Abstract
The continuous improvement of crop plants is essential for agriculture in the coming decades and relies on the use of genetic variability through breeding. However, domestication and modern breeding have reduced diversity in the crop germplasm. Global gene banks conserve diversity, but these resources remain underexplored owing to a lack of efficient strategies to isolate important alleles. Here we describe a large-scale allele-mining project at the molecular level. We first selected a set of 1,320 bread wheat landraces from a database of 16,089 accessions, using the focused identification of germplasm strategy. On the basis of a hierarchical selection procedure on this set, we then isolated 7 resistance alleles of the powdery mildew resistance gene Pm3, doubling the known functional allelic diversity at this locus. This targeted approach for molecular utilization of gene bank accessions reveals landraces as a rich resource of new functional alleles. This strategy can be implemented for other studies on the molecular diversity of agriculturally important genes, as well as for molecular breeding.
Keywords: allele mining, powdery mildew, gene banks, wheat landraces
Wheat is one of the most important human food crops, and production has to be increased significantly in the next decades (1). This has to be done in a sustainable way, with less agricultural input but increased yield. Natural biodiversity has been used for enriching diversity of cultivated plants with novel alleles to improve productivity by breeding (2). For example, tomato lines with introgression of wild alleles from Lycopersicon hirsutum outperformed the original variety by 48% and 33% for yield and fruit color, respectively (3). In barley, the mlo-11 gene providing resistance against barley powdery mildew originated from Ethiopian landraces (4). During agricultural development, early domesticates were gradually replaced first by landraces and traditional varieties, and later by genetically less-diverse modern cultivars. This has resulted in genetic bottlenecks and loss of diversity in the breeding germplasm (5). Therefore, gene bank collections are essential to conserve biodiversity and thus pay big dividends to agriculture when used efficiently (6).
Despite many studies illustrating the utilization of genetic resources in plant breeding (7, 8), the global germplasm collections are underutilized for many crop species. One important reason is the sheer number of accessions stored, and these large collections cannot be phenotyped or genotyped by an average laboratory or breeding program. Thus, the major challenge to identify rare alleles from large collections is to identify a subset of accessions that is economically feasible to screen while maximizing the probability of finding the desired trait. Core collections have been widely promoted as a means of approaching large collections by defining smaller subsets that represent maximum diversity. As an alternative approach, the focused identification of germplasm strategy (FIGS) was recently suggested as a rational method that uses information about the environment from which accessions with specific traits have been collected to predict where selection pressures for adaptive traits may occur. On the basis of this information, trait-specific sets can then be assembled from large collections (9).
Genetic variation is caused by allelic diversity at the genetic loci contributing to a particular trait. Allele mining is a relatively underexplored method to identify new alleles at a known locus. However, it is being used in important plant species, such as maize and barley (ref. 10; N. Stein, et al., personal communication). Because the first wheat disease-resistance genes have been cloned (11–16), the sequence information of these genes should allow the analysis of genetic diversity at these loci and the identification of new alleles through allele mining. Pm3, existing in 7 functionally distinct alleles (Pm3a to Pm3g), is the only wheat powdery mildew resistance gene cloned to date (13, 14, 16). In addition to the alleles from the bread wheat gene pool, a new functional allele recently has been described in a wild tetraploid wheat accession (17). All Pm3 alleles encode coiled-coil (CC), nucleotide binding site (NBS), and leucine-rich repeat (LRR) proteins. The high sequence conservation of the Pm3 alleles suggested their recent evolution from the ancestral sequence Pm3CS that is a susceptible Pm3 allele (16).
Here we describe the successful and efficient screening of gene bank accessions for the molecular identification of allelic variants at the Pm3 locus. We report the cloning of 7 previously undescribed functional Pm3 alleles from a targeted subset of wheat landraces that was established by FIGS, demonstrating its successful use in combination with allele mining. We also found that at least 2 of these Pm3 alleles confer slow-acting resistance. The strategy described here can be implemented for other diversity and molecular breeding studies involving agriculturally important traits.
Results
Establishment and Screening of a Focused Set of Wheat Landraces for Pm3-Based Powdery Mildew Resistance.
To maximize the chances of finding functional diversity of powdery mildew resistance while limiting the number of accessions to a workable size, we used FIGS to define a subset of accessions (FIGS powdery mildew set). From a virtual collection of 16,089 accessions, we identified 1,320 accessions drawn from 323 geographic sites with potentially high selection pressure for powdery mildew resistance. To select the resistant accessions, the complete set of 1,320 landraces was screened with different powdery mildew isolates (18). A total of 211 accessions that showed complete or intermediate resistance against at least one powdery mildew race were further analyzed at the molecular level. They were first screened for the presence of a Pm3-like gene with a diagnostic sequence-tagged site marker and second for the presence of the already-known Pm3 alleles (18, 19). This led to the identification of 111 landraces as candidates for the isolation of Pm3 alleles that were positive for the Pm3 diagnostic fragment but lacked any of the known Pm3 alleles.
Cloning of Pm3 Alleles from Selected Wheat Landraces.
The isolation of Pm3 alleles was performed on 56 landraces completely resistant to at least one powdery mildew isolate, whereas lines with intermediate resistance were not considered further. The Pm3 coding sequences were successfully amplified from 45 landraces, cloned, and sequenced. In the remaining landraces, amplification of a Pm3 sequence was not possible, perhaps due to the absence of a coding gene or low sequence homology at the primer binding sites. The analysis of sequence diversity led to the identification of 16 previously unknown Pm3 allelic sequences, because several landraces possessed identical alleles (Fig. 1 and supporting information Table S1). Among the 45 sequences, 9 were identical to the susceptible Pm3CS (16), suggesting that the observed resistance is not due to a Pm3 type of gene but is caused by other known or still uncharacterized Pm genes. Among the remaining 36 landraces from which the previously undescribed Pm3 alleles were isolated, 24 accessions originated from Turkey, with the majority from Eastern parts of the country; the remainder originated from Afghanistan (mostly localized in the Bamian Province), Pakistan, Azerbaijan, and Turkmenistan (9, 1, 1, and 1, respectively; Table S1). In good correspondence with this distribution, 8 of the 16 previously undescribed alleles originated from landraces collected in Turkey and 4 alleles from Afghanistan (Table S1). Different landraces with identical Pm3 alleles always originated from the same country, except Pm3_13636, which was found in 2 landraces originating from Afghanistan and Turkey. However, for alleles found in multiple accessions, the collection sites were not necessarily geographically close within the country (e.g., the accessions from Turkey; Table S1).
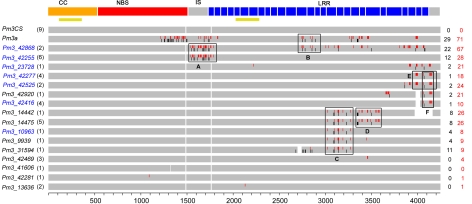
Fig. 1.
Schematic representation of sequence alignment (exons) of previously undescribed Pm3 alleles with the known alleles. This figure presents the Pm3 gene structure and the alignment of previously undescribed Pm3 alleles with the known alleles Pm3CS and Pm3a. The domains encoded by the Pm3 alleles are depicted at the top [CC, NBS, Interspacer (IS), and LRR]. Red bars in the Pm3 alleles and numbers in red indicate the polymorphic nucleotides as compared with Pm3CS, leading to non-synonymous changes in the protein. Black bars and numbers in black represent the polymorphic nucleotides leading to synonymous mutations. Boxes A, B, C, D, E, and F indicate the putative gene conversion tracts among these alleles. Numbers in parentheses correspond to the number of landraces possessing that particular Pm3 allele. Golden bars indicate the regions used for VIGS constructs. The functional alleles Pm3_42416, Pm3_42525, Pm3_23728, Pm3_42255, Pm3_10963, Pm3_42277, and Pm3_42868 are labeled in blue.
Sequence Diversity of the Previously Unknown Pm3 Alleles.
The DNA sequence comparison of the 16 previously undescribed Pm3 sequences with the known alleles Pm3a to Pm3g and Pm3CS showed an overall high similarity (Fig. 1). The previously undescribed alleles also consist of 2 exons separated by an intron of 200 bp and encode resistance proteins with an NBS and an LRR domain associated at the N-terminus with a CC domain. Nine of the previously undescribed alleles are 4442 bp long, corresponding to the size of the ancestral reference sequence Pm3CS, whereas 7 alleles bear insertions and deletions (InDels) (Pm3_42868, Pm3_42255, Pm3_42416, Pm3_42920, Pm3_31594, Pm3_41606, and Pm3_14442) making them variable in size. None of these InDels altered the ORFs except for Pm3_14442, which is a pseudogene missing 294 bp, resulting in a frame shift. Illegitimate recombination seems to be the cause of deletions: we have identified 4-bp imperfect repeat motifs at the breakpoint of the deletions in Pm3_42416 and Pm3_42920 that could have served as template sequence for illegitimate recombination (20). In Pm3_31594, a perfect 3-bp repeat was found at the breakpoint of the deletion, also suggesting illegitimate recombination.
The CC-NBS encoding region of the new alleles is highly conserved, with the exception of a 3-bp deletion and a single base change in the NBS regions of Pm3_41606 and Pm3_42281, respectively. The intron sequence was identical among the previously undescribed alleles and the known Pm3 alleles, except for 5 previously uncharacterized alleles (Pm3_42416, Pm3_42277, Pm3_42525, Pm3_42920, and Pm3_23728) that differed by maximally 6 bp. The comparison of allelic sequences showed 2 groups of sequences. The first group of 4 sequences (Pm3_42469, Pm3_41606, Pm3_42281, and Pm3_13636) has very few or only a single polymorphic residue(s) compared with the reference sequence Pm3CS. In the second group, polymorphic residues are present as sequence blocks (blocks A–F, Fig. 1) completely or partially shared between alleles. This existence of defined sequence blocks indicates the frequent sequence exchange between alleles, possibly by gene conversion. The differences in proteins encoded by the previously undescribed Pm3 alleles lead to a total of 87 aa changes compared with PM3CS, in addition to the InDels. At a majority of sites, different alleles have a single residue change (72 of 87 positions), with 13 sites having 2 alternative residues and 1 site having 3 alternative residues (R/D/Y at position 1332 instead of W in PM3CS).
Functional Analysis of Pm3 Candidate Resistance Genes by Virus-Induced Gene Silencing.
To determine whether the resistance observed in landraces with previously undescribed alleles is based specifically and solely on the Pm3 alleles, we used virus-induced gene silencing (VIGS) to suppress the Pm3 gene expression. We used 2 silencing constructs carrying fragments of the CC (BSMV.Pm3_CC) and the LRR domain (BSMV.Pm3_LRR), respectively (golden bars in Fig. 1), and a control construct (BSMV.Lr10_CC) silencing the Lr10 leaf rust resistance gene by a fragment of its CC region. The landraces carrying previously undescribed Pm3 allelic sequences (Pm3_42416, Pm3_10963, Pm3_42525, Pm3_23728, Pm3_42469, and Pm3_42255) were infected with these constructs and further challenged with avirulent Bgt isolate 98275. The leaves of landraces carrying the alleles Pm3_42416 and Pm3_42255 (IG42416 and IG42255) lost resistance to Bgt isolate 98275 after infection with BSMV.Pm3_CC and BSMV.Pm3_LRR (Fig. 2) but remained resistant when inoculated with the control BSMV.Lr10_CC. This demonstrates that Pm3_42416 and Pm3_42255 confer the observed powdery mildew resistance in these lines. In contrast, resistance was not altered in the landraces with the alleles Pm3_42469, Pm3_42525, and Pm3_10963, indicating that here the resistance might result either from a gene different from Pm3 or from a combination of Pm3 and additional genes. In the case of landrace IG23728 (Pm3_23728), results were not conclusive, possibly owing to a heterogenous seed mixture for this accession. Because of this relatively high number of inconclusive results (4 of 6 genes), we used transient transformation for further functional studies on the candidate alleles.
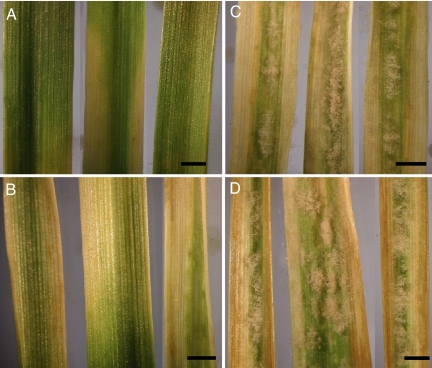
Fig. 2.
BSMV-mediated virus induced silencing of Pm3_42416 and Pm3_42255. (A) The landrace containing Pm3_42416 infected with powdery mildew but not with the virus served as resistant control. (B) Infection with BSMV.Lr10_CC (control viral construct) did not alter the observed resistance against mildew. (C and D) The resistant landrace turned susceptible to powdery mildew when infected with BSMV.Pm3_CC and BSMV.Pm3_LRR, respectively. Similar observations were made for Pm3_42255. (Scale bars, 0.5 cm.)
Identification of Functional Pm3 Alleles by Transient Transformation.
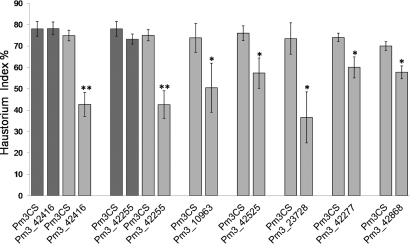
In total, 13 previously undescribed alleles were tested by transient transformation: 8 alleles against isolate Bgt 98275, 4 with Bgt 97011, and 1 with Bgt 96224. It was not possible to identify an appropriate isolate for Pm3_14475, Pm3_13636, and Pm3_42281 because several independent landraces with these alleles behaved differently for the tested isolates, suggesting that the observed resistance was not due to the Pm3 allele. We used the nonfunctional Pm3CS allele (16) as a control. Seven alleles (Pm3_42416, Pm3_42525, Pm3_23728, Pm3_42255, Pm3_10963, Pm3_42277, and Pm3_42868) showed a significant reduction in the haustorium index in comparison with Pm3CS (Fig. 3). Transformation with the remaining 6 alleles (Pm3_42920, Pm3_9939, Pm3_42469, Pm3_31594, Pm3_14442, and Pm3_41606) did not result in a reduction of the haustorium index (Fig. S1). To check for race specificity of the resistance conferred by the previously undescribed alleles, the 2 alleles showing the most significant reduction in haustorium index (Pm3_42416 and Pm3_42255) were also tested against the virulent isolate 97019. No reduction of haustorium indices was observed compared with Pm3CS (Fig. 3), demonstrating that the observed activity was not due to overexpression but to race specificity of gene action.
Fig. 3.
Functional analysis of the previously undescribed Pm3 alleles in the transient transformation assay. The graph shows the transient assay results for the 7 functional Pm3 alleles tested with corresponding avirulent Bgt isolates, in comparison with the susceptible control Pm3CS. The haustorium index (percentage of cells with haustoria) is indicated by the mean ± SD of 3 independent experiments, each contributing at least 50 interactions. *Significant differences at P = 0.05; **significant differences at P = 0.001. Transient assay results for Pm3_42416 and Pm3_42255, upon infection with the virulent isolate, are represented by dark gray bars.
In conclusion, the alleles Pm3_42416, Pm3_42255, Pm3_23728, Pm3_10963, Pm3_42525, Pm3_42277, and Pm3_42868 are previously uncharacterized, functionally active forms of Pm3 that are called Pm3l, Pm3m, Pm3n, Pm3o, Pm3p, Pm3q, and Pm3r, respectively. Five of these 7 alleles were isolated from landraces originating from Turkey, whereas Pm3_10963 and Pm3_23728 were from Afghanistan and Turkmenistan, respectively (Table S1).
Are the Previously Uncharacterized Pm3 Alleles Late-Acting Resistance Genes?
To determine whether the relatively high haustorium indices of previously undescribed alleles in comparison with the known Pm3 alleles (13, 14) are due to late action of the previously undescribed alleles, we performed lactophenol trypan blue staining. The resistance in landraces with alleles Pm3_42416 and Pm3_42255 was silenced by Pm3-specific VIGS, thereby allowing detailed study of functional activity and specificity of these 2 genes in planta. Therefore, we specifically monitored pathogen growth and cell death [hypersensitive response (HR)] in these landraces at 6 different time points [2, 3, 4, 5, 6, and 7 days after inoculation (dpi)]. Cultivar Chul carrying Pm3b was included as a comparison with a known allele.
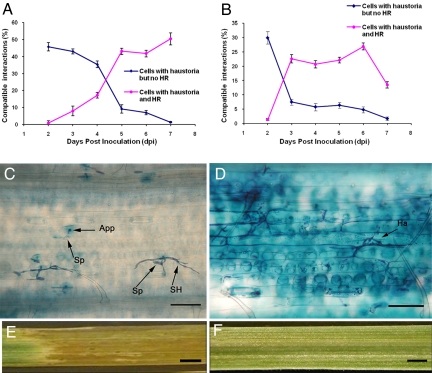
Formation of secondary hyphae and haustoria in the attacked host epidermal cells was observed at a frequency of 46% and 30% at 2 dpi for Pm3_42416 and Pm3_42255, respectively. Thus, the relatively low rate of resistance observed in the transient assay at 2 dpi is in agreement with the low degree of resistance observed in planta at this time point. For IG42416 and IG42255, almost all cells having a haustorium were associated with hypersensitive cell death at 5 dpi and 3 dpi, respectively (Fig. 4). Resistance triggered by the 2 studied genes was thus mainly based on a late hypersensitive cell death occurring after fungal penetration. For Chul, no susceptible interactions were observed at any time point, indicating a rapid Pm3b-mediated resistance already at 2 dpi. These experiments demonstrated that, although the resistance induction of these 2 alleles is slower than the activity of the known alleles, they confer full resistance and are agronomically useful genes.
Fig. 4.
Time course analysis of pathogen growth on the landraces with the Pm3_42416 or Pm3_42255 allele. (A and B) Percentages of cells with haustoria in the epidermal cells of landraces with Pm3_42416 (A) and Pm3_42255 (B). Blue lines indicate the haustorium index calculated from the cells with haustorium but no HR; pink lines mark the cells with haustoria that also exhibit HR. These observations were recorded at different time points (2, 3, 4, 5, 6, and 7 dpi) indicated at the bottom. (C) Microscopic view of cells from the landrace with Pm3_42416 at 2 dpi. This shows the occurrence of interactions with and without haustoria formation, whereas none of these leads to cell death at this stage. Arrows indicate spore (Sp), secondary hyphae (SH), and appressorium (App). (D) The cells with haustoria are accompanied by HR at 5 dpi. A blue-stained cell indicates cell death resulting from HR. Arrow indicates haustorium (Ha). (E and F) Macroscopic view of the infected leaf segments from landraces with Pm3_42416 and Pm3_42255 at 8 dpi, respectively. (Scale bars, 100 μm in C and D, 0.5 cm in E and F.)
One possible reason for the slow response could be low or delayed expression of these genes. Therefore, semiquantitative RT-PCR was carried out to compare levels of differentially expressed mRNAs in the landraces possessing the 2 previously undescribed alleles (Pm3_42416 and Pm3_42255) and some of the known rapidly acting Pm3 alleles (Pm3b, Pm3e, and Pm3g or the susceptible allele Pm3CS). No difference in the expression levels of the Pm3 alleles was observed (Fig. S2).
To determine whether the previously undescribed Pm3 alleles have unique specificities compared with known Pm3 alleles, we characterized the landraces carrying Pm3_42416 and Pm3_42255 with a set of 6 additional (total 10) powdery mildew isolates (Table S2). On the basis of the data, we conclude that these 2 previously undescribed alleles encode new Pm3 specificities and thus broaden the spectrum of available resistance genes for breeding.
Discussion
Pm3 Allele Mining in a Subset of Landraces Specifically Selected for the Isolation of Powdery Mildew Resistance Genes.
With more than 560,000 wheat accessions held in nearly 40 gene banks globally (The Global Crop Diversity Trust, 2007; http://www.croptrust.org/documents/web/Wheat-Strategy-FINAL-20Sep07.pdf), it is not possible to screen the entire collection for specific traits. Thus, the problem is to find the most effective way to choose a subset of lines to screen. The set should be of a manageable size for handling and molecular analysis, with a reasonable probability for containing the relevant trait. Core collections have been proposed as a strategy to provide representation of the genetic diversity of a crop in a collection (21). However, core collections aim to maximize genetic diversity, whereas breeders and biologists are usually interested only in one or a few traits at a time from a genetic resource collection (22). This was also the case in this study: we were specifically interested in previously undescribed functional alleles of the gene Pm3.
Our large-scale allele mining for powdery mildew resistance on the FIGS set of landraces demonstrated the effectiveness of FIGS to identify a manageable and diverse set of material for screening. Forty percent of the collection sites chosen in the process yielded resistant accessions, and almost 16% of the accessions carried resistance with a high frequency of variation for the Pm3 loci. This is an excellent result from a subset of approximately 8% of the starting collection of more than 16,000 accessions. In the search for more variation in the powdery mildew resistance loci, these results can be used in an iterative process to further refine the FIGS selection process by feeding the data of the resistant accessions into the next round of selection. For example, matching the geo-coordinates of the accessions originating from eastern Turkey or Bamian province in Afghanistan to that of collection sites of gene bank accessions may help to identify additional wheat lines with powdery mildew resistance.
The 7 previously undescribed, functionally active Pm3 alleles extend the previously known Pm3 allelic series and in fact double the number of functionally active Pm3 alleles in bread wheat to 14. Thus the Pm3 alleles now represent one of the largest allelic series of a resistance gene in plants. Pm3 alleles exhibit a high level (>97%) of sequence identity among themselves (ref. 16 and this study), which is in contrast to the RPP13 locus of downy mildew resistance in Arabidopsis, which shows high polymorphism between alleles (23). The allelic series of Mla locus of barley and the flax rust resistance L locus also show a lower level of homology among alleles. Sequence identity of 94% was reported between the 6 cloned Mla alleles (16, 24–26), and 8 of 13 L alleles were found to be >90% identical (27).
A Combined Strategy for Functional Analysis of the Previously Undescribed Pm3 Alleles.
We have used VIGS as well as a transient transformation assay for functional analysis of previously undescribed Pm3 alleles. Although VIGS has proven to be an effective method for several dicots (28), it is only recently that its successful use in monocots such as barley and wheat has been demonstrated. VIGS was shown to silence the barley phytoene desaturase gene (29) and was successfully used for silencing of leaf rust resistance genes Lr21 (30) and Lr1 (15). In our study, we found that VIGS was an effective strategy to assign function to the previously undescribed alleles. However, VIGS will only give conclusive results if the resistance in a line of interest is based specifically on the silenced gene. According to our data, in a majority of lines resistant to powdery mildew the resistance was not due to the Pm3 allele, or there was more than 1 resistance gene present. As we learnt from transient assays, 3 of the Pm3 alleles present in the 4 lines that showed no silencing by VIGS turned out to be active resistance alleles. In these 3 lines, at least 1 additional Pm gene must be present, masking the effect of Pm3 silencing. Because of the frequency in the gene pool of such lines with multiple Pm genes, transient or stable transformation seems to be best suited for functional analysis of a candidate resistance gene. Nevertheless, if a gene can be silenced by VIGS, the resistance in the donor landrace can immediately be attributed to the gene. In such plant accessions, the particular gene can then be studied in detail, allowing assessment of resistance activity and specificity, as was demonstrated for 2 of our previously undescribed Pm3 alleles.
Transient transformation has previously been found to be an effective method to study gene function in wheat (13, 14, 31). When tested with this method, 7 previously undescribed Pm3 alleles showed significant reduction in the haustorium index as compared with the susceptible Pm3CS. Among the tested genes were also the 2 genes Pm3_42416 and Pm3_42255, which according to VIGS are the only mildew-resistance genes in the accessions from which they were isolated. These 2 genes were found to be late acting, whereas the known Pm3 alleles provide a rapid resistance response without formation of haustoria (ref. 13 and this study). Complete resistance by these 2 alleles in fact occurred later than 2 dpi when observation was made in the transient assay. This explains the relatively low reduction of the haustorium indices by these alleles in the transient assay. Thus, these genes are not weak alleles but simply have a different time-course of activity, making them interesting for future functional studies at the molecular level. A late hypersensitive cell death–associated resistance has also been reported for some barley powdery mildew resistance genes, for example Mla3 and Mla7 (25, 32), and might be a widespread phenomenon in allelic series of R genes.
Sequence Diversity Lies in the LRR Region of Pm3 Alleles.
The isolation of 16 previously undescribed alleles was possible because of the conservation of Pm3 gene structure and the high level of sequence identity among the Pm3 alleles. The 100% conservation of the N-terminal region encoding a CC domain among the previously undescribed as well as the known Pm3 alleles suggests a highly conserved function of this domain in Pm3 resistance. The alleles have a mosaic pattern of sequence blocks, which probably are the result of rearrangement of variation present in the ancestral alleles or which might have arisen from gene conversion events. The finding of variability mainly in the LRR domain of the previously undescribed alleles is in agreement with the suggested role of this domain in recognition specificity (33). In the case of flax rust resistance locus L, the major sequence variation among the L alleles was also found in the LRR domain (27). The previously undescribed Pm3 alleles also show differences that originated from InDels and point mutations. InDels were also found in the functional flax L alleles (27) and among the various members of the Mla family (34). On the basis of the identification of conserved small repeats flanking the InDels in Pm3 alleles, we propose that these InDels have originated from illegitimate recombination. Illegitimate recombination has been suggested to be a major evolutionary mechanism that is at the basis of the size variability of the LRR domain of R proteins (35).
Application of Allele Mining in FIGS Sets for Breeding and Basic Research.
Plant breeding relies on the identification of genetic diversity present in the crop gene pools that can provide functional variants of genes. Our data suggest that FIGS is an effective sampling strategy for the efficient and targeted establishment of subsets from gene bank collections. Allele mining on such subsets then offers a rapid approach to identify new alleles as compared with traditional ways of identifying new sources of resistance. However, the absence of molecular sequence information for the majority of agronomically important genes is a major limiting factor. For accessing the diversity stored in gene banks, a concentrated effort is essential to identify the genes involved in agronomically relevant traits. Only then will allele mining in FIGS sets fully develop its potential.
The previously undescribed genes double the number of the known functional Pm3 alleles in bread wheat to 14. The previously undescribed alleles provide additional diversity for studies on the molecular function and specificity of the Pm3 alleles while enriching the genetic basis for resistance breeding in wheat. From results of this pilot project, we conclude that FIGS combined with an allele mining approach bears a high potential to be applied to other important crop plants for targeting important traits or genes.
Materials and Methods
Plant Material and FIGS.
A FIGS approach was used to assemble a set of 1,320 bread wheat landraces from a total of 16,089 accessions present in 3 different gene banks of the Australian Winter Cereal Collection, the International Center for Agriculture Research in the Dry Areas, and the N.I. Vavilov Institute of Plant Industry (Russia). The 1,320 accessions originated from Turkey (420), Iran (393), Afghanistan (292), Pakistan (131), Armenia (34), Turkmenistan (16), Russia (9), India (6), Azerbaijan (10), Uzbekistan (1), Serbia and Montenegro (3), Bulgaria (3), Macedonia (1), and Romania (1).
The eco-geographic profile of 400 accessions with known powdery mildew resistance was used as a template to identify environmentally similar collection sites from the FIGS database of 16,089 landraces (refs. 9 and 18; Street K, et al., unpublished data). Individual accessions were selected using multivariate statistical procedures that determined how eco-geographically similar the collection site of a given accession was to the resistant set template.
Powdery Mildew Infections and Isolates.
The powdery mildew infections and the scoring were performed as described by Kaur, et al. (18). The choice of isolates used for screening and characterization was made on the basis of their virulence/avirulence patterns on the lines carrying known Pm3 alleles (Table S2).
Isolation of Pm3 Alleles.
Alleles were amplified by using Pm3 locus-specific, long-range PCR amplification, followed by nested long-range PCR (13, 14) using PfuUltra high-fidelity DNA polymerase (Stratagene). Amplified fragments were cloned into the multiple cloning site of expression vector PGY1 (31). DNA sequencing was performed with an Applied Biosystems Capillary Sequencer 3730.
Sequence Analysis.
Sequence assembly was performed using the Gap4 program of the Staden Package. The ClustalX software (36) was used for sequence alignments, which were further analyzed in the program Genedoc (http://www.nrbsc.org/gfx/genedoc/index.html). The different R protein domains (CC, NBS, and LRR) were determined according to Meyers, et al. (37) and Yahiaoui, et al. (13).
Virus-Induced Gene Silencing.
PCR-amplified fragments from the Pm3 gene were inserted into the γ-subfragment of the viral genome (29, 30). The infectious constructs of barley stripe mosaic virus RNAs were prepared by in vitro transcription using T7 DNA-dependent RNA polymerase (mMESSAGE mMACHINE T7 Kit; Ambion).
Single-Cell Transient Transformation Assay and Microscopy.
Transient gene expression assays (13, 31) were performed as described by Yahiaoui, et al. (16). Leaves of the powdery mildew–susceptible line Chancellor were bombarded with a 1:1 (wt/wt) mixture of pUbiGUS containing the GUS reporter and the PGY1 control vector containing the Pm3CS gene or the previously undescribed Pm3 alleles. Eight alleles (Pm3_42416, Pm3_42920, Pm3_9939, Pm3_10963, Pm3_42525, Pm3_23728, Pm3_42255, and Pm3_42469) were tested against isolate Bgt 98275, 4 alleles (Pm3_31594, Pm3_42277, Pm3_42868, and Pm3_14442) with Bgt 97011, and 1 allele (Pm3_41606) with Bgt 96224. For selection of the powdery mildew isolate for the transient assay, we took advantage of the fact that the same allele was identified in several landraces. Therefore, the isolate to which all independent landraces with identical alleles showed resistance was used to infect the bombarded leaves.
Staining for hypersensitive response was performed on leaf segments of resistant landraces using lactophenol trypan blue.
Semiquantitative RT-PCR.
Total RNA was extracted from leaves of 10-day-old seedlings using TRIzol reagent (Invitrogen Life Technologies). For RT, 2 μg of total RNA was denatured at 70 °C for 5 min in the presence of 0.06 μg of oligo(dT)20 primers. RT was done with the M-MLV reverse transcriptase kit (Promega). Aliquots of the reverse transcripts (2 μL from 1:8 dilution) were then amplified in a 25-μL PCR containing 0.2 mM specific oligonucleotide primers.
Supplementary Material
Acknowledgments.
We thank the gene banks of Australian Winter Cereal Collection, the International Center for Agriculture Research in the Dry Areas (Syria), and the N.I. Vavilov Institute of Plant Industry (Russia) for providing the seed material; Dr. S. Scofield for help with setting up the VIGS system; Drs. S. Bieri and C. Loutre for the modified barley stripe mosaic virus RNA with Pm3 and Lr10 fragments, respectively; and Dr. S. Travella for help with semiquantitative RT-PCR. This project was funded by FP6 EU Grant “Bioexploit” and by Swiss National Science Foundation Grant 3100–105620.
Footnotes
The authors declare no conflict of interest.
Data deposition: The previously undescribed Pm3 sequences have been deposited in the Genbank database (accession nos. Pm3_9939 = FJ212300, Pm3_42255 = FJ212301, Pm3_42868 = FJ212302, Pm3_31594 = FJ212303, Pm3_13636 = FJ212304, Pm3_41606 = FJ212305, Pm3_42281 = FJ212306, Pm3_14475 = FJ212307, Pm3_10963 = FJ212308, Pm3_42469 = FJ212309, Pm3_23728 = FJ212310, Pm3_42277 = FJ212311, Pm3_42416 = FJ212312, Pm3_42525 = FJ212313, Pm3_42920 = FJ212314, and Pm3_14442 = FJ212315).
This article contains supporting information online at www.pnas.org/cgi/content/full/0904152106/DCSupplemental.
References
- 1.Hoisington D, et al. Plant genetic resources: What can they contribute toward increased crop productivity? Proc Natl Acad Sci USA. 1999;96:5937–5943. doi: 10.1073/pnas.96.11.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gur A, Zamir D. Unused natural variation can lift yield barriers in plant breeding. PLoS Biol. 2004;2:e245. doi: 10.1371/journal.pbio.0020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernacchi D, et al. Advanced backcross QTL analysis of tomato. II. Evaluation of near-isogenic lines carrying single-donor introgressions for desirable wild QTL-alleles derived from Lycopersicon hirsutum and L pimpinellifolium. Theor Appl Genet. 1998;97:170–180. [Google Scholar]
- 4.Piffanelli P, et al. A barley cultivation associated polymorphism conveys resistance to powdery mildew. Nature. 2004;430:887–891. doi: 10.1038/nature02781. [DOI] [PubMed] [Google Scholar]
- 5.Tanksley SD, McCouch SR. Seed banks and molecular maps: Unlocking genetic potential from the wild. Science. 1997;277:1063–1066. doi: 10.1126/science.277.5329.1063. [DOI] [PubMed] [Google Scholar]
- 6.Johnson RC. Gene banks pay big dividends to agriculture, the environment and human welfare. PLoS Biol. 2008;6:e148. doi: 10.1371/journal.pbio.0060148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu J, Bai G, Cai S, Dong Y, Ban T. New Fusarium head blight resistant sources from Asian wheat germplasm. Crop Sci. 2008;48:1090–1097. [Google Scholar]
- 8.Singrun C, Rauch P, Morgounov A, Hsam S, Zeller F. Identification of powdery mildew and leaf rust resistance genes in common wheat (Triticum aestivum L. ). Wheat varieties from the Caucasus, Central and Inner Asia. Genet Resources Crop Evol. 2004;51:355–370. [Google Scholar]
- 9.Mackay MC, et al. Focused identification of germplasm strategy—FIGS. In: Black CK, Panozzo JF, Rebetzke GJ, editors. Cereals 2004. Proceedings of the 54th Australian Cereal Chemistry Conference and the 11th Wheat Breeders' Assembly; 21–24 September 2004; Canberra, Australian Capital Territory. Melbourne: Royal Australian Chemical Institute; 2004. pp. 138–141. [Google Scholar]
- 10.Harjes CE, et al. Natural genetic variation in lycopene epsilon cyclase tapped for maize biofortification. Science. 2008;319:330–333. doi: 10.1126/science.1150255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang L, et al. Map-based cloning of leaf rust resistance gene Lr21 from the large and polyploid genome of bread wheat. Genetics. 2003;164:655–664. doi: 10.1093/genetics/164.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feuillet C, et al. Map-based isolation of the leaf rust disease resistance gene Lr10 from the hexaploid wheat (Triticum aestivum L.) genome. Proc Natl Acad Sci USA. 2003;100:15253–15258. doi: 10.1073/pnas.2435133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yahiaoui N, Srichumpa P, Dudler R, Keller B. Genome analysis at different ploidy levels allows cloning of the powdery mildew resistance gene Pm3b from hexaploid wheat. Plant J. 2004;37:528–538. doi: 10.1046/j.1365-313x.2003.01977.x. [DOI] [PubMed] [Google Scholar]
- 14.Srichumpa P, Brunner S, Keller B, Yahiaoui N. Allelic series of four powdery mildew resistance genes at the Pm3 locus in hexaploid bread wheat. Plant Physiol. 2005;139:885–895. doi: 10.1104/pp.105.062406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cloutier S, et al. Leaf rust resistance gene Lr1, isolated from bread wheat (Triticum aestivum L. ) is a member of the large psr567 gene family. Plant Mol Biol. 2007;65:93–106. doi: 10.1007/s11103-007-9201-8. [DOI] [PubMed] [Google Scholar]
- 16.Yahiaoui N, Brunner S, Keller B. Rapid generation of new powdery mildew resistance genes after wheat domestication. Plant J. 2006;47:85–98. doi: 10.1111/j.1365-313X.2006.02772.x. [DOI] [PubMed] [Google Scholar]
- 17.Yahiaoui N, Kaur N, Keller B. Independent evolution of functional Pm3 resistance genes in wild tetraploid wheat and domesticated bread wheat. Plant J. 2009;57:846–856. doi: 10.1111/j.1365-313X.2008.03731.x. [DOI] [PubMed] [Google Scholar]
- 18.Kaur N, Street K, Mackay M, Yahiaoui N, Keller B. Molecular approaches for characterization and use of natural disease resistance in wheat. Eur J Plant Pathol. 2008;121:387–397. [Google Scholar]
- 19.Tommasini L, Yahiaoui N, Srichumpa P, Keller B. Development of functional markers specific for seven Pm3 resistance alleles and their validation in the bread wheat gene pool. Theor Appl Genet. 2006;114:165–175. doi: 10.1007/s00122-006-0420-1. [DOI] [PubMed] [Google Scholar]
- 20.Devos KM, Brown JKM, Bennetzen JL. Genome size reduction through illegitimate recombination counteracts genome expansion in Arabidopsis. Genome Res. 2002;12:1075–1079. doi: 10.1101/gr.132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortiz R. No just seed repositories: A more pro-active role for gene banks. Gene Conserve. 2002;1:21–24. [Google Scholar]
- 22.Mackay MC. One core collection or many? In: Hodgkin T, Brown AHD, van Hintum ThJL, Morales AAV, editors. Core Collections of Plant Genetic Resources. Oxford: John Wiley & Sons; 1995. pp. 199–210. [Google Scholar]
- 23.Rose LE, et al. The maintenance of extreme amino acid diversity at the disease resistance gene, RPP13 in Arabidopsis thaliana. Genetics. 2004;166:1517–1527. doi: 10.1534/genetics.166.3.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou FS, et al. Cell-autonomous expression of barley Mla1 confers race-specific resistance to the powdery mildew fungus via a Rar1-independent signaling pathway. Plant Cell. 2001;13:337–350. doi: 10.1105/tpc.13.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen QH, et al. Recognition specificity and RAR1/SGT1 dependence in barley Mla disease resistance genes to the powdery mildew fungus. Plant Cell. 2003;15:732–744. [Google Scholar]
- 26.Halterman DA, Wise RP. A single-amino acid substitution in the sixth leucine rich repeat of barley MLA6 and MLA13 alleviates dependence on RAR1 for disease resistance signaling. Plant J. 2004;38:215–226. doi: 10.1111/j.1365-313X.2004.02032.x. [DOI] [PubMed] [Google Scholar]
- 27.Ellis JG, Lawrence GJ, Luck JE, Dodds PN. Identification of regions in alleles of the flax rust resistance gene L that determine differences in gene-for-gene specificity. Plant Cell. 1999;11:495–506. doi: 10.1105/tpc.11.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burch-Smith TM, Anderson JC, Martin GB, Dinesh-Kumar SP. Applications and advantages of virus-induced gene silencing for gene function studies in plants. Plant J. 2004;39:734–746. doi: 10.1111/j.1365-313X.2004.02158.x. [DOI] [PubMed] [Google Scholar]
- 29.Holzberg S, Brosio P, Gross C, Pogue GP. Barley stripe mosaic virus-induced gene silencing in a monocot plant. Plant J. 2002;30:315–327. doi: 10.1046/j.1365-313x.2002.01291.x. [DOI] [PubMed] [Google Scholar]
- 30.Scofield SR, Huang L, Brandt AS, Gill BS. Development of a virus-induced gene-silencing system for hexaploid wheat and its use in functional analysis of the Lr21-mediated leaf rust resistance pathway. Plant Physiol. 2005;138:2165–2173. doi: 10.1104/pp.105.061861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schweizer P, Christoffel A, Dudler R. Transient expression of members of the germin-like gene family in epidermal cells of wheat confers disease resistance. Plant J. 1999;20:541–552. doi: 10.1046/j.1365-313x.1999.00624.x. [DOI] [PubMed] [Google Scholar]
- 32.Boyd LA, Smith PH, Foster EM, Brown JKM. The effects of allelic variation at the Mla resistance locus in barley on the early development of Erysiphe graminis f. sp. hordei and host responses. Plant J. 1995;7:959–968. [Google Scholar]
- 33.DeYoung BJ, Innes RW. Plant NBS-LRR proteins in pathogen sensing and host-defense. Nat Immunol. 2006;7:1243–1249. doi: 10.1038/ni1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei F, Wing RA, Wise RP. Genome dynamics and evolution of the Mla (powdery mildew) resistance locus in barley. Plant Cell. 2002;14:1903–1917. doi: 10.1105/tpc.002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wicker T, Yahiaoui N, Keller B. Illegitimate recombination is a major evolutionary mechanism for initiating size variation in plant resistance genes. Plant J. 2007;51:631–641. doi: 10.1111/j.1365-313X.2007.03164.x. [DOI] [PubMed] [Google Scholar]
- 36.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyers BC, Kozik A, Griego A, Kuang H, Michelmore RW. Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell. 2003;15:809–834. doi: 10.1105/tpc.009308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.