Abstract
Across sexually reproducing species, males and females are in conflict over the control of reproduction. At the heart of this conflict in a number of taxa is male harassment of females for mating opportunities and female strategies to avoid this harassment. One neglected consequence that may result from sexual harassment is the disruption of important social associations. Here, we experimentally manipulate the degree of sexual harassment that wild female guppies (Poecilia reticulata) experience by establishing replicated, semi-natural pools with different population sex ratios. We quantify the effects of sexual harassment on female social structure and the development of social recognition among females. When exposed to sexual harassment, we found that females had more disparate social networks with limited repeated interactions when compared to females that did not experience male harassment. Furthermore, females that did not experience harassment developed social recognition with familiar individuals over an 8-day period, whereas females that experienced harassment did not, an effect we suggest is due to disruption of association patterns. These results show that social network structure and social recognition can be affected by sexual harassment, an effect that will be relevant across taxonomic groups and that we predict will have fitness consequences for females.
Keywords: familiarity, Poecilia reticulata, sexual conflict, sexual harassment, social network, social recognition
1. Introduction
Sexual conflict, where males and females differ in their reproductive interests, is widespread across sexually reproducing species (Arnqvist & Rowe 2005). This conflict is rooted in male reproductive success generally being limited by their access to females, while female reproductive success is often dependent on the availability of energetic resources for offspring production (Emlen & Oring 1977). A resultant conflict over the outcome of male–female interactions can thus develop such that the optimal reproductive scenario is different for males and females (Chapman 2006) and we see the development of behaviours and adaptations that can benefit one sex while being costly to the other (Moore et al. 2003). Central to recognizing and understanding sexual conflict and its implications for evolutionary processes such as sexual selection and sexually antagonistic coevolution is a thorough analysis of the costs that females incur from male-mating strategies (Arnqvist & Rowe 2005; Hosken & Stockley 2005).
Sexual harassment, i.e. repeated male coercion of females to obtain a mating (Clutton-Brock & Parker 1995), promotes sexual conflict because of the asymmetry in costs to males and females. Well-examined costs to females of sexual harassment include energetic costs (Clutton-Brock & Langley 1997), increased predation risk (Rowe 1994), reduced feeding opportunities (Magurran & Seghers 1994a) and physical injury (Blanckenhorn et al. 2002). Such costs may decrease female lifetime fitness and increase mortality (Meader & Gilburn 2008; Sakurai & Kasuya 2008), and selection has resulted in females developing a number of strategies to reduce their exposure to male harassment. For example, females may avoid habitats that contain high densities of males (Darden & Croft 2008), form alliances to defend themselves from male attacks (Silk 2007b) or accept advances from males where resistance would otherwise probably result in death or injury (Mesnick & Leboeuf 1991). A continued conflict between the sexes thus exists, which is a profound evolutionary driving force (Chapman et al. 2003).
One as of yet neglected consequence that may result from sexual harassment is the disruption of important social associations due to the presence of harassing males and female avoidance strategies. Social animals are often interconnected into complex heterogeneous social networks (Croft et al. 2008), and the structure of social networks can have important fitness implications for individuals as it sets the stage for key behaviours such as foraging, mating and antipredator behaviour (Krause et al. 2007). If male sexual harassment disrupts female social structure it may lead to fitness costs for females (Silk 2007a,b). Previous work has demonstrated that sexual harassment can influence female habitat use, activity and movement patterns across a range of taxa (Trillmich & Trillmich 1984; Krupa et al. 1990; Stone 1995; Darden & Croft 2008). For example, in Grevy's zebra (Equus grevyi), rates of female movement are increased with increased sexual harassment (Sundaresan et al. 2007). As a result, one can hypothesize that female avoidance strategies in response to male sexual harassment will influence female–female social network structure by, for example, breaking female–female social ties and generating more dispersed female–female social networks (i.e. fewer connections between females). A disruption of female social ties could conceivably have implications for the opportunity for females to develop social familiarity and social recognition where repeated or prolonged encounters are required for recognition to develop (see Griffiths & Ward 2006; Ligout & Porter 2006 for reviews). While there is growing interest in social components of fitness (Silk 2007a,b) and the mechanisms underpinning social networks in animals (Croft et al. 2008), the potential effect and implications of sexual harassment on female social structure remains unknown.
Here, we examine directly how males in a population influence female social network structure and how this in turn influences the development of social recognition among females. We use the Trinidadian guppy (Poecilia reticulata), a species of small freshwater fish where we see extremely high levels of male harassment of females (Magurran 2005), as a model system. In this species, males spend the majority of their time in pursuit of females, employing both courtship and forced or coerced mating tactics (Magurran 2005). Previous research has documented high energetic costs of harassment to females, as indicated by decreased foraging opportunities (Magurran & Seghers 1994a), and females are known to employ a number of behaviours to reduce this harassment, including fleeing from harassing males (Magurran & Seghers 1994b) and occupying habitats that contain a low density of males such as areas of high predation risk (Croft et al. 2006b; Darden & Croft 2008). Social familiarity is known to be important in structuring guppy social networks (Croft et al. 2004, 2006a) and they are capable of individual recognition (Ward et al. 2009). Under laboratory conditions, familiarity among females can take up to 12 days to develop, suggesting that repeated encounters are important for the development of social recognition in this species (Griffiths & Magurran 1997).
We undertake two experiments in which we establish replicated populations of fish in semi-natural pools under two different sex ratios. In the first experiment, we collect information on patterns of social association between all fish in the population and use social network analysis to quantify the population social fine structure (Croft et al. 2008). We then compare this structure between populations that have experienced different sex ratios and quantify the effect of males on female–female social structure. In the second experiment, we quantify the effect of males on the development of female–female social familiarity by recording shoaling preferences by females for others from the same or a different population over a 12-day period, testing the hypothesis that males will disrupt the opportunity for females to develop social recognition.
2. Material and methods
(a) Study population
The study was carried out using wild-caught guppies from the lower portion of the Turure River (10°40′20′ N 61°09′60′ W) in the Northern Mountain Range Trinidad from May–June 2007 and April–May 2008. Adult male and female guppies were caught in two-metre seine nets from pools in the river spaced over a distance of 400 m and individually marked using visual implant elastomer (Croft et al. 2003). All experiments were carried out in outdoor semi-natural pools (180 cm diameter, algae-coated substrate from the river of origin; aged tap water 14 cm deep).
(b) The effect of males on female social network structure
For each replicate, eight large females were placed in an experimental pool (N=12) on day 0 and left to acclimatize for 24 hours. On day 1, one of two treatments was assigned to each experimental pool. For treatment 1 (mixed-sex treatment, N=6), five male guppies (23.1±0.2 mm) were added to the pool with the eight large females (34.0±0.4 mm); and for treatment 2 (same-sex treatment, N=6), five small (male-sized) females (25.7±0.5 mm) were added to the pool with the eight large females (34.0±0.3 mm). The fish were left to acclimatize for 24 hours, after which the social network structure of each population was documented by recording shoal composition (photographed using a Nikon D40x digital camera) once per minute for a 15-minute sampling period in each quarter of the pool by an observer positioned at the side of the pool.
For each sampling event, any two fish were defined as associating if they were observed in the same shoal (defined as being within four body lengths of each other (see Croft et al. 2004)). We used a Newman-weighted association index (Newman 2001) to quantify pairwise associations in which pairs of individuals observed in a group of size g are given a weighting
reflecting the fact that a given pair in a small group is more likely to be interacting than a pair in a large group. These association weights were then accumulated over the sampling period and the matrix of associations was used to construct social networks (Croft et al. 2008). For each replicate, the association matrix was used to construct two types of network: (i) a large-female social network (LFSN) that only included the large females from a given pool and (ii) an all-inclusive social network (AISN) that included all individuals from a given pool. For each LFSN, we calculated the average unweighted degree measured as the average of the total number of network neighbours that each female in the network had. We also calculated the average Newman association index for each of these networks. For each AISN, we calculated the average-weighted degree of classes of individuals (i.e. males and large females), measured as the average of the sum of each individual's associations with others in the network (i.e. the edges linking each individual to each of its network neighbours; Wasserman & Faust 1994). Lower values of weighted degree for an individual indicate lower levels of association for that individual. For each AISN, we also calculated the average-weighted reach of classes of individuals (again, males and large females). The weighted reach between a pair of individuals is the value of the weakest connection along the strongest path (greatest sum of ties) between those two individuals (Wasserman & Faust 1994), and indicates how great the connectivity among individuals is with lower values indicating lower connectivity. These two measures indicate how central a node is in the network and are referred to as centrality measures (Wasserman & Faust 1994). Average degree, average-weighted degree and average reach were calculated in UCINET 6 for Windows (Analytic Technologies, Inc.).
We tested for differences in LFSN structure between treatments using a multivariate general linear model with Newman association index and unweighted degree as dependent variables. For the AISNs, we combined the weighted reach and weighted degree into a single measure of centrality using a principal component analysis (PCA) and used the first component for further analysis. We tested for treatment differences in large female centrality with an independent samples t-test and for sex differences in centrality in the mixed-sex networks with a paired t-test. All statistical tests were carried out in SPSS v. 14.0 for Windows (SPSS, Inc.).
(c) The effect of males on female–female familiarity
For the experiment testing the effect of males on the development of female–female social recognition, pools were populated with fish (day 0) in the same two sex ratios as in the first experiment (eight large females (33.5±0.2 mm) plus five males (25.1±0.2 mm) and eight large females (33.3±0.2 mm) plus five small females (28.5±0.2 mm)); this time with 12 replicates of each treatment (i.e. 24 networks total). In this experiment, all fish in a replicate were placed in the pools simultaneously on day 0. Since we wanted to test for social recognition among females, we had to control for the possible effect of habitat odour cues that could be used to ‘recognize’ familiars during shoaling trials (see Ward et al. 2005). Distinctive habitat odour cues were likely to develop in each of our semi-natural pools and to control for this in our experimental design, we subdivided each 180 cm diameter pool to give two semicircular experimental pools. We used a mesh barrier that restricted fish movement between pool halves and prevented the fish from the two halves from having any tactile or visual contact. The water within each pool was circulated daily to ensure adequate mixing of the halves. One replicate of a treatment was then placed in one-half with another replicate of the same treatment in the other half. In this way, the unfamiliar stimulus fish used during shoaling trials could come from the same pool as the test fish, but without the test fish ever having encountered them.
Shoaling trials were run under natural light conditions on days 4, 8 and 12. During trials, we measured the time that focal females spent with a shoal of two familiar females versus a shoal of two unfamiliar females during a 10-minute test period. Fish were tested in a 40×20 cm tank containing water taken from their own pool. Shoal fish compartments (5×20 cm) were created by erecting a perforated acetate barrier at either end of the tank, leaving a 30×20 cm open field between the shoal fish compartments as the test arena. Focal fish were recorded as shoaling with stimulus fish if they were within 5 cm of the barrier to a shoal compartment. Two females from each replicate were tested singly on each test day and their shoaling times averaged for further analysis, so that only one value for each replicate was included in the analysis per test day. Each female acted as a test fish and as a stimulus shoal fish only once to avoid pseudoreplication.
We tested for the development of familiarity among large females using a repeated-measures general linear model (SPSS v. 14.0) as in Griffiths & Magurran (1997). We used the proportion of time spent with each stimulus shoal as our dependent variable and treatment and familiarity as the main effects with day as the within-subject effect.
3. Results
(a) The effect of males on female social network structure
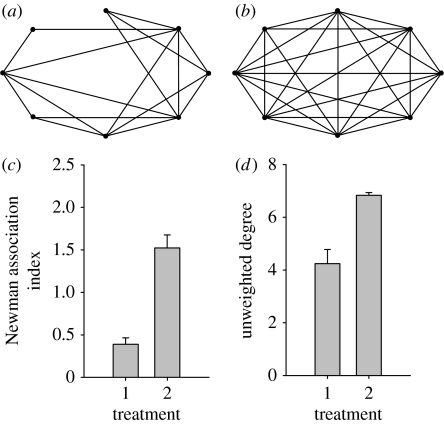
We found striking differences between treatments in the network structure of the LFSNs (F2,9=23.975, p<0.0001; figure 1a,b). There was a lower overall level of association (fewer repeated interactions as indicated by a lower average Newman association index) among females in the presence of males compared to when there were no males present (F1,10=44.258, p<0.0001, figure 1c), and large females had contact with fewer other large females in the network (as indicated by a lower average unweighted degree) when males were present compared with when they were not (F1,10=22.810, p=0.001; figure 1c).
Figure 1.
Example of LFSNs observed (a) in the mixed-sex treatment and (b) in the same-sex treatment. (c–d) Summary of network measures on female–female associations illustrating (c) the average Newman index and (d) the average unweighted degree for LFSNs in the two treatments. Error bars represent standard error. 1, mixed sex; 2, same sex.
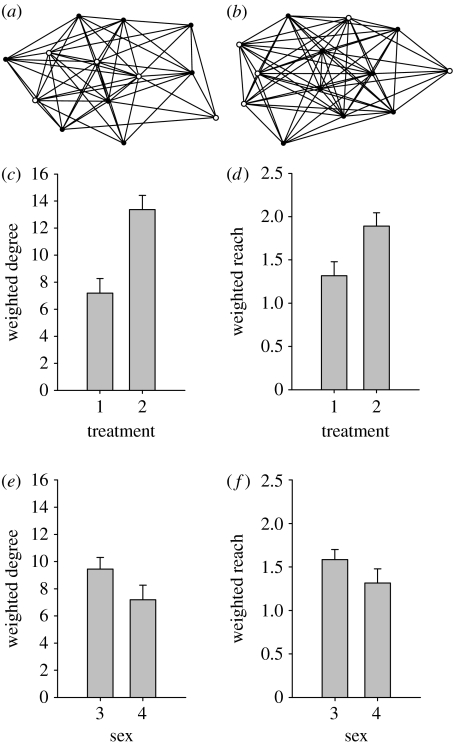
In the analysis of the AISNs, averages of weighted degree and weighted reach show that large females in the same-sex social networks are more central in the network than large females in mixed-sex social networks (t-test on PCA component 1: 95.3% of variance with a 0.98 contribution of each variable, t10=−3.318, p=0.008; figure 2a–c). They also show that this is because males occupy the central positions in the mixed-sex networks when compared with the large females in those networks (paired t-test on PCA component 1, t5=3.775, p=0.013; figure 2b,d).
Figure 2.
(a) Example of AISNs observed in the mixed-sex treatment (large females (filled circles); males (unfilled circles)) and (b) in the same-sex treatment (large females (filled circles); small females (unfilled circles)). (c–f) Summary of network measures on associations illustrating (c) the average weighted degree and (d) the average weighted reach for AISNs in the two treatments, and (e) the average weighted degree and (f) the average weighted reach for males and females in AISNs in the mixed-sex treatment. Error bars represent standard error. (1, mixed sex; 2, same sex; 3, male; 4, female.)
(b) The effect of males on female–female familiarity
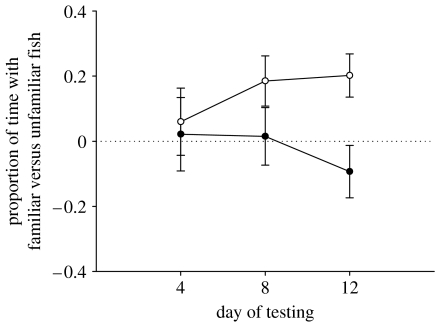
We found a significant effect of the interaction between day, treatment and familiarity in our repeated-measures analysis (F2,46=4.270, p=0.021), and the results of a post hoc analysis of variance (table 1) revealed that large females in the same-sex treatment exhibited recognition of familiars on day 8 and day 12, while those from the mixed-sex treatment did not (figure 3); although there was a tendency for these latter females to do so on day 12 with a slight negative preference (figure 3) that differed from the preference exhibited by females in the same-sex treatment (t21=−2.796, p=0.011).
Table 1.
Results of post hoc analyses of variance with familiarity as the main effect to test the interaction day×treatment×familiarity in the repeated-measures general linear model (see text).
| treatment | day | F1,22 | p-value |
|---|---|---|---|
| mixed sex | 4 | 0.115 | 0.738 |
| 8 | 0.024 | 0.878 | |
| 12 | 2.900 | 0.103 | |
| same sex | 4 | 0.684 | 0.417 |
| 8 | 11.500 | 0.003 | |
| 12 | 18.200 | <0.0001 |
Figure 3.
Differences in the proportion of time focal fish spent with familiar fish and unfamiliar fish of the total time they spent shoaling (familiar minus unfamiliar) (same-sex treatment (unfilled circles) and mixed-sex treatment (filled circles)). Positive values indicate a bias for shoaling with familiar individuals. Error bars represent standard error.
4. Discussion
In this study, we found that males greatly influenced female social structure, the ability of females to develop social recognition and even female–female association preferences. In populations where females experienced sexual harassment, they had a lower degree of association with other females and were more peripheral in the social network. Furthermore, females that were housed with males did not develop familiarity with females from the same social network, although there was a tendency for them to do so after 12 days with what appeared to be a preference for unfamiliar females. By contrast, females that did not experience sexual harassment developed social recognition and displayed a significant preference for others from within their own network after 8 days. Overall, our results suggest that male sexual harassment impacts social network structure and has implications for the development of social recognition and female social bonds, an effect that is likely to be relevant for a diverse array of taxa.
Across animal taxa, individuals exhibit non-random social associations that ultimately define the social structure of populations (Croft et al. 2008). Understanding why certain patterns of association develop and how inter-individual associations and interactions affect population-level structure (e.g. connectedness and fragmentation) is essential in our endeavour to unravel the functions and implications of social organization. The results of our first experiment show that males have a previously unconsidered effect on female–female social network structure, weakening female–female ties and leading to females occupying more peripheral positions in the social network. Previous work on guppies has shown that females may experience up to one forced copulation attempt (a sneaky mating) from males every minute (Magurran & Seghers 1994b), and that this sexual harassment constrains female behaviour leading to lost feeding opportunities (Magurran & Seghers 1994a). The disruption of female social structure most probably results from males influencing female shoaling and foraging activities by harassing and chasing individual females. While disruption due to sexual harassment from males probably has the largest effect on social network structure, females reducing their ties with other females due to sexual interest in males could potentially be a factor in this equation. For example, females may approach males to solicit mating events and thus reduce female–female encounters. However, in the guppy system, such events are rare in comparison with the level of male coercion of females to obtain matings (Magurran & Seghers 1994b), and sexual harassment is thus more likely to constrain female behaviour and determine social structure.
Social associations in animals are often cultivated over time through repeated interactions, which may be particularly important for the development of social recognition (Ligout & Porter 2006). Previous work on guppies suggests that social familiarity may take an extended time period to develop, up to 12 days (Griffiths & Magurran 1997). However, more recent work on three-spined sticklebacks (Gasterosteus aculeatus) suggests that recognition can be achieved quickly when habitat odour differs between familiar and unfamiliar fish (Ward et al. 2005). Our experimental set-up allowed us to control for the effect of habitat-mediated familiarity and shows that the development of social familiarity between females is disrupted by the presence of males, presumably due to the observed limited contact between females. The hindrance of the development of inter-individual familiarity is likely to have fitness consequences (Griffiths & Ward 2006; Ligout & Porter 2006; Silk 2007a). For example, previous work with fish has shown that shoals of familiar individuals outperform randomly assembled shoals in foraging tasks (Morrell et al. 2008) and display more coordinated antipredator behaviour, which is thought to lead to a reduced risk of predation (Chivers et al. 1995). Social familiarity is also known to be important for mediating aggression (Utne-Palm & Hart 2000) and stabilizing group hierarchy (Höjesjö et al. 1998). Thus, one may hypothesize that female groups with reduced social familiarity due to male harassment may suffer decreased foraging success, increased predation risk and increased aggression.
Although marginally non-significant, our results also suggest that females that experience male harassment may indeed develop social recognition, but it undoubtedly takes them longer to do so. Furthermore, while we observe positive preference for familiar individuals between females in the absence of male harassment, females that experienced sexual harassment actually preferentially associated with unfamiliar individuals once familiarity started to develop. Social preferences for familiar individuals in fish and other taxa are well documented (see Griffiths & Ward 2006; Ligout & Porter 2006 for reviews in fish and mammals). However, a preference for associating with unfamiliar individuals has been reported in at least one study, where it appeared to be linked with unfavourable environmental conditions (Frommen et al. 2007). It is possible that the observed tendency for a preference for unfamiliar individuals in our study is also a response to unfavourable conditions, i.e. high levels of male harassment perhaps leading to decreased foraging success. Preferring to associate with unfamiliar individuals may be a way for individuals to change their social environment in an attempt to ensure more favourable conditions. The role of the social and ecological environment in driving preference for unfamiliar versus familiar individuals certainly makes for an interesting avenue for future research.
A female's fitness is influenced by many ecological factors. The results presented here provide the first insight into the effect of male sexual harassment on the disruption of female social networks and social recognition, which we predict will have fitness consequences and represent a key currency in the trade-off between potentially costly male avoidance behaviour and acceptance of male sexual advances. Our work leads us to hypothesize that in wild populations where females experience sexual harassment, social network structure and social recognition will differ as a function of male density and the opportunity for females to avoid sexual harassment. Future studies testing these predictions in wild populations are eagerly anticipated. In conclusion, in a recent paper Rankin & Kokko (2007) ask ‘do males matter?’ in the context of population dynamics, from our results, we think that we can answer this very simply for population social dynamics: ‘yes, they do’.
Acknowledgements
We thank Mathew Edenbrow for assistance in the field and S. Alonzo and two anonymous referees for their insightful comments on a previous version of the manuscript. Funding was provided to D.P.C. by NERC (NE/E001181/1).
References
- Arnqvist G., Rowe L. Princeton University Press; Princeton, NJ: 2005. Sexual conflict. [Google Scholar]
- Blanckenhorn W.U., Hosken D.J., Martin O.Y., Reim C., Teuschl Y., Ward P.I. The costs of copulating in the dung fly Sepsis cynipsea. Behav. Ecol. 2002;13:353–358. doi:10.1093/beheco/13.3.353 [Google Scholar]
- Chapman T. Evolutionary conflicts of interest between males and females. Curr. Biol. 2006;16:R744–R754. doi: 10.1016/j.cub.2006.08.020. doi:10.1016/j.cub.2006.08.020 [DOI] [PubMed] [Google Scholar]
- Chapman T., Arnqvist G., Bangham J., Rowe L. Sexual conflict. Trends Ecol. Evol. 2003;18:41–47. doi:10.1016/S0169-5347(02)00004-6 [Google Scholar]
- Chivers D.P., Brown G.E., Smith R.J.F. Familiarity and shoal cohesion in fathead minnows (Pimephales promelas)—implications for antipredator behaviour. Can. J. Zool. 1995;73:955–960. doi:10.1139/z95-111 [Google Scholar]
- Clutton-Brock T., Langley P. Persistent courtship reduces male and female longevity in captive tsetse flies Glossina morsitans morsitans Westwood (Diptera: Glossinidae) Behav. Ecol. 1997;8:392–395. doi:10.1093/beheco/8.4.392 [Google Scholar]
- Clutton-Brock T.H., Parker G.A. Sexual coercion in animal societies. Anim. Behav. 1995;49:1345–1365. doi:10.1006/anbe.1995.0166 [Google Scholar]
- Croft D.P., Albanese B., Arrowsmith B.J., Botham M., Webster M., Krause J. Sex biased movement in the guppy (Poecilia reticulata) Oecologia. 2003;137:62–68. doi: 10.1007/s00442-003-1268-6. doi:10.1007/s00442-003-1268-6 [DOI] [PubMed] [Google Scholar]
- Croft D.P., Krause J., James R. Social networks in the guppy (Poecilia reticulata) Proc. R. Soc. Lond. B. 2004;271:S516–S519. doi: 10.1098/rsbl.2004.0206. doi:10.1098/rsbl.2004.0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft D.P., James R., Thomas P.O.R., Hathaway C., Mawdsley D., Laland K.N., Krause J. Social structure and co-operative interactions in a wild population of guppies (Poecilia reticulata) Behav. Ecol. Sociobiol. 2006a;59:644–650. doi:10.1007/s00265-005-0091-y [Google Scholar]
- Croft D.P., Morrell L.J., Wade A.S., Piyapong C., Ioannou C.C., Dyer J.R.G., Chapman B.B., Yan W., Krause J. Predation risk as a driving force for sexual segregation: a cross-population comparison. Am. Nat. 2006b;167:867–878. doi: 10.1086/504853. doi:10.1086/504853 [DOI] [PubMed] [Google Scholar]
- Croft D.P., James R., Krause J. Princeton University Press; Princeton, NJ: 2008. Exploring animal social networks. [Google Scholar]
- Darden S.K., Croft D.P. Male harassment drives females to alter habitat use and leads to segregation of the sexes. Biol. Lett. 2008;4:449–451. doi: 10.1098/rsbl.2008.0308. doi:10.1098/rsbl.2008.0308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlen S.T., Oring L.W. Ecology, sexual selection, and the evolution of mating systems. Science. 1977;197:215–223. doi: 10.1126/science.327542. doi:10.1126/science.327542 [DOI] [PubMed] [Google Scholar]
- Frommen J.G., Luz C., Bakker T.C.M. Nutritional state influences shoaling preference for familiars. Zoology. 2007;110:369–376. doi: 10.1016/j.zool.2007.06.002. doi:10.1016/j.zool.2007.06.002 [DOI] [PubMed] [Google Scholar]
- Griffiths S.W., Magurran A.E. Familiarity in schooling fish: how long does it take to acquire? Anim. Behav. 1997;53:945–949. doi:10.1006/anbe.1996.0315 [Google Scholar]
- Griffiths S.W., Ward A.J.W. Learned recognition of conspecifics. In: Brown C., Laland K.N., Krause J., editors. Fish learning & behaviour. Blackwell Publishing Ltd; Oxford, UK: 2006. pp. 139–157. [Google Scholar]
- Höjesjö J., Johnsson J.I., Petersson E., Jarvi T. The importance of being familiar: individual recognition and social behavior in sea trout (Salmo trutta) Behav. Ecol. 1998;9:445–451. doi:10.1093/beheco/9.5.445 [Google Scholar]
- Hosken D.J., Stockley P. Sexual conflict. Curr. Biol. 2005;15:R535–R536. doi: 10.1016/j.cub.2005.07.014. doi:10.1016/j.cub.2005.07.014 [DOI] [PubMed] [Google Scholar]
- Krause J., Croft D.P., James R. Social network theory in the behavioural sciences: potential applications. Behav. Ecol. Sociobiol. 2007;62:15–27. doi: 10.1007/s00265-007-0445-8. doi:10.1007/s00265-007-0445-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa J.J., Leopold W.R., Sih A. Avoidance of male giant water striders by females. Behaviour. 1990;115:247–253. doi:10.1163/156853990X00590 [Google Scholar]
- Ligout S., Porter R.H. Social recognition in mammals: mechanisms and sensorial bases. Prod. Anim. 2006;19:119–133. [Google Scholar]
- Magurran A.E. Oxford series in ecology and evolution. Oxford University Press; Oxford, UK: 2005. Evolutionary ecology: the Trinidadian guppy. [Google Scholar]
- Magurran A.E., Seghers B.H. A cost of sexual harassment in the guppy, Poecilia reticulata. Proc. R. Soc. Lond. B. 1994a;258:89–92. doi:10.1098/rspb.1994.0147 [Google Scholar]
- Magurran A.E., Seghers B.H. Sexual conflict as a consequence of ecology—evidence from guppy, Poecilia reticulata, populations in Trinidad. Proc. R. Soc. Lond. B. 1994b;255:31–36. doi:10.1098/rspb.1994.0005 [Google Scholar]
- Meader S.J., Gilburn A.S. Asymmetrical costs of sexual conflict in the seaweed fly, Coelopa frigida. Ecol. Entomol. 2008;33:380–384. doi:10.1111/j.1365-2311.2007.00980.x [Google Scholar]
- Mesnick S.L., Leboeuf B.J. Sexual-behavior of male northern elephant seals 2. Female response to potentially injurious encounters. Behaviour. 1991;117:262–280. doi:10.1163/156853991X00571 [Google Scholar]
- Moore A.J., Gowaty P.A., Moore P.J. Females avoid manipulative males and live longer. J. Evol. Biol. 2003;16:523–530. doi: 10.1046/j.1420-9101.2003.00527.x. doi:10.1046/j.1420-9101.2003.00527.x [DOI] [PubMed] [Google Scholar]
- Morrell L.J., Croft D.P., Dyer J.R.G., Chapman B.B., Kelley J.L., Laland K.N., Krause J. Association patterns and foraging behaviour in natural and artificial guppy shoals. Anim. Behav. 2008;76:855–864. doi:10.1016/j.anbehav.2008.02.015 [Google Scholar]
- Newman M.E.J. Scientific collaboration networks. II. Shortest paths, weighted networks, and centrality. Phys. Rev. E. 2001;64 doi: 10.1103/PhysRevE.64.016132. doi:10.1103/PhysRevE.64.016132 [DOI] [PubMed] [Google Scholar]
- Rankin D.J., Kokko H. Do males matter? The role of males in population dynamics. Oikos. 2007;116:335–348. doi:10.1111/j.0030-1299.2007.15451.x [Google Scholar]
- Rowe L. The costs of mating and mate choice in water striders. Anim. Behav. 1994;48:1049–1056. doi:10.1006/anbe.1994.1338 [Google Scholar]
- Sakurai G., Kasuya E. The costs of harassment in the adzuki bean beetle. Anim. Behav. 2008;75:1367–1373. doi:10.1016/j.anbehav.2007.09.010 [Google Scholar]
- Silk J.B. The adaptive value of sociality in mammalian groups. Phil. Trans. R. Soc. B. 2007a;362:539–559. doi: 10.1098/rstb.2006.1994. doi:10.1098/rstb.2006.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk J.B. Social components of fitness in primate groups. Science. 2007b;317:1347–1351. doi: 10.1126/science.1140734. doi:10.1126/science.1140734 [DOI] [PubMed] [Google Scholar]
- Stone G.N. Female foraging responses to sexual harassment in the solitary bee Anthophora plumipes. Anim. Behav. 1995;50:405–412. doi:10.1006/anbe.1995.0255 [Google Scholar]
- Sundaresan S.R., Fischhoff I.R., Rubenstein D.I. Male harassment influences female movements and associations in Grevy's zebra (Equus grevyi) Behav. Ecol. 2007;18:860–865. doi:10.1093/beheco/arm055 [Google Scholar]
- Trillmich F., Trillmich K.G.K. The mating systems of pinnipeds and marine iguanas—convergent evolution of polygyny. Biol. J. Linn. Soc. 1984;21:209–216. doi:10.1111/j.1095-8312.1984.tb02062.x [Google Scholar]
- Utne-Palm A.C., Hart P.J.B. The effects of familiarity on competitive interactions between threespined sticklebacks. Oikos. 2000;91:225–232. doi:10.1034/j.1600-0706.2000.910203.x [Google Scholar]
- Ward A.J.W., Holbrook R.I., Krause J., Hart P.J.B. Social recognition in sticklebacks: the role of direct experience and habitat cues. Behav. Ecol. Sociobiol. 2005;57:575–583. doi:10.1007/s00265-004-0901-7 [Google Scholar]
- Ward A.J.W., Webster M.M., Magurran A.E., Currie S., Krause J. Species and population differences in social recognition between fishes: a role for ecology? Behav. Ecol. 2009 doi:10.1093/beheco/arp025 [Google Scholar]
- Wasserman S., Faust K. Cambridge University Press; New York, NY: 1994. Social network analysis: methods and applications. [Google Scholar]