.Fesus L, Szondy Z, Uray I. Probing the molecular program of apoptosis by cancer chemopreventive agents. J Cell Biochem Suppl. 1995;22:151–61. doi: 10.1002/jcb.240590820. [DOI] [PubMed] [Google Scholar]
- 12.Samaha HS, Kelloff GJ, Steele V, Rao CV, Reddy BS. Modulation of apoptosis by sulindac, curcumin, phenylethyl 3-methylcaffeate and 6-phenylhexyl isothiocyanate: apoptotic index as a biomarker of colon cancer chemoprevention and promotion. Cancer Res. 1997;57:1301–5. [PubMed] [Google Scholar]
- 13.Wright SC, Zhong J, Larrick JW. Inhibition of apoptosis as a mechanism of tumour promotion. FASEB J. 1994;8:654–60. doi: 10.1096/fasebj.8.9.8005393. [DOI] [PubMed] [Google Scholar]
- 14.Endo Y, Tsurugi K. Mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. Nucleic Acids Symp Ser. 1986;17:187–90. [PubMed] [Google Scholar]
- 15.Bagaria A, Surendranath K, Ramagopal UA, Ramakumar S, Karande AA. Structure – function analysis and insights into the reduced toxicity of abrus precatorius agglutinin I in relation to abrin. J Biol Chem. 2006;281:34465–74. doi: 10.1074/jbc.M601777200. [DOI] [PubMed] [Google Scholar]
- 16.Hedge R, Podder SK. Studies on the variants of the protein toxins: ricin and abrin. Anal Biochem. 1992;204:155–64. doi: 10.1111/j.1432-1033.1992.tb16618.x. [DOI] [PubMed] [Google Scholar]
- 17.Klebe RJ, Grant GM, Grant AM, Garcia AM, Giambernardi TA, Taylor GP. RT-PCR without RNA isolation. Biotechniques. 1996;21:1094–100. doi: 10.2144/96216rr02. [DOI] [PubMed] [Google Scholar]
- 18.Christopher SP. The significance of spontaneous and induced apoptosis in gastrointestinal tract of mice. Cancer Metast Rev. 1992;11:179–95. doi: 10.1007/BF00048063. [DOI] [PubMed] [Google Scholar]
- 19.Daniel RC, Bernand WS. Etoposide induced cytotoxicity in two Human T- cell leukaemic lines: delayed loss of membrane permeability rather than DNA fragmentation as an indicator of programmed cell death. Cancer Res. 1993;53:4887–96. [PubMed] [Google Scholar]
- 20.Manske JM, Buchsbaum DJ, Vallera DA. The role of ricin B-chain in the intracellular trafficking of anti-CD5 immunotoxins. J Immunol. 1989;142:1755–66. [PubMed] [Google Scholar]
- 21.Williams JM, Lea N, Lord JM, Robert LM, Milford DV, Taylor CM. Comparison of ribosome inactivating proteins in the induction of apoptosis. Toxicol Lett. 1997;91:121–7. doi: 10.1016/s0378-4274(97)03879-4. [DOI] [PubMed] [Google Scholar]
- 22.Verveckon W, Kleff S, Pfuller U, Bussing A. Induction of apoptosis by mistletoe- I lectin and its subunits. Int J Biochem cell Biol. 2000;32:317–26. doi: 10.1016/s1357-2725(99)00135-1. [DOI] [PubMed] [Google Scholar]
- 23.Hajto T, Hostanska K, Berki T, Palinkas L, Boldizar F, Nemeth P. Oncopharmocological perspectives of a plant lectin (Viscum album agglutinin –I): overview of recent results from in vitro experiments and in vivo models and their possible relevance for clinical applications. Evid Based Complement Alternat Med. 2005;2:59–67. doi: 10.1093/ecam/neh058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li JC, Kaminskas E. Progressive formation of DNA lesions in cultured Ehrlich ascityes tumour cells with hydroxyurea. Cancer res. 1987;47:2755–8. [PubMed] [Google Scholar]
- 25.Babu TD, Kuttan G, Padsikkala J. Cytotoxic and antitumour properties of certain taxa of Umbelliferae with special reference to Centella asiatica (Linn) urban. J Etanopharmacology. 1995;48:53–57. doi: 10.1016/0378-8741(95)01284-k. [DOI] [PubMed] [Google Scholar]
- 26.Chan LN, Li JS, Liu SY. Differential effects of abrin on normal and tumor cells. J Cell Physiol. 1985;123:132–8. doi: 10.1002/jcp.1041230119. [DOI] [PubMed] [Google Scholar]
- 27.Ohba H, Moriwaki S, Bakalova R, Yasuda S, Yamasaki N. Plant-derived abrin-a induces apoptosis in cultured leukemic cell lines by different mechanisms. Toxicol Appl Pharmacol. 2004;195:182–93. doi: 10.1016/j.taap.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Bennet CE, Glassman AB, Witten M. DNA and protein synthesis in normal and transformed lymphocytes exposed to abrin. Pharmacol Biochem Behav. 1982;16:185–8. doi: 10.1016/0091-3057(82)90034-x. [DOI] [PubMed] [Google Scholar]
- 29.Strachan T, Read AP. Human Molecular Genetics. 2nd. New York: John Wiley; 1999. [Google Scholar]
- 30.Qu X, Qing L. Abrin induces HeLa cell apoptosis by cytochrome c release and caspase activation. J Biochem Mol Biol. 2004;37:445–53. doi: 10.5483/bmbrep.2004.37.4.445. [DOI] [PubMed] [Google Scholar]
- 31.Narayanan S, Surolia A, Karande AA. Ribosome-inactivating protein and apoptosis: abrin causes cell death via mitochondrial pathway in Jurkat cells. Biochem J. 2004;377:233–40. doi: 10.1042/BJ20030797. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]