Abstract
While the ancient Egyptians and Greeks used honey for wound care, and a broad spectrum of wounds are treated all over the world with natural unprocessed honeys from different sources, Medihoney™ has been one of the first medically certified honeys licensed as a medical product for professional wound care in Europe and Australia. Our experience with medical honey in wound care refers only to this product. In this review, we put our clinical experience into a broader perspective to comment on the use of medical honey in wound care. More prospective randomized studies on a wider range of types of wounds are needed to confirm the safety and efficacy of medical honey in wound care. Nonetheless, the current evidence confirming the antibacterial properties and additional beneficial effects of medical honey on wound healing should encourage other wound care professionals to use CE-certified honey dressings with standardized antibacterial activity, such as Medihoney™ products, as an alternative treatment approach in wounds of different natures.
Keywords: Medical honey, wound care, MRSA
Introduction
In our paediatric oncology department at the Children's Hospital Medical Centre, University of Bonn, Germany most patients suffer from a profoundly suppressed immune system, due to their underlying disease (i.e. leukemia) (1) and the chemotherapy they undergo. This frequently results in wound healing problems (2,3), leaving the patient susceptible to wound infections. Unfortunately these infections spread easily in immunocompromized patients and can cause secondary potentially life-threatening bloodstream infections (4).
Five years ago a 12-year old patient was submitted to our unit. Doctors at another hospital had removed an abdominal lymphoma, leaving an open drainage site on his abdomen. On admission, his wound was infected with methicillin-resistant Staphylococcus aureus (MRSA). In order to avoid nosocomial spread, the patient was immediately isolated, a difficult situation for the child to comprehend with significant additional costs from the perspective of the hospital. Although the patient was scheduled to receive chemotherapy, treatment could not commence until the infection cleared. The wound was treated with a local antiseptic (octenidin) for 12 days. Since no improvement occurred, we decided to use an Australian medical honey (Medihoney™), which contains leptospermum honey, a type with excellent in vitro activity against MRSA (5–7). The wound was free of bacteria two days later, and the chemotherapy against the underlying illness could be started.
While the ancient Egyptians and Greeks used honey for wound care, a broad spectrum of wounds are treated all over the world with natural unprocessed honeys from different sources (8–13), Medihoney™ has been one of the first medically certified honeys licensed as medical product for professional wound care in Europe and Australia (14,15). Our experience with medical honey in wound care refers only to this product. We were asked by one of the editors of eCAM to put our clinical experience (2,16) into a broader perspective and to comment on the use of medical honey in wound care (Table 1).
Table 1.
Key information from this review
| What makes medical honey effective? | Medical honey is hygroscopic, meaning it draws moisture out of the environment and thus dehydrates bacteria. The enzyme glucose oxidase, produces gluconic acid and minute amounts of hydrogen peroxide when in contact with the wound surface. In addition, each charge contains light- and heat-stable, in vitro confirmed antibacterial properties from Leptospermum spp. honeys. The pH level of honey is low (mean 4.4). Bacterial colonization or infection and recalcitrant wound healing situations are often accompanied by high pH values in wound exudates and lowering the pH speeds healing. |
| Why is medical honey irradiated? | Honey may contain spores from Clostridium botulinum, which are inactivated. The ready to use product is delivered sterile. |
| What is Medihoney™? | Medihoney is a mixture of two honeys derived from Australia and New Zealand containing glucose oxidase and Leptospermum compounds which contribute to its antibacterial activity. |
| Legal background | Medihoney™ is licensed for wound care in Australia, Europe and the USA. In Europe a CE-certification exists declaring Medihoney™ as medical product. |
| Practical advances | Medical honey dressings are easy to change without pain and without harm to the regenerating tissue. Malodour from recalcitrant wounds as a result of critical colonization and partial tissue necrosis is successfully abandoned with medical honey due to its antibacterial, anti-inflammatory and debriding effects. Medical honey can be used in all different stages of wound healing, in many different types of wounds and even in patients with diabetes. Medical honey is well accepted by most patients and their families. |
| Drawbacks | Some patients (5 out of 100) experience pain after the application of medical honey to the wound. In some of them, treatment with medical honey has to be stopped. Few patients with pre-existing atopic diseases show local atopic reactions. No severe systemic atopic reaction due to medical honey has been reported in the literature. Medical honey has to be kept in the wound for 12–24 h a day. Thus, it is combined with particular dressings like calcium alginates of hydrofiber dressings, which add substantially to the overall cost of treatment. Depending on the amount of exudate, the dressing with medical honey has to be changed up to 2 times a day in acute inflammatory wounds. The most often practised change interval for medical honey dressings is every 24–48 h. |
| Scientific evidence# | For Medihoney™: one prospectively randomized controlled study considering the prevention of catheter-related bacteremias in patients with dialysis catheters. Many in vitro studies and animal studies confirming the antibacterial properties and the positive effects on wound healing. Growing body of clinical experiences and observational studies derived from independent wound care facilities all over the world. |
#For the medical use of honey in general please refer to the outstanding review of Peter Molan from 2006 (15)
What Makes Medical Honey Effective?
Honey works differently from antibiotics, which attack the bacteria's cell wall or inhibit intracellular metabolic pathways. Honey is hygroscopic, meaning it draws moisture out of the environment and thus dehydrates bacteria. Its sugar content is also high enough to hinder the growth of microbes, but the sugar content alone is not the sole reason for honey's antibacterial properties.
When honey is diluted with water, reducing its high sugar content, it still inhibits the growth of many different bacterial species that cause wound infections (14,15,17–20).
Leptospermum spp. are known by a number of common names in Australia and New Zealand including Tea Tree, Manuka, Goo Bush and Jelly Bush. A least 79 species of Leptospermum have been described in Australia and New Zealand (21). The medical honey, our clinical experience refers to (Medihoney™), consists of standard mixture of different Leptospermum spp. honeys (Fig. 1), and exhibits a standard antibacterial activity as confirmed by appropriate in vitro testing methods (22).
Figure 1.
Pictures of different Leptospermum spp. plants from Australia.
Unlike glucose oxidase, the antibacterial properties from Leptospermum spp. honeys are light- and heat-stable. Neither type of activity is influenced by the final sterilizing procedure of gamma-irradiation (23). Over 100 substances are candidates for the particular antibacterial property of these honeys, but the active ingredient has not yet been identified. Even if the hydrogen peroxide activity is blocked and the osmotic effect of honey is circumvented by dilution, selected honeys from Leptospermum spp. still display significant antibacterial effects. Natural honey of other sources can vary as much as 100-fold in the potency of their antibacterial activity (which is due to hydrogen peroxide) (5–7,24–27).
In addition to its antibacterial properties, medical honey hastens the healing of wounds through its anti-inflammatory effects. The amount of wound exudate is related to the activity of the local inflammatory process, in particular in wounds, which are colonized or infected with bacteria. Thus, the anti-inflammatory action of honey reduces oedema and the amount of exudate by down regulating the inflammatory process. It also reduces pain, as the pain in wounds results from the nerve endings being sensitized by prostaglandins produced in the process of inflammation, as well from the pressure on tissues resulting from oedema.
Due to its high sugar content, honey also prevents pain on dressing changes as it keeps the wound surface moist by mobilizing the oedema from the surrounding tissues.
The anti-inflammatory action of honey has been extensively observed clinically (19,28,29) and in animal models (15). Animals do not demonstrate any placebo effects, as they are incapable of having attitudes influence their healing process, such as believing that natural products would be more effective, or from hearing via the news media of the effectiveness of honey in wound treatment (30). Thus, these observational and histological studies can be cited as convincing evidence for the positive results with honey not being due to a placebo effect. Similarly, this can be concluded also for the observations in wounds in animal models of honey stimulating the rate of angiogenesis, granulation and epithelialization (31,32), which would explain the findings in clinical trials that honey speeds up the healing process (15).
Although the mechanism(s) by which honey speeds up the healing process have not been determined, there are some findings, which provide possible explanations. One way in which honey may work is through its stimulation of an inflammatory response in leukocytes (28,29,33), as inflammation is what triggers the cascade of cellular events that give rise to the production of growth factors which control angiogenesis and proliferation of fibroblasts and epithelial cells. Very recently, Tonks et al. discovered a 5.8 kDA component of manuka honey which stimulates the production of TNF-α in macrophages via Toll-like receptor 4 (34).
Another mechanism may be related to the pH level of honey being low (3.4–5.5; mean 4.4) (35,36). Bacterial colonization or infection and recalcitrant wound healing situations are often accompanied by pH values >7.3 in wound exudates (37,38). It has been demonstrated that acidification of wounds speeds healing (39), this being attributed to the low pH increasing the amount of oxygen off-loaded from hemoglobin in the capillaries. More recently it has also been attributed to suppression of protease activity in wounds by getting away from the neutral pH that is the optimum for their activity (37). Excessive protease activity in a wound can slow or prevent healing by destroying growth factors (which are proteins) and destroying the protein fibres and fibronectin in the wound matrix, attachment to which activates fibroblasts and is necessary for the migration of these and of epithelial cells (http://www.worldwidewounds.com/2005/august/Schultz/Extrace-Matric-Acute-Chronic-Wounds.html). This protease activity results from excessive inflammation. The anti-inflammatory activity of honey would thus remove this impediment to healing, as would the antibacterial activity working through removing infecting bacteria stimulating the inflammatory response.
The remarkable debriding action of honey that has been noted by many would also assist in this by removing slough which is a rich source of bacteria to stimulate an inflammatory response (19).
Last but not least, medical honey successfully manages the problem of malodour from chronic colonized wounds, which results in severe discomfort and social isolation in these patients. We relate the experience of one female patient with relapsed breast cancer after chemotherapy and irradiation. Her husband informed us, that other members of the family would not enter her room, because her wound smelled like a ‘dead rabbit’. This malodour problem was successfully eliminated with the application of medical honey in 2 weeks.
Why is the Final Product Irradiated?
Clostridium botulinum spores pervade our environment, existing in the soil, air, dust and raw agricultural products. Due to the possibility of Clostridial contamination and many reports of infant botulism in the literature since the first description of this syndrome in 1976, it has been recommended by paediatricians, not to feed infants with honey and to place warning labels on packaging, as done in the United Kingdom and Norway (36).
In deep wound cavities the possibility exists of an anaerobic environment, where the spores could proliferate and produce botulinum toxin. Negative effects such as paralysis and cardiac arrhythmia have been described related to systemic effects of the toxin. To eliminate botulism spores with heat, honey must be heated to 120°C (248°F) for 10 min, which results in adverse changes to some of honeys’ beneficial properties. Since spores have occasionally been found in honey, each batch of Medihoney™ is gamma irradiated to inactivate spores such as those from Clostridium spp. This does not have a detrimental impact on the antibacterial activity of honey. On the other hand, irradiating honey is only a safety measure on the side of caution since we (40) could not detect a single case report in the literature of C. botulinum wound infection related to the use of non-irradiated honey in wound care (41).
Medihoney is not an Antiseptic
An ideal wound antiseptic would meet the following criteria (42):
Fast onset of bactericidal action and a remnant, broad spectrum effect against bacteria and fungi, even under the unfavorable conditions of exudating, colonized or infected wounds.
Enhancement and acceleration of the physiologic process of wound healing (debridement, granulation), even if applied for prolonged periods.
No adverse local or systemic effects (allergy, toxicity related to absorption).
Moderate cost even if applied two times daily.
Medihoney meets all of the above criteria except ‘a fast onset of activity’, as it does not seem to produce the desired reduction of bacteria (more than five log steps) and fungi in 1–10 min to be qualified as an antiseptic.
What About Conventional Wound Care Products in Paediatric Oncology?
Some of the parents in our unit have at first been sceptical about the benefits of honey in wound care. However, the scientific evidence for using conventional wound care products in paediatric oncology patients is non-existent, since no prospective randomized studies have been performed in this particular population and no research has been done on the long-term effects of modern conventional treatments such as silver dressings.
There has been a report of a Silver-coated dressing which caused raised liver enzymes and an argyria-like syndrome in an adolescent burn patient (43). Another common treatment is povidone iodine, which has the advantage of antiseptic properties and is well suited for skin disinfection prior to invasive procedures. However, the antiseptic activity of iodine products is hampered by interactions with the protein content of the wound exudate and severe adverse effects of systemic absorption of iodine on thyroid function must be considered in infants and toddlers as well as in adult patients with latent hyperthyreosis.
In principle, the same problem exists with alcohol-containing antiseptics, since they are almost completely absorbed and need to be metabolized by the children, who are treated concomitantly with many other drugs. In contrast, medical honey does not display the problem of systemic absorption and thus can even be utilized in patients with diabetes mellitus without adverse effects on blood glucose levels.
Medical Honey in Severely Immunocompromized Patients?
One of our patients with acute myeloic leukemia in relapse had a wound infection with methicillin-resistant coagulase-negative staphylococci after thoracic surgery for invasive Aspergillus infection of the lung, but was allocated to allogenic bone marrow transplantation. Using medical honey, the infection was eliminated.
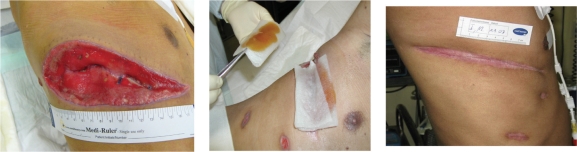
The patient continued to receive medical honey applications during and after the transplantation (Fig. 2), leading to a successful healing without further local or systemic complications.
Figure 2.
Thoracotomy wound of a patient with acute myeloic leukemia after surgical site infection (Medihoney™ was applied on a calcium-alginate dressing and left in the wound for 24 h).
Medical Honey for Recalcitrant Wounds
Many of the 150 wound care patients we have treated with medical honey had previously received specialist attention for recalcitrant wounds (2,3,16,44). When treatment with honey was commenced, their wound status changed to healing from non-healing (2,3,16,44). Similar findings have been reported by others (19,45).
Medihoney™ for the protection Of Catheter Entry Sites
The clinical usefulness of hemodialysis catheters is limited by increased infectious morbidity and mortality. Topical antiseptic agents, such as mupirocin, are effective at reducing this risk but have been reported to select for antibiotic-resistant strains. Johnson et al. (46) performed a randomized, controlled trial comparing the effect of thrice-weekly exit-site application of Medihoney™ versus mupirocin on infection rates in patients who were receiving hemodialysis via tunnelled, cuffed central venous catheters. A total of 101 patients were enrolled. The incidences of catheter-associated bacteremia in honey-treated (n = 51) and mupirocin-treated (n = 50) patients were comparable (0.97 versus 0.85 episodes per 1000 catheter-days, respectively; NS). No exit-site infections occurred. During the study period, 2% of staphylococcal isolates within the hospital were mupirocin resistant. Thrice-weekly application of standardized antibacterial honey to hemodialysis catheter exit sites was safe, cheap, and effective and resulted in a comparable rate of catheter-associated infection to that obtained with mupirocin (although the study was not adequately powered to assess therapeutic equivalence).
The effectiveness of honey against antibiotic-resistant microorganisms and its low likelihood of selecting for further resistant strains suggest that this agent may represent a satisfactory alternative means of chemoprophylaxis in patients with central venous catheters. In our paediatric oncology unit, all entry sites of tunnelled central venous catheters, which show any sign of inflammation or dehiscence, are covered with medical honey.
Honey Treatment for Prevention of Oral Mucositis and for Gingivitis
Many cancer patients suffer from mucositis, a side effect of chemotherapy that attacks the entire gastrointestinal tract from the mouth to the anus. The cancer treatment breaks down the epithelial cells lining the tract, leaving the patient prone to ulcerations and infections. These important and beneficial cells replicate and divide rapidly, which is why in a typical healthy individual wounds in the mouth heal quickly. Chemotherapy does not distinguish between healthy and malignant cells, attacking all that reproduce rapidly, including these epithelial cells.
Thus 20–40% of all cancer patients receiving intensive chemotherapy suffer from mucositis, the number climbs to 80% when chemotherapy and radiation are combined, and staggers even higher in patients receiving treatment for cancer in the head and neck area. Open sores in cancer patients suffering from mucositis leave them susceptible to infection. In a study conducted in 2003, Biswal et al. (47) investigated the use of honey in 40 adult patients with head and neck cancer. Patients consumed 20 ml (one and one-third teaspoon) of pure honey 15 min before, 15 min after and 6 h post-treatment. There was significant reduction in the symptomatic grade three-fourth mucositis (which describes the necessity for morphine infusion and parenteral nutrition) among honey-treated patients compared with controls; i.e. 20% versus 75% (P < 0.001).
The compliance of the honey-treated group of patients was better than controls. A total of 55%patients treated with topical honey showed no change or a positive gain in body weight compared with only 25% in the control arm (P = 0.05). According to a recent review, honey has potential for the treatment of periodontal disease, mouth ulcers and other problems of oral health (48), and a trial has demonstrated a statistically significant difference between chewing gelled honey and chewing chewing-gum in decreasing the number of bleeding sites on gums with gingivitis (49).
Use of Honey for Wound Care in Neonates
Due to the safeness of Medihoney™, we used this product for wound care in premature neonates. One infant patient had a recalcitrant wound after surgical intervention for meningomyelocele. The wound stagnated, and during intensive care treatment, it became colonized with multiresistant Klebsiella oxitoca. Before treatment with Medihoney™ was initiated, the child had received numerous intravenous antibiotics and many other conventional wound care treatments to clear the wound, spending the first 3 months of its life in the hospital. Medihoney™ dressings cleared the wound of the pathogen and allowed the patient to be discharged after 3 weeks. The wound healed completely without further surgical intervention. Viardi et al. (40) published an impressive case series including nine neonates with polymicrobial wound infection after surgery for congenital heart disease. All infants were considered as treatment failures, since chlorhexidine 0.05% plus fusidic acid ointment plus systemic antibiotics did not result in any improvement of the sternal wound infection. All infants showed marked clinical improvement after 5 days of treatment with topical application of 5–10 ml of fresh unprocessed (non-pasteurized, non-irradiated) honey twice daily. The wounds were closed, clean and sterile in all infants after 21 days of honey application. There were no adverse reactions to the treatment. In a recent review for Neonatal Network, Susan Bell reviewed the current literature dealing with the medical use of medical honey in neonatal wound care. Nurses in her unit are using medical honey for this purpose since 2005. She came to the conclusion that the scientific evidence regarding the use of honey for wound treatment in neonates and infants is interesting, but still not strong. The sample sizes in the available clinical studies were small; there were no comparison groups and no randomization. She stated that double-blinded randomized controlled clinical trials with sufficient power are needed to determine the efficacy of honey in both initial wound management and secondary treatment of infected and poorly healing wounds. It has to be questioned, how double or even single (on the side of the patent) blinding can be established in studies, which investigate medical honey for wound care and compare it with other conventional modes of treatment.
Honey Treatment Against Viral Disease
Al-Waili et al. published a cross over trial (9) in which 16 adult patients with a history of recurrent attacks of herpetic lesions, 8 labial and 8 genital, were treated by topical application of honey for one attack and acyclovir cream for another attack. For labial herpes, the mean duration of attacks and pain, occurrence of crusting and mean healing time with honey treatment were 35, 39, 28 and 43% shorter, respectively, than with acyclovir treatment. For genital herpes, the mean duration of attacks and pain, occurrence of crusting and mean healing time with honey treatment were 53, 50, 49 and 59% shorter, respectively, than with acyclovir. Two cases of labial herpes and one case of genital herpes remitted completely with the use of honey. No side effects were observed with repeated applications of honey, whereas three patients developed local itching with acyclovir. The authors came to the conclusion that topical honey application is safe and effective in the management of the signs and symptoms of recurrent lesions from labial and genital herpes (9). According to this experience, we treat children and adults with recurrent herpetic lesions on the lips with medical honey, as soon as a new lesion is developing. In addition, we use topical medical honey in addition to systemic acyclovir in immunocompromized patients with zoster to prevent secondary bacterial skin infection and to accelerate healing of the herpetic lesions.
Medical Honey in Chronic Ocular Surface Diseases
Albietz et al. (50) recently published the results of a study that assessed the effect of Medihoney™ antibacterial honey on the ocular flora in patients with dry eye caused by tear deficiency and/or Meibomian gland disease. In this prospective, open-label pilot study, bacteria isolated from the eyelid margin and conjunctiva were identified and quantified before and at 1 and 3 months after initiation of treatment with topical application of antibacterial honey thrice daily. The total colony-forming units (CFUs) isolated from each of the dry eye subgroups before antibacterial honey use was significantly greater than the total CFU isolated from the non-dry eye group.
Use of Medihoney™ Antibacterial honey significantly reduced total CFUs for the eyelids and the conjunctiva of dry eye subjects from baseline at month 1 and 3. At month 3, the total CFUs for all dry eye subgroups were not significantly different from those of the non-dry eye group. A review of the medical uses of honey (8) has cited several other reports of honey, an ancient treatment for sore eyes, being found to be successful in modern ophthalmology.
Comparing Different Types of Honey
A comparison of different types of honey, which is beyond the scope of this article, may be an important issue in future trials (24). In particular the results obtained by Prof. Descotte from France, presented during several international Apitherapy meetings, point into this direction; unfortunately they have not been published in a medline listed journal. Irish et al. (51) found Jarrah honey (derived from Eucalyptus marginata), which contains higher amounts of glucose oxidase, to be significantly more effective against Candida spp. in vitro; the activity of the other honeys including Medihoney™ against Candida spp. with minimal inhibitory concentrations above 40% (w/v) were not distinguishable from the activity of an artificial honey.
Minimal Requirements for Clinical Use
From a professional medical perspective honey used in wound care should have a proven antibacterial activity against the most important pathogens in wound infection (S. aureus, Pseudomonas aeruginosa) (16,52) measured with an appropriate microbiological in vitro method (5,25,27,51,52). In addition, it should be irradiated (23) in particular if it is used in deep or partially necrotic wounds (53) which possess a potential of anaerobic wound conditions. Surely, we do not criticize the emergency care use of raw honey directly derived from local bee keepers in countries with extremely limited medical resources (54) in which physicians or other health care workers do not have access to conventional wound care products (13,55–57).
Who Should Supervise the Use of Medical Honey in Clinical Practice?
For safety reasons, wound care with medical honey should always be supervised by a physician or an experienced wound care nurse in patients with chronic complicated wounds or with significant comorbidities. The ‘honey approach’ to wound care must be part of a comprehensive wound care concept. Any comorbidity which contributes to the problems in wound healing should be diagnosed and treated thoroughly with state of the art interventions (e.g. bypass surgery in patients with reduced arterial perfusion, compressive bandages in patients with chronic venous ulcers, sufficient control of blood glucose levels in patients with diabetic food syndrome) (58).
Honey Must be Kept in Contact to the Wound
Medical honey dressings should keep the honey in contact with the wound for at least 12 h, but preferably for 24 h. Some patients apply the wound dressing overnight, so as not to restrict their mobility during the day. If the dressing is inappropriate, the honey may be washed out of the wound by exudate. The clinical benefits of medical honey including antibacterial protection, wound cleaning and pH modulation may as a result be reduced. From our experience (2) and the experience of others (59), the best way to keep the honey in the wound is to soak medical honey into a calcium-alginate or hydrofiber dressing, which forms a gel with the honey as it absorbs the exudate. The various registered medical honey products available, some in the form of prepared honey-impregnated dressings, have been described (14,45).
When to Use Systemic Antibiotics Concomitantly
In case of an infected wound or in case of colonization with a multiresistant bacterial isolate, a hospital grade, licensed antiseptic (e.g. octenidine) is applied to the wound in addition to Medihoney™ on the first 2 days of treatment. In patients with neutropenia (neutrophil count <0.5 × 109 l−1 or leukocyte counts <1 × 109 l−1 and no differential count available) systemic antibiotics have to be administered concomitantly after a local wound swab has been taken if fever or local sign reveals soft tissue infection. With hospitals facing increasing problems of bacterial resistance, it is important to note that the use of medical honey has never been observed to foster bacterial resistance (20).
Frequency of Dressing Changes
The frequency of dressing changes depends on the amount of exudate. In early stages, fresh surgical wounds infected with pathogenic bacteria may necessitate a dressing change twice a day. In stable wound care situations, the Medihoney™ dressing has been left in place for up to 7 days (58,60). Prospective studies confirming the safety of such an extended dressing change interval are not yet available.
Compliance Issues
Dunford et al. (61) undertook a four-center feasibility study to determine whether Medihoney™ is an acceptable treatment for patients with leg ulcers in terms of pain relief, odour control and overall patient satisfaction. A total of 40 patients whose leg ulcers had not responded to 12 weeks of compression therapy were recruited. Medihoney dressings were applied on their ulcers for the 12-week study period. All other aspects of their care, including the use of compression bandaging, remained unchanged. Overall, ulcer pain and size decreased significantly and odorous wounds were deodorized promptly.
These results revealed a high patient acceptance for and concordance with this treatment. The same first author reported the care of an adolescent with multiple wounds, infected with resistant bacterial pathogens after meningococcal sepsis and limb amputation (62). Before the use of medical honey, each dressing change had to be performed under general anaesthesia to alleviate pain and anxiety. Shortly after the introduction of medical honey dressings, the wounds improved and dressing changes could be performed without any analgesic medication. We have had similar experiences with children in our clinic. If the medical honey dressing is completely moistened with sterile Ringer solution, it can easily and painlessly be removed without any negative attachment to the wound. In our unit and our wound care ambulance, parents or relatives are educated in the aseptic procedure of the dressing change and thus, they are enabled to perform it at home.
Adverse Effects
There are two important adverse effects related to the use of medical honey. We have observed in about 5% of our patients stinging pain after administration. This problem may be circumvented by the conditioning of the wound surface with a sterile anaesthetic cream. Unfortunately, local anaesthetics cause vasoconstriction. Thus, the application of an anaesthetic to the wound surface may result in an objectionable reduction of local perfusion. In some patients who experienced pain after administration, treatment with medical honey had to be stopped (16) or postponed to later phases of wound healing after the acute inflammatory process has been controlled by other approaches. Two of 150 patients from our patient population showed reproducible local atopic reactions to Medihoney™. Both children had an underlying atopic disposition. We are not aware of any severe systemic atopic reaction related to the use of medical honey from our experience and from the published literature.
Cost Issues
Ingle et al. (63) from South Africa performed a randomized controlled trail to investigated the use of natural honey (monofloral aloe honey) in shallow wounds, donor sites for skin grafting and partial thickness burns in 44 goldmine workers. They compared this group with another group of 43 workers whose wounds were treated with IntraSite™ Gel (Smith and Nephew Ltd). They did not find significant differences in healing time, adverse events (itching, pain) and satisfaction with treatment but the average cost of the use of honey was 4% that of IntraSite™ treatment. More prospective studies investigating the overall cost of treatment with medical honey compared with conventional wound care approaches are awaited (64) in particular in chronic, recalcitrant wounds. In clinical practice, the use of calcium alginate or hydrofiber gauzes and semipermeable dressings to keep the honey in the wound has to be added to the acquisition cost of the medical honey. To contain the demands on personnel resources, it would be of outstanding interest to develop ready to use medical honey dressings, which have to be changed only every 2–3 days even in acute wound care situations.
WoundViewer© Database
We recently developed a database for the standardized prospective collection of datasets considering the clinical use of medical honey products in wound care to foster the collection of clinical evidence to help design prospective comparative studies in the near future.
Conclusion
More prospective randomized studies on a wider range of types of wounds are needed to confirm the safety and efficacy of medical honey in wound care (65). Nonetheless, the current evidence (15) confirming the antibacterial properties and additional beneficial effects of medical honey on wound healing should encourage other wound care professionals to use CE-certified honey dressings with standardized antibacterial activity, such as Medihoney™ products, as an alternative treatment approach in wounds of different natures.
Acknowledgements
The research of K.T. was made possible by the generous support of the Humboldt Foundation through a German Chancellor Scholarship to the author. The development of the WoundvViewer© database for the prospective standardized documentation of wound care courses in patients treated with medical honey has been financially supported by Medihoney Inc. (http:/www.medihoney.com).
References
- 1.Lehrnbecher T, Foster C, Vazquez N, Mackall CL, Chanock SJ. Therapy-induced alterations in host defense in children receiving therapy for cancer. J Pediatr Hematol Oncol. 1997;19:399–417. doi: 10.1097/00043426-199709000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Simon A, Sofka K, Wiszniewsky G, Blaser G, Bode U, Fleischhack G. Wound care with antibacterial honey (Medihoney) in pediatric hematology-oncology. Support Care Cancer. 2006;14:91–7. doi: 10.1007/s00520-005-0874-8. [DOI] [PubMed] [Google Scholar]
- 3.Gaur AH, Liu T, Knapp KM, Daw NC, Rao BN, Neel MD, et al. Infections in children and young adults with bone malignancies undergoing limb-sparing surgery. Cancer. 2005;104:602–10. doi: 10.1002/cncr.21212. [DOI] [PubMed] [Google Scholar]
- 4.Gaur AH, Flynn PM, Shenep JL. Optimum management of pediatric patients with fever and neutropenia. Indian J Pediatr. 2004;71:825–35. doi: 10.1007/BF02730723. [DOI] [PubMed] [Google Scholar]
- 5.Lusby PE, Coombes AL, Wilkinson JM. Bactericidal activity of different honeys against pathogenic bacteria. Arch Med Res. 2005;36:464–7. doi: 10.1016/j.arcmed.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 6.Cooper RA, Molan PC, Harding KG. The sensitivity to honey of gram-positive cocci of clinical significance isolated from wounds. J Appl Microbiol. 2002;93:857–63. doi: 10.1046/j.1365-2672.2002.01761.x. [DOI] [PubMed] [Google Scholar]
- 7.Cooper RA, Molan PC, Harding KG. Antibacterial activity of honey against strains of Staphylococcus aureus from infected wounds. J R Soc Med. 1999;92:283–5. doi: 10.1177/014107689909200604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molan PC. Why honey is effective as a medicine. 1. Its use in modern medicine. Bee World. 1999;80:80–92. [Google Scholar]
- 9.Al-Waili NS. Topical honey application vs. acyclovir for the treatment of recurrent herpes simplex lesions. Med Sci Monit. 2004;10:MT94–8. [PubMed] [Google Scholar]
- 10.Al-Waili NS. Topical application of natural honey, beeswax and olive oil mixture for atopic dermatitis or psoriasis: partially controlled, single-blinded study. Complement Ther Med. 2003;11:226–34. doi: 10.1016/s0965-2299(03)00120-1. [DOI] [PubMed] [Google Scholar]
- 11.Al-Waili NS. Therapeutic and prophylactic effects of crude honey on chronic seborrheic dermatitis and dandruff. Eur J Med Res. 2001;6:306–8. [PubMed] [Google Scholar]
- 12.Al-Waili NS. Clinical and mycological benefits of topical application of honey, olive oil and beeswax in diaper dermatitis. Clin Microbiol Infect. 2005;11:160–3. doi: 10.1111/j.1469-0691.2004.01013.x. [DOI] [PubMed] [Google Scholar]
- 13.Al-Waili NS. Investigating the antimicrobial activity of natural honey and its effects on the pathogenic bacterial infections of surgical wounds and conjunctiva. J Med Food. 2004;7:210–22. doi: 10.1089/1096620041224139. [DOI] [PubMed] [Google Scholar]
- 14.Molan PC, Betts JA. Clinical usage of honey as a wound dressing: an update. J Wound Care. 2004;13:353–6. doi: 10.12968/jowc.2004.13.9.26708. [DOI] [PubMed] [Google Scholar]
- 15.Molan PC. The evidence supporting the use of honey as a wound dressing. Int J Low Extrem Wounds. 2006;5:40–54. doi: 10.1177/1534734605286014. [DOI] [PubMed] [Google Scholar]
- 16.Blaser G, Santos K, Bode U, Vetter H, Simon A. Treatment of MRSA-colonized or infected wounds with Medihoney™ antibacterial honey products. J Wound Care. 2007;16:325–8. doi: 10.12968/jowc.2007.16.8.27851. [DOI] [PubMed] [Google Scholar]
- 17.Molan PC. The role of honey in the management of wounds. J Wound Care. 1999;8:415–8. doi: 10.12968/jowc.1999.8.8.25904. [DOI] [PubMed] [Google Scholar]
- 18.Molan PC. Potential of honey in the treatment of wounds and burns. Am J Clin Dermatol. 2001;2:13–9. doi: 10.2165/00128071-200102010-00003. [DOI] [PubMed] [Google Scholar]
- 19.Molan PC. Re-introducing honey in the management of wounds and ulcers - theory and practice. Ostomy Wound Manage. 2002;48:28–40. [PubMed] [Google Scholar]
- 20.Blair S, Carter D. The potential for honey in the management of wound and infection. J Australian Infection Control. 2005;10:24–31. [Google Scholar]
- 21.Thompson J. A revision of the genus Leptospermum (Myrtaceae). National herbarium of New South Wales, Royal Botanic Gardens, Sydney, Australia. Telopea (Syd.) 1988;3:301–449. [Google Scholar]
- 22.Bang LM, Buntting C, Molan P. The effect of dilution on the rate of hydrogen peroxide production in honey and its implications for wound healing. J Altern Complement Med. 2003;9:267–73. doi: 10.1089/10755530360623383. [DOI] [PubMed] [Google Scholar]
- 23.Molan PC, Allen KL. The effect of gamma-irradiation on the antibacterial activity of honey. J Pharm Pharmacol. 1996;48:1206–9. doi: 10.1111/j.2042-7158.1996.tb03922.x. [DOI] [PubMed] [Google Scholar]
- 24.Molan PC. The antibacterial activity of honey. 2. Variation in the potency of the antibacterial activity. Bee World. 1992;73:59–76. [Google Scholar]
- 25.French VM, Cooper RA, Molan PC. The antibacterial activity of honey against coagulase-negative staphylococci. J Antimicrob Chemother. 2005;56:228–31. doi: 10.1093/jac/dki193. [DOI] [PubMed] [Google Scholar]
- 26.Cooper R, Molan P. The use of honey as an antiseptic in managing Pseudomonas infection. J Wound Care. 1999;8:161–4. doi: 10.12968/jowc.1999.8.4.25867. [DOI] [PubMed] [Google Scholar]
- 27.Cooper RA, Halas E, Molan PC. The efficacy of honey in inhibiting strains of Pseudomonas aeruginosa from infected burns. J Burn Care Rehabil. 2002;23:366–70. doi: 10.1097/00004630-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Tonks AJ, Cooper RA, Jones KP, Blair S, Parton J, Tonks A. Honey stimulates inflammatory cytokine production from monocytes. Cytokine. 2003;21:242–7. doi: 10.1016/s1043-4666(03)00092-9. [DOI] [PubMed] [Google Scholar]
- 29.Tonks A, Cooper RA, Price AJ, Molan PC, Jones KP. Stimulation of TNF-alpha release in monocytes by honey. Cytokine. 2001;14:240–2. doi: 10.1006/cyto.2001.0868. [DOI] [PubMed] [Google Scholar]
- 30.Shmueli A, Shuval J. Are users of complementary and alternative medicine sicker than non-users? Evid Based Complement Alternat Med. 2007;4:251–5. doi: 10.1093/ecam/nel076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta S, Singh H, Varshney A, Prakash P. Therapeutic efficacy of honey in infected wounds in buffaloes. Indian J Anim Sci. 1992;62:521–3. [Google Scholar]
- 32.Bergman A, Yanai J, Weiss J, Bell D, David M. Acceleration of wound healing by topical application of honey. An animal model. Am J Surg. 1983;145:374–6. doi: 10.1016/0002-9610(83)90204-0. [DOI] [PubMed] [Google Scholar]
- 33.Abuharfeil N, Al-Oran R, Abo-Shehada M. The effect of bee honey on proliferative activity of human B- and T-lymphocytes and the activity of phagocytes. Food Agri Immunol. 1999;11:169–77. [Google Scholar]
- 34.Tonks AJ, Dudley E, Porter NG, Parton J, Brazier J, Smith EL, et al. A 5.8-kDa component of manuka honey stimulates immune cells via TLR4. J Leukoc Biol. 2007;82:1147–55. doi: 10.1189/jlb.1106683. [DOI] [PubMed] [Google Scholar]
- 35.Molan PC. The antibacterial activity of honey. 1. The nature of the antibacterial activity. Bee World. 1992;73:5–28. [Google Scholar]
- 36.Aureli P, Franciosa G, Fenicia L. Infant botulism and honey in Europe: a commentary. Pediatr Infect Dis J. 2002;21:866–8. doi: 10.1097/00006454-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 37.Rushton I. Understanding the role of proteases and pH in wound healing. Nurs Stand. 2007;21:68–72. doi: 10.7748/ns2007.04.21.32.68.c4499. [DOI] [PubMed] [Google Scholar]
- 38.Schneider LA, Korber A, Grabbe S, Dissemond J. Influence of pH on wound-healing: a new perspective for wound-therapy? Arch Dermatol Res. 2007;298:413–20. doi: 10.1007/s00403-006-0713-x. [DOI] [PubMed] [Google Scholar]
- 39.Rendel M, Mayer C, Weninger W, Tschachler E. Topically applied of lactic acid increases spontaneous secretion of vascular endothelial growth factor by human constructed epidermis. Br J Dermatol. 2001;145:3–9. doi: 10.1046/j.1365-2133.2001.04274.x. [DOI] [PubMed] [Google Scholar]
- 40.Vardi A, Barzilay Z, Linder N, Cohen HA, Paret G, Barzilai A. Local application of honey for treatment of neonatal postoperative wound infection. Acta Paediatr. 1998;87:429–32. doi: 10.1080/08035259850157048. [DOI] [PubMed] [Google Scholar]
- 41.Bell SG. The therapeutic use of honey. Neonatal Netw. 2007;26:247–51. doi: 10.1891/0730-0832.26.4.247. [DOI] [PubMed] [Google Scholar]
- 42.Kramer A, Daeschlein G, Kammerlander G, Abdriessen A, Aspöck C, Bergemann R, et al. Consensus recommendation for the choice of antiseptic agents in wound care (Article in German) Hygiene und Medizin. 2004;29:147–57. [Google Scholar]
- 43.Trop M, Novak M, Rodl S, Hellbom B, Kroell W, Goessler W. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648–52. doi: 10.1097/01.ta.0000208126.22089.b6. [DOI] [PubMed] [Google Scholar]
- 44.Cooper RA, Molan PC, Krishnamoorthy L, Harding KG. Manuka honey used to heal a recalcitrant surgical wound. Eur J Clin Microbiol Infect Dis. 2001;20:758–9. doi: 10.1007/s100960100590. [DOI] [PubMed] [Google Scholar]
- 45.Visavadia BG, Honeysett J, Danford MH. Manuka honey dressing: An effective treatment for chronic wound infections. Br J Oral Maxillofac Surg. 2006 doi: 10.1016/j.bjoms.2006.09.013. Electronically published on November 17, 2006, [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Johnson DW, van Eps C, Mudge DW, Wiggins KJ, Armstrong K, Hawley CM, et al. Randomized, controlled trial of topical exit-site application of honey (Medihoney) versus mupirocin for the prevention of catheter-associated infections in hemodialysis patients. J Am Soc Nephrol. 2005;16:1456–62. doi: 10.1681/ASN.2004110997. [DOI] [PubMed] [Google Scholar]
- 47.Biswal BM, Zakaria A, Ahmad NM. Topical application of honey in the management of radiation mucositis: a preliminary study. Support Care Cancer. 2003;11:242–8. doi: 10.1007/s00520-003-0443-y. [DOI] [PubMed] [Google Scholar]
- 48.Molan PC. The potential of honey to promote oral wellness. Gen Dent. 2001;49:584–9. [PubMed] [Google Scholar]
- 49.English H, Pack A, Molan P. The effects of manuka honey on plaque and gingivitis: a pilot study. J Int Acad Periodontol. 2004;6:63–7. [PubMed] [Google Scholar]
- 50.Albietz JM, Lenton LM. Effect of antibacterial honey on the ocular flora in tear deficiency and Meibomian gland disease. Cornea. 2006;25:1012–9. doi: 10.1097/01.ico.0000225716.85382.7b. [DOI] [PubMed] [Google Scholar]
- 51.Irish J, Carter DA, Shokohi T, Blair SE. Honey has an antifungal effect against Candida species. Med Mycol. 2006;44:289–91. doi: 10.1080/13693780500417037. [DOI] [PubMed] [Google Scholar]
- 52.Mullai V, Menon T. Bactericidal activity of different types of honey against clinical and environmental isolates of Pseudomonas aeruginosa. J Altern Complement Med. 2007;13:439–41. doi: 10.1089/acm.2007.6366. [DOI] [PubMed] [Google Scholar]
- 53.Yapucu Gunes U, Eser I. Effectiveness of a honey dressing for healing pressure ulcers. J Wound Ostomy Continence Nurs. 2007;34:184–90. doi: 10.1097/01.WON.0000264833.11108.35. [DOI] [PubMed] [Google Scholar]
- 54.Mphande AN, Killowe C, Phalira S, Jones HW, Harrison WJ. Effects of honey and sugar dressings on wound healing. J Wound Care. 2007;16:317–9. doi: 10.12968/jowc.2007.16.7.27053. [DOI] [PubMed] [Google Scholar]
- 55.Subrahmanyam M. A prospective randomised clinical and histological study of superficial burn wound healing with honey and silver sulfadiazine. Burns. 1998;24:157–61. doi: 10.1016/s0305-4179(97)00113-7. [DOI] [PubMed] [Google Scholar]
- 56.Subrahmanyam M. Honey impregnated gauze versus polyurethane film (OpSite) in the treatment of burns—a prospective randomised study. Br J Plast Surg. 1993;46:322–3. doi: 10.1016/0007-1226(93)90012-z. [DOI] [PubMed] [Google Scholar]
- 57.Subrahmanyam M. Honey-impregnated gauze versus amniotic membrane in the treatment of burns. Burns. 1994;20:331–3. doi: 10.1016/0305-4179(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 58.Gethin G, Cowman S. Case series of use of Manuka honey in leg ulceration. Int Wound J. 2005;2:10–5. doi: 10.1111/j.1742-4801.2005.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.White R, Cooper R, Molan PC, editors. Honey: A Modern Wound Management Product. Aberdeen, UK: Wounds, UK; 2005. [Google Scholar]
- 60.Gethin G. Is there enough clinical evidence to use honey to manage wounds? J Wound Care. 2004;13:275–8. doi: 10.12968/jowc.2004.13.7.26637. [DOI] [PubMed] [Google Scholar]
- 61.Dunford CE, Hanano R. Acceptability to patients of a honey dressing for non-healing venous leg ulcers. J Wound Care. 2004;13:193–7. doi: 10.12968/jowc.2004.13.5.26614. [DOI] [PubMed] [Google Scholar]
- 62.Dunford C, Cooper R, Molan P. Using honey as a dressing for infected skin lesions. Nurs Times. 2000;96(Suppl 14):7–9. [PubMed] [Google Scholar]
- 63.Ingle R, Levin J, Polinder K. Wound healing with honey–a randomised controlled trial. S Afr Med J. 2006;96:831–5. [PubMed] [Google Scholar]
- 64.Canter PH, Coon JT, Ernst E. Cost-effectiveness of complementary therapies in the United Kingdom-a systematic review. Evid Based Complement Alternat Med. 2006;3:425–32. doi: 10.1093/ecam/nel044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wahner-Roedler DL, Vincent A, Elkin PL, Loehrer LL, Cha SS, Bauer BA. Physicians’ attitudes toward complementary & alternative medicine and their knowledge of specific therapies: a survey at an academic medical center. Evid Based Complement Alternat Med. 2006;3:495–501. doi: 10.1093/ecam/nel036. [DOI] [PMC free article] [PubMed] [Google Scholar]