Abstract
Trans-generational effects on immunity are well known in vertebrates and are considered in many evolutionary and ecological theories of species interaction. Maternal effects have been identified to be of special importance, and are now recognized as a mechanism for adaptive phenotypic response to environmental heterogeneity. We have previously shown that exposure to dietary non-pathogenic bacteria can induce several aspects of immune response in an insect herbivore, the cabbage semilooper (Trichoplusia ni). Here, we test the effects of this exposure on the immune status of the next generation, measuring immune parameters on three different levels—enzyme activities, protein expression and transcript abundance. We also monitored fitness-related traits which are often negatively correlated with increased immunocompetence. We found evidence for trans-generational priming on all these levels, with immune system parameters that are clearly not transmitted in a 1 : 1 ratio from parent to offspring, but rather in a complex manner with a strong but not exclusive maternal component. These findings indicate that trans-generational priming is a complex and multifaceted phenomenon, potentially playing a role as a long-term but non-genetic mode of environmental adaptation.
Keywords: innate immunity, trans-generational priming, insect herbivore, maternal effects
1. Introduction
The phenotype of an organism is not only determined by its genotype, but also by interactions with the environment and epigenetic factors (Gotthard & Nylin 1995; Ghalambor et al. 2007; Nussey et al. 2007; Poulin & Thomas 2008). Epigenetic inheritance is increasingly recognized as playing a role in shaping the phenotype and life history of an organism (Poulin & Thomas 2008). Maternal effects especially have been reported in many different taxa as a response to various environmental factors, including pathogens. In many cases it has been shown that these kind of maternal effects are adaptive, optimizing life-history traits in the long term (Mousseau & Fox 1998).
Pathogens and parasites profoundly influence the fitness of their hosts. The ability to cope with infections is a life-history trait with a high degree of phenotypic plasticity, and its phenotypic costs result in well-documented trade-offs with fitness (Schmid-Hempel 2005). Under certain conditions such trade-offs could be ameliorated if information about the likelihood of future infection was available. Sorci & Clobert (1995) suggested that in a relatively stable environment, infection levels experienced by mothers are good indicators for what the offspring are likely to encounter. Trans-generational priming of the immune system could, therefore, result in lower mortality of progeny by reducing the delay required by independent induction of progeny immune response, and possibly increasing efficiency by priming those aspects of the immune response most appropriate to dealing with the particular stress encountered by the parents.
In vertebrates it is known that immunocompetent mothers can transmit pathogen resistance to their offspring (Grindstaff et al. 2003). Mucosal immune responses in the foetal gut are primed by maternal gut flora (Blümer et al. 2007). Evidence for trans-generational immune priming has also been reported in arthropods namely mealworm beetle (Tenebrio molitor; Moret 2006), bumble-bee (Bombus terrestris; Moret & Schmid-Hempel 2001), mosquito (Anopheles stephensi; Grech et al. 2007), Mediterranean flour moth (Ephestia kuehniella; Rahman et al. 2004), and the crustacean Daphnia magna (Little et al. 2003). By contrast, studies on peach-potato aphid (Myzus persicae) did not provide evidence for trans-generational priming of resistance to its parasitoid (Diaeretiella rapae; Vorburger et al. 2008). The heritability of immune function, specifically the encapsulation response, has also been studied (Collins et al. 1986; Fellowes et al. 1998; Cotter & Wilson 2002).
We have previously shown that exposure to dietary non-pathogenic bacteria during the larval stage can induce several aspects of the immune response in the insect Trichoplusia ni (Freitak et al. 2007). Trichoplusia ni is a lepidopteran generalist herbivore established worldwide, except for Australia and the tropics and its broad host range includes tomato, potato, maize, cotton and many other plants. Here, we assess the extent to which components of an immune response can be seen in the unexposed progeny of exposed individuals. Reciprocal crosses were made with parents grown on sterile artificial diet or bacteria-laden diet to study trans-generational maternal and paternal effects. We examined different aspects of the immunity and physiology of the insect by measuring enzyme activities, haemolymph protein profiles and transcript levels of a number of immune-related genes in different tissues, as well as fitness-related parameters including larval developmental time, pupal mass and survival. Instead of the parental immune status being faithfully mirrored in the next generation, we observed a complex, mixed-pattern scenario, with strong maternal effects in many aspects. Thus, the pattern of trans-generational priming affects diverse aspects of the progeny immune response in a highly selective manner.
2. Material and methods
(a) Animals
Cabbage semilooper (T. ni) eggs were obtained from Entopath Inc. (USA). Eggs were placed in plastic cups with artificial diet and allowed to hatch, and were grown at room temperature (23°C) at 16 L : 8 D cycle, at 55 per cent relative humidity. To estimate the impact of constant presence of bacteria in the diet, two treatment groups were formed for the parental generation: larvae were fed on artificial diet (Freitak et al. 2007) with or without bacteria. Larvae in both treatments may have initially ingested some bacteria when consuming the eggshell after hatching or from other environmental sources. Bacterial diet was prepared by soaking with overnight cultures (OD600=4; 2.5 ml/40 cm2) of Escherichia coli and Micrococcus luteus (approx. 80 μg per 125 g of diet). Old diet was removed and fresh diet (sterile or freshly laden with bacteria) was provided every 3 days to keep the difference between treatments of the bacterial concentration approximately constant in time. Larvae were not given a choice of different types of food, to avoid affects of changes in feeding behaviour on immune responses as shown by Lee et al. (2006).
Adults originating from different diets were mated to estimate the effect of parental diet on the physiology and immune system of offspring. Four different crosses were made, females and males grown on bacteria-free diet (N♀N♂), females and males grown on bacterial diet (B♀B♂), females grown on bacterial diet and males on bacteria-free diet (B♀N♂) and females grown on bacteria-free diet and males on bacterial diet (N♀B♂). Mixed mating of 30 males and 30 females were carried out in 21×13×13 cm cages. All the larvae from first and second generation were kept at the same densities to avoid density-dependent effects such as those shown by Barnes & Siva-Jothy (2000). Eggs were collected and allowed to hatch and fed on artificial bacteria-free diet. All the experiments were carried out using early last instar larvae. For measuring fitness-related traits, a total of 400 larvae were used, with 100 larvae per cross.
(b) Lytic activity in the haemolymph
For estimation of the total lytic activity of the haemolymph, a lytic zone assay was performed. 12×12 cm petri dishes were filled with 35 ml of autoclaved Sörensen buffer with 21 mg lyophilisized M. luteus cells (Sigma) and 2.1 mg streptomycin sulphate (Calbiochem) with a final concentration of 1.5 per cent agar. Wells within plates (2 mm diameter) were made by puncturing the agar with a plastic pipette and removing the agar plug by suction. Haemolymph samples (4 μl) were pipetted directly into the wells and the plates were incubated for 24 hours at 37°C. In total, haemolymph samples from 79 larvae were analysed, with 20 larvae per cross, with the exception of the B♀N♂ cross, where 19 animals were sampled. A dilution series of chicken egg-white lysozyme (Sigma) (2, 1, 0.750, 0.500, 0.250, 0.125, 0.62 and 0.31 mg ml−1) was added to each plate as a control and a calibration curve was created based on these standards. Lytic activity was determined as the radius of the clear zone around a sample well.
(c) Pro-phenoloxidase quantity and phenoloxidase activity in the haemolymph
Haemolymph was collected via puncturing the dorso-lateral part of the larval abdomen and 20 μl was diluted into 200 μl ice cold phosphate-buffered saline (PBS) and vortexed immediately. In total, haemolymph from 80 larvae were used, with 20 animals per cross. For measuring phenoloxidase (PO) activity, we followed the procedures described in Barnes & Siva-Jothy (2000) and in Moret & Siva-Jothy (2003). For pro-phenoloxidase (proPO) activity measurements, the method described in Adamo (2004) was used. Samples were centrifuged at 4°C at 9000g for 7 min, supernatant removed and kept for further analysis at −80°C. PO assays were carried out in 96-well plates using the Infinite 200 microplate reader (Tecan). 100 μl of supernatant was mixed with 200 μl of 0.4 mM L-Dopa (Sigma) and changes in absorbance were measured at 30°C for 60 min at 490 nm. To measure total activity of enzyme per volume of the haemolymph accounted for by both (already activated) PO and (unactivated) proPO, 30 μl of supernatant was incubated with 70 μl of chymotrypsin solution (2 mg chymotrypsin (Calbiochem, Germany) in 1.5 ml PBS) to activate the proPO for 20 min at 30°C and changes in absorbance were measured at 30°C for 60 min at 490 nm. To estimate the additional PO activity accounted for by activation of proPO, readings measured without chymotrypsin digestion were subtracted from the total activity seen after incubation with chymotrypsin. For both measurements the same samples were used. Enzyme activity was measured as the slope (Vmax value) of the reaction curve during the linear phase of the reaction (15–45 min after adding the substrate).
(d) Protein expression in the haemolymph
Protein expression patterns in the haemolymph were analysed with sodium dodecyl sulphate polyacrylamide gradient gel electrophoresis (SDS-PAGE) with 4–12 per cent gradient gels performed in a XT-MES buffer system. Previous experiments showed that the major protein constituents of the haemolymph can change drastically, both qualitatively and quantitatively. For example, arylphorin makes up a large amount of haemolymph total protein content and we know from previous experiments (Freitak et al. 2007), that also after correcting for protein concentration in the sample, the same proteins will be highly abundant as compared with controls. In the current study, we wanted to estimate the effect of the parental diet on both quantitative and qualitative changes of haemolymph proteins. This can be best achieved by comparing the protein profiles in the same volume of haemolymph. However, we have observed the same banding pattern differences after correcting for protein concentrations. Two microlitres of haemolymph sample were diluted into 50 μl of ice-cold 4 per cent SDS containing Tris–HCl buffer with EDTA-free protease inhibitor cocktail (Pierce), directly frozen in liquid N2 and stored at −20°C until further use. All the samples were analysed as described in Freitak et al. (2007).
(e) RNA isolation
Dissected insect midguts and the rest of the bodies (except for the head capsules) were ground using a motorized hand pestle and total RNA was isolated using TRIzol Reagent (Invitrogen) according to the manufacturers' protocol. For isolating RNA from the haemocytes, haemolymph samples from approximately 15 larvae, (approx. 25 μl per larvae) were pooled in 1 ml phosphate buffer and centrifuged at 4°C at 9000g for 10 min. Supernatant was removed, the cell pellet was dissolved in TRIzol and RNA was isolated according to the manufacturers' protocol. An additional DNAse (Turbo DNAse, Ambion) treatment was included prior to the second purification step to eliminate any contaminating DNA. A second purification step was performed with RNeasy MinElute CleanUp Kit (Qiagen). RNA integrity was verified on an Agilent 2100 Bioanalyzer using RNA Nano chips (Agilent). RNA quantity was determined photospectrometrically using a NanoDrop ND-1000 Spectrophotometer (Thermo Scientific).
(f) Quantitative real-time PCR
500 ng of DNA-free total RNA was converted into single-stranded DNA using a mix of random and oligo-dT20 primers according to the ABgene protocol (ABgene). Real-time PCR oligonucleotide primers were designed using the online Primer3 internet-based interface (http://frodo.wi.mit.edu). Primers were designed by the rules of highest maximum efficiency and sensitivity to be followed to avoid formation of self- and heterodimers, hairpins and self-complementarity. Gene-specific primers (sequences provided in the electronic supplementary material, §1) were designed on the basis of sequence obtained for selected T. ni genes and several additional genes as potential house-keeping genes to serve as the endogenous control (normalizer). Q-RT-PCR was done in optical 96-well plates on a MX3000P Real-Time PCR Detection System (Stratagene) using the Absolute QPCR SYBR green Mix (ABgene) to monitor double-stranded DNA synthesis in combination with ROX as a passive reference dye included in the PCR master mix. For estimating gene expression differences, three biological replicates per cross, consisting of 15 pooled larvae were used. For adult samples, three biological replicates per diet and sex, consisting of 15 pooled individuals were used.
(g) Statistical analysis
Statistical analyses were performed with the software package SPSS v. 15 (SPSS Inc.). Normality of the data was estimated by using Shapiro-Wilcoxon and Levene's tests. In the case where assumptions for normality and homogeneity were not violated, our hypotheses were tested using an ANOVA model, otherwise a non-parametric Kruskall–Wallis ANOVA test was used. In the case of qRT-PCR data, the 2−ΔΔCT method was used (Livak & Schmittgen 2001).
3. Results
(a) Immune-related changes in the haemolymph
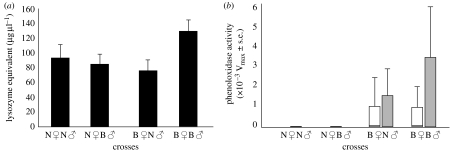
Haemolymph samples were taken from 14-day old (early stage last instar) larvae. The effect of the absence or presence of bacteria in the diet of the parental generation on the general antibacterial activity was not significant (F3,78=1.99, p=0.124, n=79; figure 1a). Although the mean general antibacterial activity is higher in offspring of the B♀B♂ cross (129.2 μg of lysozyme) in comparison to other crosses (N♀N♂, 94.1 μg of lysozyme; N♀B♂, 84.6 μg of lysozyme; B♀N♂, 74.6 μg of lysozyme), the difference is not statistically significant after Bonferroni corrections on the post hoc analysis (B♀B♂ versus B♀N♂, p=0.149; B♀B♂ versus N♀B♂, p=0.469 and B♀B♂ versus N♀N♂, p=0.609).
Figure 1.
Enzyme activities in the haemolymph of last instar T. ni larvae originating from N♀N♂, B♀B♂, B♀N♂ and N♀B♂ crosses. (a) General antibacterial activity measured as the diameter of the lytic zone on agar plates with lyophilized M. luteus and transformed into lysozyme equivalents (μg μl−1). Results represent mean values ±s.e. (b) proPO (open bars) and PO (filled bars) activities measured in the haemolymph samples. Vmax is measured as the maximum change in optical density per minute ±s.e.
A significant effect of bacteria in the parental diet was observed on the PO activity in the haemolymph of second generation T. ni larvae, but not on the additional PO activity accounted for by activation of proPO. The activity of PO was significantly affected by parental diet (Kruskal–Wallis ANOVA, H3,79=10.87, p=0.012), whereas that accounted for by activation of proPO was only marginally affected (Kruskall–Wallis ANOVA, H3,79=6.7, p=0.082; n=80; figure 1b).
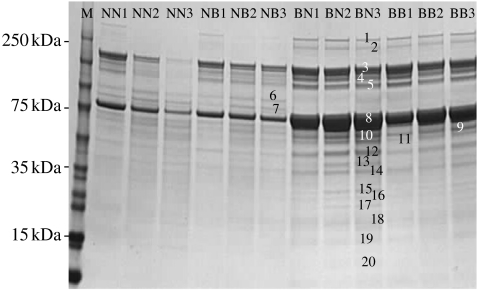
We found strong maternal effects on protein expression in the larval haemolymph. In order to detect changes in total as well as relative protein amounts, equal volumes of haemolymph were loaded per individual rather than standardizing on total protein content. The analysis of the protein profiles of one-dimensional protein gel electrophoresis (one-dimensional SDS-PAGE) resulted in 20 differentially expressed bands, 18 of which were more pronounced in B♀B♂ and B♀N♂ crosses, and two in N♀B♂ and N♀N♂ crosses. Based on mass-spectrometry data, three proteins were identified, including storage proteins, such as arylphorin and vitellogenin, but also juvenile hormone suppressing protein involved in developmental processes, all of which were highly expressed in B♀B♂ and B♀N♂ crosses (figure 2, details for protein identification are summarized in the electronic supplementary material, §2).
Figure 2.
SDS gel electrophoresis of T. ni last instar larvae haemolymph proteins stained with Coomassie blue. Equal amounts of larval haemolymph samples from three individuals per cross are presented: BB1–BB3 from B♀B♂ cross; BN1–BN3 from B♀N♂ cross; NB1–NB3 from N♀B♂ cross; NN1–NN3 from N♀N♂ cross. Protein bands identified as differentially expressed are vitellogenin (3, 4 and 7), arylphorin (8 and 10) and juvenile hormone suppressive protein (11 and 12). 17 additional unknown proteins were observed as differentially expressed.
(b) Tissue-specific gene expression in second generation
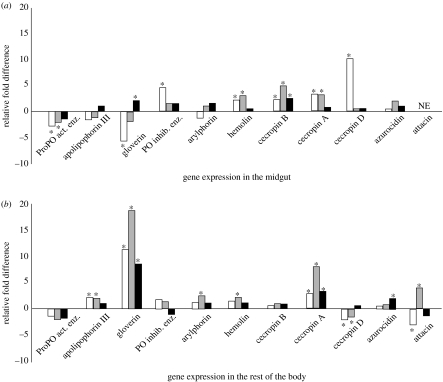
Differential expression of immune response-related genes was examined in the midgut, rest of the body, haemocytes and whole larvae of the crosses. A number of immune inducible genes were upregulated in the B♀B♂ cross larval midguts and the rest of the body, but also in the rest of the body of B♀N♂ and N♀B♂ crosses (figure 3a,b). In addition, apolipophorin III and arylphorin were upregulated in the rest of the body of B♀N♂ cross larvae. Apolipophorin III and beta-glycan recognizing protein were upregulated in the haemocytes of larvae from B♀N♂ cross. In the cases where we used pooled larvae to study differential gene expression, only a metalloprotease showed higher expression in the B♀N♂ cross. We examined the expression pattern of 12 immune inducible genes in haemocytes, only two of them showed higher expression in B♀N♂ cross, namely arylphorin III (more than twofold) and β-glucan recognition protein (more than twofold) (expression patterns for all studied genes are shown in the electronic supplementary material, §3).
Figure 3.
Differential gene expression in last instar T. ni larvae originating from different crosses. (a) The effect of parental diet on the relative gene expression in midgut tissue of offspring originating from different crosses in T. ni. (b) The effect of parental diet on the relative gene expression in rest of the body of offspring originating from different crosses in T. ni. In both cases relative gene expression in offspring of N♀N♂ cross is set to 1, values are mean ±s.d., NE—not expressed. Open bars, N♀B♂; grey bars, B♀N♂; black bars, B♀B♂; and asterisks, more than twofold difference in gene expression.
(c) Immunity-related gene expression in the parental generation
To compare the changes in immune status reflecting gene expression we also examined transcripts for immune inducible genes in parental generation actually exposed to bacterial diet. We have previously found that the transcription of several immune inducible genes is influenced in the midgut tissue by bacterial feeding in larvae (Freitak et al. 2007). At the same time in the rest of the body of larvae grown on bacterial diet, only 2 out of 17 examined genes showed different expression, namely attacin was highly upregulated (more than 100-fold) and cobatoxin was downregulated.
Expression of 24 immune response-related genes was studied in the adult stage of T. ni moths. Six of them were upregulated in the animals grown on bacterial diet and one downregulated. Among the upregulated genes were attacin (more than 10-fold), cecropin A (more than threefold), a chemosensory-like protein (sixfold), cecropin D, lebocin and azurocidin (expression patterns for all studied genes are shown in the electronic supplementary material, §4 and 5) All of these are directly involved in immune response, having a function in recognizing bacteria and acting on pathogen cell-wall integrity (Steiner et al. 1988; Sun et al. 1990; Lee et al. 1996; Carlsson et al. 1998; Liu et al. 2000; Kang et al. 2002). Haemolin, a protein with sequence homology to mammalian immunoglobulins by virtue of shared Ig-C2 domains, was at the same time significantly downregulated. Haemolin is one of the first proteins to appear in the haemolymph of the giant silkmoth Hyalophora cecropia upon infection, binding to the surface of bacteria (Sun et al. 1990).
For a number of genes (9) gender-specific differential gene expression patterns were also examined. All of the studied genes, with exception of gallerimycin, were differentially expressed in males and females. Major upregulation of attacin, cecropin A (more than sixfold), chemosensory protein and azurocidin (more than 30-fold) was found in females fed on bacterial diet in comparison with females grown on bacteria-free diet. In the case of males, attacin, haemolin, cecropin B, chemosensory protein and lebocin (more than fivefold) were significantly upregulated in the bacteria-fed group.
(d) Effect on fitness
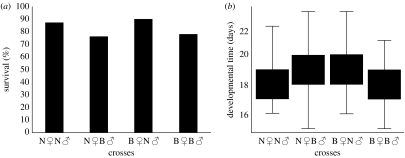
To estimate the effects of the parental diet on the life-history traits of the next generation, a number of fitness-related traits were studied. We found no effect of the different crosses on pupal mass (ANOVA, F3,323=0.3, p=0.8; n=324). However, survival into the adult stage was significantly lower among offspring originating from B♀B♂ (78%) and N♀B♂ (76%) in comparison to N♀N♂ (87%) and B♀N♂ (90%) crosses (Χ2 test, Χ23,400=9.7, p=0.021; n=400; figure 4). No effect of sex on developmental time was discovered (all tests p<0). Crosses where both parents originated from different diets (N♀B♂ and B♀N♂) had prolonged developmental times (Kruskal–Wallis ANOVA, H3.331=24.5, p>0.000; N=332; figure 4b).
Figure 4.
Effect of parental diet on the fitness-related traits in T. ni. (a) The effect of parental diet on the survival of offspring originating from different crosses in T. ni. Graph shows percentage of survival in different crosses (n=100 per each cross). Survival is reduced in B♀B♂ and N♀B♂ crosses. (b) The effect of parental diet on developmental time of the offspring originating from different T. ni crosses (n=100 per each cross). Offspring of the parents forming mixed crosses B♀N♂ and N♀B♂ have prolonged developmental times. Results represent medians with upper and lower quartiles.
4. Discussion
In the current work we present evidence that constant ingestion of bacteria during larval development has an effect on immune-related aspects of the phenotype of the next generation. The induced changes cover many aspects of the immune response, including enzyme activities, protein expression and transcript levels of many genes. These effects are predominantly but not exclusively maternal. At the transcriptional level, comparison of parental and offspring generations does not indicate a simple transfer of the changed immune and physiological status from the parents, but rather a new immune status quality in offspring.
Strong diet-related maternal effects were found on the immune system of next generation T. ni larvae. In B♀B♂ and B♀N♂ crosses, immune response-related enzyme activities were higher and a number of proteins were notably expressed. In a previous study (Freitak et al. 2007), we found that in the parental generation fed on bacterial and bacteria-free diet, lysozyme is highly active in bacterial diet-fed larvae and PO in bacteria-free diet larvae. It has been proposed that these two enzyme activities exhibit a trade-off with each other and are not displayed at high levels at the same time (Cotter & Wilson 2002), potentially due to possible mechanistic reasons and severe autoimmune effects (Cotter et al. 2004; Nappi & Christensen 2005; Sadd & Siva-Jothy 2006). We observed such a trade-off in the first, directly exposed generation but not the second generation. This could be due to additional physiological changes to cope with higher enzyme activities and increased autoimmune risk. One of the candidates enabling the insects to avoid harm caused by free radicals is vitellogenin, a protein we find to be highly expressed in the haemolymph of larvae of both B♀B♂ and B♀N♂ crosses, showing both high lytic and high PO activity. Vitellogenin is known to act as an antioxidant and to reduce oxidative stress related harmful effects to the organism (Seehuus et al. 2006). The elevated vitellogenin expression in the haemolymph of larvae whose mothers have been feeding on bacterial diet might be an adaptive induction, possibly enabling the insects to cope with high free radical stress due to elevated PO activity. Lipophorins and vitellogenins are related and are both also known to be pro-coagulants in, for e.g. crayfish (Hall et al 1999; Ma et al. 2006). As in the first generation, in the second generation arylphorin is one of the predominant haemolymph proteins. Arylphorin is highly inducible upon immune challenge (Kunkel et al. 1990; Asgari & Schmid-Hempel 2004) and bacterial feeding (Freitak et al. 2007), but at the same time its role in the immune response is still largely unknown, although growth promoting and mitosis enhancing effects have been reported (Hakim et al. 2007). Another highly abundant protein in B♀B♂ and B♀N♂ crosses was identified as juvenile hormone suppressing protein. This could be related to the high expression of vitellogenin, as a high titre of juvenile hormone leads to suppression of this protein (Pinto et al. 2000).
Strong maternal effects can also be seen at the transcript level. It is interesting to note that a number of immune response-related genes are upregulated in the rest of the body of B♀B♂ and B♀N♂ crosses, among them apolipophorin III and arylphorin, also known to be involved in storage processes. It is very interesting to see that a number of immune response-related genes known to be antimicrobial effector molecules (for e.g. gloverin, cecropins, haemolin) are highly expressed in the midgut tissue. Although no direct induction of the immune system elements occurred in the second generation, we can observe higher abundance of several markers, characteristic for immune challenged organism. Our findings support the idea that parental diet is able to alter the immune status in the next generation. Elevated expression of antimicrobial genes in the midgut tissue could be seen as an adaptive response preparing offspring to elevated bacterial load in the environment by increasing their chance for survival in the case of infection.
Alterations in immune status can lead to certain trade-offs with other life-history traits, such as developmental time and body mass (Schmid-Hempel 2005). We did not see any differences in the pupal masses between different crosses. Developmental time was prolonged in mixed crosses—B♀N♂ and N♀B♂. Mortality was higher in the B♀B♂ and N♀B♂ crosses, indicating a paternally-related cost associated with an elevated immune level and possibly an imbalance in homeostasis. Our experimental design kept larval densities constant, so we could not observe effects of varying density on immune status as seen in other studies (Barnes & Siva-Jothy 2000; Wilson et al. 2002). Nor was there any opportunity in our experimental design for larvae to vary their diet choice in response to increased immune challenge, as seen by Lee et al. (2006).
A number of studies claiming to show adaptive immunity and trans-generational immune priming in invertebrates have been strongly criticized recently, as lacking mechanistic evidence (Hauton & Smith 2007). The critics argue that in many cases only survival has been considered as a measure of successful immune response, and usually single enzyme or protein measurements have been used as markers reflecting the immune status. Several other studies have presented additional life-history parameters (for e.g. body mass, survival, egg mass, etc.) as evidence of adaptive priming of the immune system, but at the same time do not show any measurable immune markers. The complexity of the response we have observed in T. ni provides further evidence that approaches focusing on only one or two immune parameters in assessing trans-generational effects may give an incomplete and possibly unrepresentative picture.
Encountering non-pathogenic bacteria in food is sufficient to trigger changes in the immune status of the next generation but this effect can only be seen on some immune markers and not on others. The immune response is multifaceted and involves coordination and reorganization of many aspects of physiology, the outcome being a complex pattern of induction and repression of different factors. These immune system parameters are clearly not transmitted in a 1 : 1 ratio from parent to offspring, but are selectively transmitted in a complex manner with a strong but not exclusively maternal component. Comparing and linking enzyme activities in the haemolymph with protein expression and transcriptional changes in various tissues fails to show a homogeneous and/or simple pattern. There are several possible reasons for this observation: (i) regulation takes place at the post-translational level, (ii) despite an observed regulation at the transcriptional level, translation into proteins is regulated differently, or (iii) some of the candidate genes are not regulated by the parental treatment. Pro-PO exemplifies the first situation; although transcription of pro-PO was not increased on progeny of bacteria-fed individuals, PO activity, which is known to be post-translationally regulated, did increase.
The mechanism of transmission of this information is completely unknown, but could involve maternal transmission of proteins and/or RNA in the egg cytoplasm, as well as epigenetic mechanisms such as DNA methylation. Determining the relative importance of these possible mechanisms remains a challenging topic for future research.
Acknowledgements
We thank Stephanie Fuhr for substantial help in insect rearing. We are grateful to A. Svatos and A. Muck for the MS support. We thank D. Schnabelrauch and H. Ringys-Beckstein for essential technical assistance. This work was supported by the International Max Planck Research School (IMPRS) and the Max Planck Society.
Supplementary Material
III RT-qPCR results for hemocytes. IV RT-qPCR results for rest of the bodies originating from parental generation. V RT-qPCR results for adults
References
- Adamo S. Estimating disease resistance in insects: phenoloxidase and lysozyme-like activity and disease resistance in the cricket Gryllus texensis. J. Insect Physiol. 2004;50:209–216. doi: 10.1016/j.jinsphys.2003.11.011. doi:10.1016/j.jinsphys.2003.11.011 [DOI] [PubMed] [Google Scholar]
- Asgari S., Schmid-Hempel O. Isolation of an imaginal disc growth factor homologue from Pieris rapae and its expression following parasitization by Cotesia rubecula. J. Insect Physiol. 2004;50:687–694. doi: 10.1016/j.jinsphys.2004.05.003. doi:10.1016/j.jinsphys.2004.05.003 [DOI] [PubMed] [Google Scholar]
- Barnes A., Siva-Jothy M.T. Density-dependent prophylaxis in the mealworm beetle Tenebrio molitor L. (Coleoptera: Tenebrionidae): cuticular melanisation is an indicator of investment in immunity. Proc. R. Soc. Lond. B. 2000;267:177–182. doi: 10.1098/rspb.2000.0984. doi:10.1098/rspb.2000.0984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blümer N., Pfefferle P.I., Renz H. Development of mucosal immune function in the intrauterine and early postnatal environment. Curr. Opin. Gastroenterol. 2007;23:655–660. doi: 10.1097/MOG.0b013e3282eeb428. doi:10.1097/MOG.0b013e3282eeb428 [DOI] [PubMed] [Google Scholar]
- Carlsson A., Nystrom T., de Cock H., Bennich H. Attacin—an insect immune protein—binds LPS and triggers the specific inhibition of bacterial outer-membrane protein synthesis. Microbiology. 1998;144:2179–2188. doi: 10.1099/00221287-144-8-2179. [DOI] [PubMed] [Google Scholar]
- Collins F.H., Sakai R.K., Vernick K.D., Paskewitz S., Seeley D.C., Miller L.H., Collins W.E., Campbell C.C., Gwadz R.W. Genetic selection of a Plasmodium-refractory strain of the malaria vector Anopheles gambiae. Science. 1986;234:607–610. doi: 10.1126/science.3532325. doi:10.1126/science.3532325 [DOI] [PubMed] [Google Scholar]
- Cotter S.C., Wilson K. Heritability of immune function in the caterpillar Spodoptera littoralis. Heredity. 2002;88:229–234. doi: 10.1038/sj.hdy.6800031. doi:10.1038/sj.hdy.6800031 [DOI] [PubMed] [Google Scholar]
- Cotter S.C., Hails R.S., Cory J.S., Wilson K. Density-dependent prophylaxis and condition-dependent immune function in Lepidopteran larvae: a multivariate approach. J. Anim. Ecol. 2004;72:283–293. doi:10.1111/j.0021-8790.2004.00806.x [Google Scholar]
- Fellowes M.D., Kraaijeveld A.R., Godfray H.C. Trade-off associated with selection for increased ability to resist parasitoid attack in Drosophila melanogaster. Proc. R. Soc. Lond. B. 1998;265:1553–1558. doi: 10.1098/rspb.1998.0471. doi:10.1098/rspb.1998.0471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitak D., Wheat C.W., Heckel D.G., Vogel H. Immune system responses and fitness costs associated with consumption of bacteria in larvae of Trichoplusia ni. BMC Biol. 2007;5:56. doi: 10.1186/1741-7007-5-56. doi:10.1186/1741-7007-5-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalambor C.K., McKay J.K., Carroll S.P., Reznick D.N. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 2007;21:394–407. doi:10.1111/j.1365-2435.2007.01283.x [Google Scholar]
- Gotthard K., Nylin S. Adaptive plasticity and plasticity as an adaptation: a selective review of plasticity in animal morphology and life history. Oikos. 1995;74:3–17. doi:10.2307/3545669 [Google Scholar]
- Grech K., Maung L.A., Read A.F. The effect of parental rearing conditions on offspring life history in Anopheles stephensi. Malar. J. 2007;6:130. doi: 10.1186/1475-2875-6-130. doi:10.1186/1475-2875-6-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindstaff J.L., Brodie E.D., III, Ketterson E.D. Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transmission. Proc. R. Soc. Lond. B. 2003;270:2309–2319. doi: 10.1098/rspb.2003.2485. doi:10.1098/rspb.2003.2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim R.S., et al. Growth and mitogenic effects of arylphorin in vivo and in vitro. Arch. Insect Biochem. Physiol. 2007;64:63–73. doi: 10.1002/arch.20155. doi:10.1002/arch.20155 [DOI] [PubMed] [Google Scholar]
- Hall M., Wang R., van Antwerpen R., Sottrup-Jensen L., Söderhäll K. The crayfish plasma clotting for clot formation in crustacean blood. Proc. Natl Acad. Sci. USA. 1999;96:1965–1970. doi: 10.1073/pnas.96.5.1965. doi:10.1073/pnas.96.5.1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauton C., Smith V.J. Adaptive immunity in invertebrates: a straw house without a mechanistic foundation. Bioessays. 2007;29:1138–1146. doi: 10.1002/bies.20650. doi:10.1002/bies.20650 [DOI] [PubMed] [Google Scholar]
- Kang D., Lundström A., Liu G., Steiner H. An azurocidin-like protein is induced in Trichoplusia ni larval gut cells after bacterial challenge. Dev. Comp. Immunol. 2002;26:495–503. doi: 10.1016/s0145-305x(02)00009-5. doi:10.1016/S0145-305X(02)00009-5 [DOI] [PubMed] [Google Scholar]
- Kunkel J.G., Grossniklaus-Buergin C., Karpells S.T., Lanzrein B. Arylphorin of Trichoplusia ni: characterization and parasite-induced precocious increase in titer. Arch. Insect Biochem. Physiol. 1990;13:117–125. doi:10.1002/arch.940130111 [Google Scholar]
- Lee W.-J., Lee J.-D., Kravchenko V.V., Ulevitch R.J., Brey P.T. Purification and molecular cloning of an inducible gram-negative bacteria-binding protein from the silkworm, Bombyx mori. Proc. Natl Acad. Sci. USA. 1996;93:7888–7893. doi: 10.1073/pnas.93.15.7888. doi:10.1073/pnas.93.15.7888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.P., Cory J.S., Wilson K., Raubenheimer D., Simpson S.J. Flexible diet choice offsets protein costs of pathogen resistance in a caterpillar. Proc. R. Soc. B. 2006;273:823–829. doi: 10.1098/rspb.2005.3385. doi:10.1098/rspb.2005.3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little T.J., O'Connor B., Colegrave N., Watt K., Read A.F. Maternal transfer of strain-specific immunity in an invertebrate. Curr. Biol. 2003;13:489–492. doi: 10.1016/s0960-9822(03)00163-5. doi:10.1016/S0960-9822(03)00163-5 [DOI] [PubMed] [Google Scholar]
- Liu G., Kang D., Steiner H. Trichoplusia ni lebocin, an inducible immune gene with a downstream insertion element. Biochem. Biophys. Res. Commun. 2000;269:803–807. doi: 10.1006/bbrc.2000.2366. doi:10.1006/bbrc.2000.2366 [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. doi:10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Ma G., Hay D., Li D., Asgari S., Schmidt O. Recognition and inactivation of LPS by lipophorin particles. Dev. Comp. Immunol. 2006;30:619–626. doi: 10.1016/j.dci.2005.09.003. doi:10.1016/j.dci.2005.09.003 [DOI] [PubMed] [Google Scholar]
- Moret Y. ‘Trans-generational immune priming’: specific enhancement of the antimicrobial immune response in the mealworm beetle, Tenebrio molitor. Proc. R. Soc. B. 2006;273:1399–1405. doi: 10.1098/rspb.2006.3465. doi:10.1098/rspb.2006.3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moret Y., Schmid-Hempel P. Immune defence in bumble-bee offspring. Nature. 2001;414:506. doi: 10.1038/35107138. doi:10.1038/35107138 [DOI] [PubMed] [Google Scholar]
- Moret Y., Siva-Jothy M.T. Adaptive innate immunity? Responsive-mode prophylaxis in the mealworm beetle, Tenebrio molitor. Proc. R. Soc. Lond. B. 2003;270:2475–2480. doi: 10.1098/rspb.2003.2511. doi:10.1098/rspb.2003.2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousseau T.A., Fox C.W. The adaptive significance of maternal effects. Trends Ecol. Evol. 1998;13:403–407. doi: 10.1016/s0169-5347(98)01472-4. doi:10.1016/S0169-5347(98)01472-4 [DOI] [PubMed] [Google Scholar]
- Nappi A.J., Chrsitensen B.M. Melanogenesis and associated cytotoxic reactions: applications to insect innate immunity. Insect Biochem. Mol. Biol. 2005;35:443–459. doi: 10.1016/j.ibmb.2005.01.014. doi:10.1016/j.ibmb.2005.01.014 [DOI] [PubMed] [Google Scholar]
- Nussey D.H., Wilson A.J., Brommer J.E. The evolutionary ecology of individual phenotypic plasticity in wild populations. J. Evol. Biol. 2007;20:831–844. doi: 10.1111/j.1420-9101.2007.01300.x. doi:10.1111/j.1420-9101.2007.01300.x [DOI] [PubMed] [Google Scholar]
- Pinto L.Z., Bitondi M.M.G., Simôes Z.L.P. Inhibition of vitellogenin synthesis in Apis mellifera workers by a juvenile hormone analogue, pyriproxyfen. J. Insect Physiol. 2000;46:153–160. doi: 10.1016/s0022-1910(99)00111-0. doi:10.1016/S0022-1910(99)00111-0 [DOI] [PubMed] [Google Scholar]
- Poulin R., Thomas F. Epigenetic effects of infection on the phenotype of host offspring: parasites reaching across host generations. Oikos. 2008;177:331–335. doi:10.1111/j.2007.0030-1299.16435.x [Google Scholar]
- Rahman R.M., Roberts H.L.S., Sarjan M., Asgari S., Schmidt O. Induction and transmission of Bacillus thuringiensis tolerance in the flour moth Ephestia kuehniella. Proc. Natl Acad. Sci. USA. 2004;101:2696–2699. doi: 10.1073/pnas.0306669101. doi:10.1073/pnas.0306669101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadd B.M., Siva-Jothy M.T. Self-harm caused by an insect's innate immunity. Proc. R. Soc. B. 2006;273:2571–2574. doi: 10.1098/rspb.2006.3574. doi:10.1098/rspb.2006.3574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Hempel P. Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol. 2005;50:529–551. doi: 10.1146/annurev.ento.50.071803.130420. doi:10.1146/annurev.ento.50.071803.130420 [DOI] [PubMed] [Google Scholar]
- Seehuus S.C., Norberg K., Gimsa U., Krekling T., Amdam G.V. Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc. Natl Acad. Sci. USA. 2006;103:962–967. doi: 10.1073/pnas.0502681103. doi:10.1073/pnas.0502681103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorci G., Clobert J. Effects of maternal parasite loead on offspring life-history traits in the common lizard (Lacerta vivipara) J. Evol. Biol. 1995;8:711–723. doi:10.1046/j.1420-9101.1995.8060711.x [Google Scholar]
- Steiner H., Andreu D., Merrifield T.B. Binding and action of cecropin and cecropin analogues: antibacterial peptides from insects. Biochim. Biophys. Acta. 1988;939:260–266. doi: 10.1016/0005-2736(88)90069-7. doi:10.1016/0005-2736(88)90069-7 [DOI] [PubMed] [Google Scholar]
- Sun S.C., Lindstrom I., Boman H.G., Faye I., Schmidt O. Hemolin: an insect-immune protein belonging to the immunoglobulin superfamily. Science. 1990;250:1729–1732. doi: 10.1126/science.2270488. doi:10.1126/science.2270488 [DOI] [PubMed] [Google Scholar]
- Vorburger C., Gegenschatz S.E., Ranieri G., Rodriguez P. Limited scope for maternal effects in aphid defence against parasitoids. Ecol. Entomol. 2008;33:189–196. doi:10.1111/j.1365-2311.2007.00949.x [Google Scholar]
- Wilson K., Thomas M.B., Blanford S., Doggett M., Simpson S.J., Moore S.L. Coping with crowds: density dependent disease resistance in desert locusts. Proc. Natl Acad. Sci. USA. 2002;99:5471–5475. doi: 10.1073/pnas.082461999. doi:10.1073/pnas.082461999 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
III RT-qPCR results for hemocytes. IV RT-qPCR results for rest of the bodies originating from parental generation. V RT-qPCR results for adults