Abstract
Many individual decisions are informed by direct comparison of the alternatives. In collective decisions, however, only certain group members may have the opportunity to compare options. Emigrating ant colonies (Temnothorax albipennis) show sophisticated nest-site choice, selecting superior sites even when they are nine times further away than the alternative. How do they do this? We used radio-frequency identification-tagged ants to monitor individual behaviour. Here we show for the first time that switching between nests during the decision process can influence nest choice without requiring direct comparison of nests. Ants finding the poor nest were likely to switch and find the good nest, whereas ants finding the good nest were more likely to stay committed to that nest. When ants switched quickly between the two nests, colonies chose the good nest. Switching by ants that had the opportunity to compare nests had little effect on nest choice. We suggest a new mechanism of collective nest choice: individuals respond to nest quality by the decision either to commit or to seek alternatives. Previously proposed mechanisms, recruitment latency and nest comparison, can be explained as side effects of this simple rule. Colony-level comparison and choice can emerge, without direct comparison by individuals.
Keywords: collective decision, radio-frequency identification, comparison, emigration, sequential search, Temnothorax
1. Introduction
Direct comparison of alternatives can contribute to individual decision-making during mate choice (Bateson & Healy 2005), foraging (Langen 1999) and shopping for consumer goods (Doyle et al. 1999). Another mechanism used in individual decision-making is sequential search, in which an animal simply chooses the first option that exceeds an acceptance threshold, irrespective of the other alternatives available. This can also contribute to mate choice (Moore & Moore 1988) and foraging decisions (Langen 1999). Which strategy is optimal depends on the costs of searching and sampling (Real 1990).
Social animals must apply decision mechanisms not only to individual choices, but also when they participate in group decisions (Conradt & List 2009). These can range from totally democratic, involving all members, to totally despotic, where one individual leads the whole group (Conradt & Roper 2005, 2009). Influential individuals might manipulate the group for their own benefit or might simply be better informed than others, for example if they have had the opportunity directly to compare alternatives and choose the better option. Conflicts over decisions that will influence overall survivorship and (inclusive) fitness of all members should be relatively low within kin groups (Bourke & Franks 1995). Such should be the case in nest choice by complete social insect colonies. Nevertheless, information asymmetry could arise if some individuals have visited multiple nest sites, while others have experienced only one.
The rock ant Temnothorax albipennis makes a collective decision when a colony emigrates to a new nest. Scouting ants that discover new nests assess them on the basis of multiple attributes (Franks et al. 2003, 2006). Some of these scouts subsequently recruit nest-mates to the new nest using tandem-running, where an informed ant leads a second ant to her destination (Möglich 1978; Richardson et al. 2007). When the number of ants in the new nest reaches a quorum, scouts begin rapid transport of the rest of the colony by carrying nest-mates and brood (Pratt et al. 2002). Colonies are able to choose the best of several nests (Mallon & Franks 2000; Franks et al. 2003). Two individual-level mechanisms for this collective choosiness have been identified. Some ants visit both nest sites, and subsequently recruit only to the better site, which has been taken as evidence for direct comparison (Mallon et al. 2001). However, ants that visit only one site still contribute to the colony decision, by starting to recruit earlier (i.e. using a shorter recruitment latency) when a nest is of higher quality (Mallon et al. 2001).
Modelling has demonstrated that differences in recruitment latency can account for choice between two equidistant nests, even in the absence of individual comparisons (Pratt & Sumpter 2006). However, T. albipennis colonies can choose the better of two nests even when it is nine times further away (Franks et al. 2008). In this case, recruitment latency differences should be cancelled out by the greater time required to travel to the new nest, so how do colonies avoid becoming trapped through the recruitment of nest-mates to a nearby adequate nest and instead emigrate to a more distant high-quality nest?
We offered radio-frequency identification (RFID)-tagged T. albipennis colonies the choice between a nearby poor-quality nest, and a more distant high-quality nest. RFID readers monitored the visits of ants to the new nests and identified ants performing recruiting tandem runs (figure 1). We tested three mechanisms that could be used in nest choice: recruitment latency; direct comparison; and a threshold rule. To test whether recruitment latency could be contributing to nest choice, we measured whether there were still nest-quality-dependent differences in individual recruitment latencies even at these distances. The other two mechanisms, direct comparison and the threshold rule, require some ants to switch between the two new nests. The direct comparison mechanism requires ‘informed’ switchers to visit both nests and directly compare their quality. We determined whether such informed ants returned rapidly to the better nest and whether they were more likely to recruit subsequently to this nest, to test whether direct comparison plays a role in nest choice. The third mechanism the ants could use is the threshold rule, with ‘uninformed’ switchers basing their decision, either to remain committed to one nest or to search for an alternative, only on the quality of the nest they are currently visiting. We tested whether such uninformed switchers were more likely to desert a low-quality than a high-quality nest, to test whether this mechanism could be significant in nest choice. Finally, we compared emigration dynamics between colonies that chose the high-quality far nest and those which chose the low-quality near nest, to investigate how these individual-level mechanisms influence the success of the colony decision process.
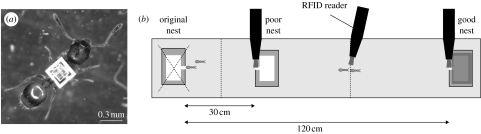
Figure 1.
Experimental procedure using RFID technology. (a) Temnothorax albipennis worker with RFID tag. (b) Experimental arena (18×180 cm) with original nest (destroyed at the start of the trial) and two new nests. The good nest has a red filter making the interior dark. Dashed lines indicate recording points for tandem runs (at 15 and 45 cm).
2. Material and methods
(a) Study species
Nine T. albipennis colonies were collected from the Dorset coast, England on 29 September 2007. Colonies were queenright and contained 100–200 workers and brood of all stages. Colonies were housed in artificial nests as in Franks et al. (2008) with a 2.0×1.5 mm entrance. Nests were placed into small Petri dishes with Fluon-coated walls. Colonies were provided with water ad libitum and fed weekly with honey solution and Drosophila melanogaster.
(b) RFID-tagging protocol
An RFID microtransponder (500×500×120 μm) with a unique ID was affixed to the thorax of every worker ant in each colony (Robinson et al. 2009). Ants were RFID tagged in a single session the day before a trial. The RFID reader (PharmaSeq, Inc., NJ) consisted of a laser that provided energy (35 mW) to the passive tags, and an antenna to detect the radio identification signal. The laser modulation frequency is 1 MHz and the wavelength is 690 nm (Hitachi HL6738MG). RFID tagging has no observable effects on the behaviour of the ants (Robinson et al. 2009).
(c) Emigration procedure
Trials were performed in a long arena with Fluon-coated sides, cleaned with water and alcohol between trials. Two new nests with the same dimensions as the original nest were placed in the arena (figure 1). The nearer nest had a clear acetate lid whereas the farther nest had a red filter covering the cavity area. A red filter makes the nest dark as ants do not have a red visual pigment (Briscoe & Chittka 2001); T. albipennis colonies prefer darker nests (Franks et al. 2006). Each nest had an RFID reader placed vertically over the entrance. A trial began with the original nest placed in the arena and then destroyed by removing the top microscope slide. The RFID readers detected ants entering and leaving the nests (86% tag read rate, see the electronic supplementary material) and handheld RFID readers were used to read the tags on tandem-running ants (100% tag read rate) as they crossed half-way lines (figure 1). The identity of the leader and follower in the tandem run was thus recorded and the direction of the tandem run was noted. This procedure did not disrupt tandem runs. Emigrations were observed until complete, or for the first 5 hours if emigration was still incomplete. During observations, the time at which the transport of a nest-mate or a brood item first occurred to each nest was recorded. RFID readers over the nest entrances logged data for 24 hours, after which the nest choice of the colony was recorded. Colonies were considered to have split if brood was present in both nests. Nine trials were performed, each with a new colony.
(d) Analysis
To test whether our colonies actively chose the good nest, even though it was further away, we compared our results to a control carried out prior to this experiment that used the same nine colonies and six additional colonies in an identical experimental set-up, but with both the near and the far nest covered with red filters, making them of equal (high) quality (full methods and results in Franks et al. 2008). The Freeman–Halton extension of the Fisher's exact probability test was used to compare colony-level nest choice to these control results (Freeman & Halton 1951). Recruitment latency was defined as the time from when an ant first enters a nest to when the same ant leads a tandem run to that nest, minus travel time (details in the electronic supplementary material). The generalized linear mixed model (GLMM) on switching included all ants that made at least two nest visits in total during the pre-decision period without following a tandem run. Ants either switched to visiting the other nest, or continued to visit the same nest. The model used a binomial error structure with quality of first nest visited and first nest discovery time as fixed factors and colony as a random factor. Non-significant interactions were omitted from the final model. For the comparison of travel times to the better nest, the travel times (near nest to far nest) of ants which made independent visits (not following tandem run) to the far nest, then the near nest, then returned to the far nest were compared with the ants that simply made independent visits to the near nest and then the far nest. GLMMs were performed in R (R v. 2.4.1 2006, R Foundation for Statistical Computing); all other statistics were performed in Minitab (Release v. 14.20 2005 Minitab, Inc.).
3. Results
Four of nine colonies chose the far (good) nest, three chose the near (poor) nest and two colonies split between far and near nests. A control using far and near nests of equal quality previously found that 14 out of 15 colonies chose the near nest and none split (Franks et al. 2008). Our results differ significantly from this control (Fisher's exact probability test, p<0.001) showing that some of our colonies actively chose the good nest, even though it was further away.
We found no significant difference in recruitment latencies between ants recruiting to near versus far nests, either for paired comparisons within ants that led tandem runs to both nests (mean±s.d: near nest 36.0±28.2 min; far nest 59.0±41.9 min; paired t-test t=0.8, d.f.=5, p=0.25), or when latencies for all 48 ants leading tandem runs are included (mean±s.d.: near nest 57.6±46.5 min; far nest 49.0±60.4 min; t-test t=1.4, d.f.=22, p=0.27; further details in the electronic supplementary material).
(a) Evidence for a threshold rule
In total, an average of 27%±11 of the ants active during the pre-decision period (ants recorded entering a new nest before transport of brood/nest-mates began to that nest), visited both available nests, showing that ants do not necessarily remain committed to the first nest they visit. How is the likelihood of an ant switching between nests affected by nest quality? More ants visit the near nest than the far nest; so to test whether switching is really quality dependent, we compared among ants that made more than one independent visit (not following a tandem run) to the new nests (figure 2a). Approximately half (41%) of ants first visiting the near nest (n=82) later switched and visited the far nest rather than continuing to visit only the near nest, whereas of the ants first visiting the far nest (n=65) only a tiny minority (3%) switched to visiting the near nest, with most ants remaining committed to the far nest during subsequent visits. These ants are uninformed when they are switching between the nests, as they do not know the quality of other nests available until they encounter them. To test whether this was simply an effect of ants discovering the near nest earlier in the emigration process, so having more time to discover the other nest before transport began, we analysed whether ants stayed or switched using a GLMM with first nest discovery time and nest quality as fixed factors. First nest discovery time had no significant effect on whether an ant switched (GLMM, t=1.36, d.f.=136, p=0.17), whereas quality of the nest first visited has a significant effect on whether the ants subsequently switched and visited the other nest (GLMM, t=3.07, d.f.=136, p<0.01). Switching ants were more active overall than other ants. Among ants active during the pre-decision phase, those which confined their visits to one nest averaged 3.5±6.6 visits, while ants visiting both nests averaged 12.5±18.1 visits in total (t-test, t=4.1, d.f.=76, p<0.0001). In addition to being more active overall, switching ants also made more visits per nest (6.3±9.0) than other ants (t-test, t=2.33, d.f.=97, p<0.05).
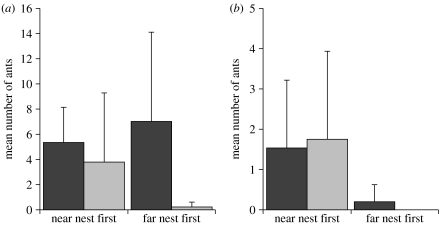
Figure 2.
Direction of uninformed switching. (a) Ants that make an independent visit to one nest, and then either continue to visit that nest only (black bars, stay) or visit the other nest (grey bars, switch). (b) Ants which make an independent visit to one nest and begin recruitment by tandem running to that nest, then either continue to visit that nest only (black bars, stay) or visit the other nest (grey bars, switch). Nine colonies, mean+s.d.
The same overall pattern is seen for ants that begin leading tandem runs to the first nest they visit, without having visited the other nest or followed a tandem run (figure 2b). Approximately half (53%) of these ants first leading a tandem run to the near nest (n=30) later switched and visited the far nest rather than continuing to visit only the near nest, whereas of the ants first visiting the far nest (n=2), none switched to visiting the near nest and all continued to visit the far nest. The very small n for ants at the far nest prevents meaningful GLMM analysis comparing the proportions switching in each direction; however, it is clear that uninformed switching from the near nest to the far nest occurs even after tandem running has started. These data support the threshold-rule hypothesis.
(b) Evidence for direct comparison?
Informed ants that recruit by tandem running only after first visiting both nests are rare (table 1). Ants that made independent visits to both nests, and then recruited to one, were equally likely to recruit to the near or the far nests, with no effect of which nest they visit first. These data do not support the direct comparison hypothesis. Three ants became informed ants after finding one nest independently and following a tandem run to the other. These then recruited by tandem running to the far nest (table 1). Switching in cases where the opportunity for direct comparison is available therefore makes up only a tiny proportion of all the switching between nests. Even if direct comparison is not used in determining recruitment effort, it could still be advantageous if ants that have had the opportunity to compare nests are able to subsequently find the superior nest more quickly than ants that had previous knowledge of the near nest only. There were no differences in the times it took informed and uninformed ants to find the far nest (mean±s.d. informed 136±69 min, uniformed 165±165 min, t-test: t=0.34, d.f.=20, p=0.73). These informed returning ants were also very rare (n=4) and actually made more repeat visits to the near nest (median=4.5) before returning to the far nest than uniformed ants (median=1; Mann–Whitney test, W=76, n=22, p<0.05). This does not support the idea that direct comparisons allow ants to return to superior alternatives more directly.
Table 1.
Informed ants that visited both nests before leading a tandem run. (TR, tandem run.)
| near nest first | far nest first | ||||
|---|---|---|---|---|---|
| lead TR to near | lead TR to far | lead TR to near | lead TR to far | Fisher's exact probability test | |
| discover both nests independently | 5 | 3 | 1 | 1 | p=0.99 |
| discover first nest independently and follow TR to other | 0 | 2 | 0 | 1 | N=3, no test performed |
These data support the hypothesis that ants switch from assessing or recruiting to a poor nest to searching for and recruiting to a better one (the threshold rule), but do not support the hypothesis that direct comparison of the two nests is a necessary part of this process.
(c) What is the effect of switching on nest choice?
Our data suggest that individual switching during assessment or recruitment (i.e. in the pre-decision phase before transport commenced) following a threshold rule should shift scouts away from the poor-quality nest towards visiting and recruiting to the higher quality nest. However, although all colonies included switching ants, not all the colonies we studied finally chose the far (good) nest. What effect does individual switching have on the collective decision? The proportion of ants in a colony switching between nests did not differ significantly between colonies that finally chose the near or far nest, or split (ANOVA F=0.65, d.f.=2,6, p=0.56). The timing of these switches, however, does differ significantly between colonies that chose the far nest, and those that stayed in the poor nest. For colonies that chose the far nest, ants finding one nest tended to switch shortly afterwards to the other nest, there is a positive correlation between the order of entering the two nests (figure 3). By contrast, for colonies that chose the near nest, there is an inverse relationship between the time of discovery of the two nests, so certain ants finding one nest early in the emigration process did not find the other nest until much later (figure 3). This difference between the switching dynamics suggests that the timing of individual switching could influence the colony-level outcome.
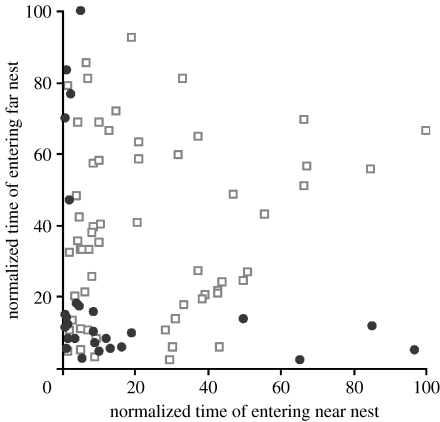
Figure 3.
Times of visits to each nest by switching ants. Times normalized with 0 as start of emigration; 100 as end of pre-decision period. Squares, ants from colonies that chose the far nest, colonies pooled. Analysis of ranked data showed a positive correlation (Spearman ρ=0.473, n=77, p<0.001). Individual trials all showed positive correlations. Circles, ants from colonies that chose the near nest, colonies pooled. Analysis of ranked data showed a significant negative correlation (Spearman ρ=−0.529, n=27, p<0.01). Individual trials all showed negative correlations. The two colonies that split are not plotted. Pooled, they showed no significant correlation; individually one was slightly positively correlated; one slightly negatively correlated.
4. Discussion
Certain models of nest choice in social insects predict that individual switching should increase the accuracy of the collective decision (Marshall et al. 2005, in press; Planqué et al. 2007), while other models give switching a much less prominent role (Pratt et al. 2002; Pratt & Sumpter 2006). Our data from RFID-tagged ants clearly show that such switching occurs during decision-making in T. albipennis; however, our data suggest that switching is not based on direct comparison by individuals, contrasting with the assumption of previous models (Pratt et al. 2002, 2005; Marshall et al. 2005; Pratt & Sumpter 2006; Planqué et al. 2007). Informed switching by ants that had the opportunity to compare both nests was rare in our experiments and did not result in ants choosing the better nest or returning to the better nest more quickly. By contrast, our data clearly show that uninformed ants finding a nest do not necessarily remain committed to it, even after recruitment has begun, but may switch to searching for alternatives, depending only on the quality of the current nest. If the current nest is low quality, this uninformed switching is common, leading to many ants discovering the better nest. If the current nest is of high quality, ants do not search for alternatives and either begin recruitment, or continue to visit the high-quality nest. Ants that continue to visit a nest can still contribute to the decision process, even if they do not recruit by tandem running, because their presence in the nest may contribute to the quorum, and therefore to the likelihood that other ants will begin rapid transport of brood and nest-mates to that nest. Ants involved in switching were very active, making many nest visits. In colonies that were able to choose the better nest, a small number of individuals initially found the poor nest and began recruitment, but then rapidly switched to searching for alternatives, resulting in recruitment to the better nest, and the redirection of the colony. Thus, the speed with which ants decided to desert a low-quality nest to search for alternatives could influence successful nest choice.
Previous studies on T. albipennis and the closely related Temnothorax curvispinosus have found that ants use a lower recruitment latency when they encounter a high-quality nest (Mallon et al. 2001; Pratt 2005; Pratt et al. 2005; Pratt & Sumpter 2006); however, we do not find this pattern in our data. The extra travel time to distant nests could easily cancel out differences in recruitment latency; however, even when we controlled for distance, we still found no differences in recruitment latency between the two nests of differing quality. Our data suggest that these previously observed differences in recruitment latency could simply be a side effect of a nest-quality-dependent decision to continue searching. All the studies that recorded recruitment latencies offered the colonies only a single new nest at a time (Mallon et al. 2001; Pratt 2005; Pratt et al. 2005; Pratt & Sumpter 2006). We suggest that an ant encountering a nest compares it to an acceptability threshold, and either rejects it and continues to search, or accepts it and begins repeat visits and recruitment by tandem running. This model would account for a long recruitment latency when only one poor nest is available, because ants encountering this nest would be likely to continue searching for alternatives, only beginning recruitment after repeated re-encounters with the same nest or when enough time had elapsed that they became less choosy. Ants encountering a good nest would be more likely to become committed rather than to continue searching, so a short latency would be seen. During recruitment latency, ants do not simply wait passively, but make trips to the new nest and in the arena (Pratt 2005). This is consistent with searching behaviour. Ants that have discovered a poor nest spend a higher proportion of the recruitment latency outside the new nest than ants that have discovered a good nest (Planqué et al. 2007 reanalysing data from Mallon et al. 2001), further supporting the idea that ants that find a poor nest search for alternatives.
This suggests a new model of nest-site choice, simpler than previous models that have incorporated remembering and comparing the quality of previous nests, emigration-stage-specific search probabilities and nest-quality-dependent acceptance probabilities (Pratt et al. 2005; Planqué et al. 2007). These three processes could be condensed to the single rule that an ant encountering a nest assesses the nest and compares it to an acceptability threshold, then either rejects it and continues to search, or accepts it and begins repeat visits and recruitment by tandem running. When two nests are available, ants that have found the poorer nest are likely to continue searching, and by random chance may encounter the good nest, which they will recruit if it reaches their threshold of acceptability. Thus apparent comparison by individuals can be explained more parsimoniously by simple thresholds, as is the case with self-organized aggregation by cockroaches (Amé et al. 2006; Halloy et al. 2007).
Temnothorax albipennis are known to exhibit ‘negative latent learning’ (Franks et al. 2007). Colonies housed in a good nest with a poor nest available will, if later forced to emigrate, avoid that poor nest and choose an equally poor unfamiliar nest, whereas if a good nest is available during reconnoitring, they will not avoid it later (Franks et al. 2007). This apparent comparison of the newly found nest with the home nest could also be explained by a simple absolute acceptability threshold used during reconnoitring to reject the poor nests, so ants would not need to learn the exact quality of the nests they have found, but simply whether they are acceptable or not. Inter-individual variation in acceptability threshold of the searching ants may be quite high. This may have implications for speed–accuracy trade-offs (Marshall et al. 2005) and for the threshold searching rule, because a small number of ants with very high thresholds could always be searching for better nests, leading to the ‘move to improve’ phenomenon where a colony leaves an undamaged nest to move to a superior site (Dornhaus et al. 2004).
In the honeybee Apis mellifera, individual switching between nests is rare, and individuals recruit persistently to one nest (Camazine et al. 1999; Seeley & Buhrman 1999). Preventing honeybees from switching between nests during the decision process does not prevent or delay nest-site choice (Visscher & Camazine 1999) probably because bees rely more on competition between recruitment processes, with individuals recruiting for longer to a better nest site (Seeley & Buhrman 1999; Seeley et al. 2000). The equivalent mechanism in ants of individuals performing more tandem runs to better sites is not seen in T. albipennis (Mallon et al. 2001; unpublished data from this study). In foraging, honeybees recruit to better sites at a higher rate (Seeley et al. 2000), but this mechanism is also not supported in T. albipennis (Mallon et al. 2001; unpublished data from this study). Why do rock ants and honeybees use such different strategies? Both honeybees and rock ants make speed–accuracy trade-offs, but their priorities differ. Bees can remain as a swarm for several days while scouting for nests but their final decision must be unanimous (Lindauer 1957). By contrast, a homeless T. albipennis colony is immediately vulnerable and a rapid decision is required. Temnothorax albipennis can be polydomous, so colony splitting, which is one potential outcome of high individual influence on group decisions (Kerth et al. 2006; Conradt & Roper 2007), is less damaging. Honeybees may therefore use a slower assessment process to generate a right-first-time consensus decision, while T. albipennis uses a rapid-action strategy, with the flexibility to redirect emigrations and reunite.
It is also possible that switching during recruitment does indeed play a role in honeybee nest-site choice, but that this behaviour has been overlooked because the studies in which switching has been found to be low have offered swarms nest sites of equal and high quality (Camazine et al. 1999; Visscher & Camazine 1999). Switching may be important only when swarms must choose between nest sites of differing quality. In early studies on house-hunting bees, Lindauer (1955, 1957) suggested that bees that visited two nests could compare their qualities and choose to recruit to the better nest; however, in the light of our results, it should be noted that bees visiting both nests and recruiting only to the better nest need not necessarily have compared the alternatives, but could use simple thresholds. Seeley & Visscher (2004) suggested that the ‘stop signal’ used by honeybees to inhibit foraging waggle dances when a nectar surplus occurs (Kirchner 1993; Nieh 1993) could also be used by scout bees that have switched from a poor-quality site to a better one. Informed scouts could thus interfere with recruitment to their previously preferred site. This hypothesis remains to be tested.
Although our data show that many individual ants investigate both options during decision-making, they suggest that the ants do not need to perform the cognitively demanding process of directly comparing two nests. If the probability of recruitment versus searching for alternatives is dependent on the nest quality, an ant would not need to take into account the quality of nests it had visited previously. This simple threshold rule has several advantages over comparative evaluation. Comparing requires an assessment of multiple options before recruitment can begin. This can be costly (Real 1990) and the ideal assessment sample size may vary (Ferguson 1989; Todd & Miller 1999). By contrast, using the threshold rule, if the very first nest the ants encounter is good enough, they can immediately start emigration to that nest, without searching for alternatives or waiting for the return of informed ants. Thus the threshold rule can provide a swift solution. In addition, animals (including humans) using comparative evaluation frequently make ‘irrational’ decisions, due to the context in which options are compared, for example distracter effects from irrelevant alternatives (Doyle et al. 1999; Bateson & Healy 2005). Also, if individuals are not consistent in their ranking of each pair of options, group-level preferences could become intransitive (e.g. option A preferred to B, B preferred to C but C preferred to A), leading to a Condorcet paradox (Regenwetter et al. 2009). The threshold rule makes an absolute assessment of nest quality that is not subject to these risks, and circumvents the necessity for memorization and comparison of every site visited. Thus, simple individual behaviours could substitute for direct comparisons while facilitating effective choice between nest sites at the colony level.
Acknowledgements
We thank M. Giurfa, E. A. Langridge, J. A. R. Marshall, T. O. Richardson, S. Perez-Espona, A. B. Sendova-Franks and N. Stroeymeyt and two anonymous reviewers for their comments on the manuscript. N.R.F. and E.J.H.R. acknowledge EPSRC grant EP/D076226/1.
Supplementary Material
Further details of RFID-tagging methods and recruitment latency analysis
References
- Amé J.M., Halloy J., Rivault C., Detrain C., Deneubourg J.L. Collegial decision making based on social amplification leads to optimal group formation. Proc. Natl Acad. Sci. USA. 2006;103:5835–5840. doi: 10.1073/pnas.0507877103. doi:10.1073/pnas.0507877103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson M., Healy S.D. Comparative evolution and its implications for mate choice. Trends Ecol. Evol. 2005;20:659–664. doi: 10.1016/j.tree.2005.08.013. doi:10.1016/j.tree.2005.08.013 [DOI] [PubMed] [Google Scholar]
- Bourke, A. F. G. & Franks, N. R. 1995 Social evolution in ants Monographs in Behaviour and Ecology. Princeton, NJ: Princeton University Press.
- Briscoe A.D., Chittka L. The evolution of color vision in insects. Annu. Rev. Entomol. 2001;46:471–510. doi: 10.1146/annurev.ento.46.1.471. doi:10.1146/annurev.ento.46.1.471 [DOI] [PubMed] [Google Scholar]
- Camazine S., Visscher P.K., Finley J., Vetter R.S. House-hunting by honey bee swarms: collective decisions and individual behaviours. Insectes Soc. 1999;46:348–360. doi:10.1007/s000400050156 [Google Scholar]
- Conradt L., List C. Group decisions in humans and animals: a survey. Phil. Trans. R. Soc. B. 2009;364:719–742. doi: 10.1098/rstb.2008.0276. doi:10.1098/rstb.2008.0276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt L., Roper T.J. Consensus decision-making in animals. Trends Ecol. Evol. 2005;20:449–456. doi: 10.1016/j.tree.2005.05.008. doi:10.1016/j.tree.2005.05.008 [DOI] [PubMed] [Google Scholar]
- Conradt L., Roper T.J. Democracy in animals: the evolution of shared group decisions. Proc. R. Soc. B. 2007;274:2317–2326. doi: 10.1098/rspb.2007.0186. doi:10.1098/rspb.2007.0186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt L., Roper T.J. Conflicts of interest and the evolution of decision sharing. Phil. Trans. R. Soc. B. 2009;364:807–819. doi: 10.1098/rstb.2008.0257. doi:10.1098/rstb.2008.0257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornhaus A., Franks N.R., Hawkins R.M., Shere H.N.S. Ants move to improve: colonies of Leptothorax albipennis emigrate whenever they find a superior nest site. Anim. Behav. 2004;67:959–963. doi:10.1016/j.anbehav.2003.09.004 [Google Scholar]
- Doyle J.R., O'Connor D.J., Reynolds G.M., Bottomley P.A. The robustness of the asymmetrically dominated effect: buying frames, phantom alternatives, and in-store purchases. Psychol. Mark. 1999;16:225–243. doi:10.1002/(SICI)1520-6793(199905)16:3<225::AID-MAR3>3.0.CO;2-X [Google Scholar]
- Ferguson T.S. Who solved the secretary problem? Stat. Sci. 1989;4:282–296. doi:10.1214/ss/1177012493 [Google Scholar]
- Franks N.R., Mallon E.B., Bray H.E., Hamilton M.J., Mischler T.C. Strategies for choosing between alternatives with different attributes: exemplified by house-hunting ants. Anim. Behav. 2003;65:215–223. doi:10.1006/anbe.2002.2032 [Google Scholar]
- Franks N.R., Dornhaus A., Metherell B., Nelson T., Lanfear S.A., Symes W. Not everything that counts can be counted: ants use multiple metrics for a single nest trait. Proc. R. Soc. B. 2006;273:165–169. doi: 10.1098/rspb.2005.3312. doi:10.1098/rspb.2005.3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N.R., Hooper J.W., Dornhaus A., Aukett P.J., Hayward A.L., Berghoff S.M. Reconnaissance and latent learning in ants. Proc. R. Soc. B. 2007;274:1505–1509. doi: 10.1098/rspb.2007.0138. doi:10.1098/rspb.2007.0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N.R., Hardcastle K.A., Collins S., Smith F.D., Sullivan K.M.E., Robinson E.J.H., Sendova-Franks A.B. Can ant colonies choose a far-and-away better nest over an in-the-way poor one? Anim. Behav. 2008;76:323–334. doi:10.1016/j.anbehav.2008.02.009 [Google Scholar]
- Freeman G.H., Halton J.H. Note on exact treatment of contingency, goodness of fit and other problems of significance. Biometrika. 1951;38:141–149. [PubMed] [Google Scholar]
- Halloy J., et al. Social integration of robots into groups of cockroaches to control self-organized choices. Science. 2007;318:1155–1158. doi: 10.1126/science.1144259. doi:10.1126/science.1144259 [DOI] [PubMed] [Google Scholar]
- Kerth G., Ebert C., Schmidtke C. Group decision making in fission–fusion societies: evidence from two-field experiments in Bechstein's bats. Proc. R. Soc. B. 2006;273:2785–2790. doi: 10.1098/rspb.2006.3647. doi:10.1098/rspb.2006.3647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner W.H. Vibrational signals in the tremble dance of the honeybee, Apis mellifera. Behav. Ecol. Sociobiol. 1993;33:169–172. doi:10.1007/BF00216597 [Google Scholar]
- Langen T.A. How western scrub-jays (Aphelocoma californica) select a nut: effects of the number of options, variation in nut size, and social competition among foragers. Anim. Cogn. 1999;2:223–233. doi:10.1007/s100710050043 [Google Scholar]
- Lindauer M. Schwarmbienen auf Wohnungssuche. Z. Vgl. Physiol. 1955;37:263–324. doi:10.1007/BF00303153 [Google Scholar]
- Lindauer M. Communication in swarm-bees searching for a new home. Nature. 1957;179:63–66. doi:10.1038/179063a0 [Google Scholar]
- Mallon E.B., Franks N.R. Ants estimate area using Buffon's needle. Proc. R. Soc. Lond. B. 2000;267:765–770. doi: 10.1098/rspb.2000.1069. doi:10.1098/rspb.2000.1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallon E.B., Pratt S.C., Franks N.R. Individual and collective decision-making during nest site selection by the ant Leptothorax albipennis. Behav. Ecol. Sociobiol. 2001;50:352–359. doi:10.1007/s002650100377 [Google Scholar]
- Marshall J.A.R., Dornhaus A., Franks N.R., Kovacs T. Noise, cost and speed–accuracy trade-offs: decision-making in a decentralized system. J. R. Soc. Interface. 2005;3:243–254. doi: 10.1098/rsif.2005.0075. doi:10.1098/rsif.2005.0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall, J. A. R., Bogacz, R., Dornhaus, A., Planqué, R., Kovacs, T. & Franks, N. R. In press. On optimal decision-making in brains and social insect colonies. J. R. Soc. Interface (doi:10.1098/rsif.2008.0511) [DOI] [PMC free article] [PubMed]
- Möglich M. Social organization of nest emigration in Leptothorax (Hym. Form.) Insectes Soc. 1978;25:205–225. doi:10.1007/BF02224742 [Google Scholar]
- Moore A.J., Moore P.J. Female strategy during mate choice—threshold assessment. Evolution. 1988;42:387–391. doi: 10.1111/j.1558-5646.1988.tb04141.x. doi:10.2307/2409241 [DOI] [PubMed] [Google Scholar]
- Nieh J.C. The stop signal of honey bees: reconsidering its message. Behav. Ecol. Sociobiol. 1993;33:51–56. doi:10.1007/BF00164346 [Google Scholar]
- Planqué R., Dornhaus A., Franks N.R., Kovacs T., Marshall J.A.R. Weighted waiting in collective decision-making. Behav. Ecol. Sociobiol. 2007;61:347–356. doi:10.1007/s00265-006-0263-4 [Google Scholar]
- Pratt S.C. Behavioral mechanisms of collective nest-site choice by the ant Temnothorax curvispinosus. Insectes Soc. 2005;52:383–392. doi:10.1007/s00040-005-0823-z [Google Scholar]
- Pratt S.C., Sumpter D.J.T. A tunable algorithm for collective decision making. Proc. Natl Acad. Sci. USA. 2006;103:15 906–15 910. doi: 10.1073/pnas.0604801103. doi:10.1073/pnas.0604801103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt S.C., Mallon E.B., Sumpter D.J.T., Franks N.R. Quorum sensing, recruitment, and collective decision-making during colony emigration by the ant Leptothorax albipennis. Behav. Ecol. Sociobiol. 2002;52:117–127. doi:10.1007/s00265-002-0487-x [Google Scholar]
- Pratt S.C., Sumpter D.J.T., Mallon E.B., Franks N.R. An agent-based model of collective nest site choice by the ant Leptothorax albipennis. Anim. Behav. 2005;70:1023–1036. doi:10.1016/j.anbehav.2005.01.022 [Google Scholar]
- Real L. Search theory and mate choice. I. Models of single-sex discrimination. Am. Nat. 1990;136:376–405. doi:10.1086/285103 [Google Scholar]
- Regenwetter M., Grofman B., Popova A., Messner W., Davis-Stober C.P., Cavagnaro D.R. Behavioural social choice: a status report. Phil. Trans. R. Soc. B. 2009;364:833–843. doi: 10.1098/rstb.2008.0259. doi:10.1098/rstb.2008.0259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson T.O., Sleeman P.A., McNamara J.M., Houston A.I., Franks N.R. Teaching with evaluation in ants. Curr. Biol. 2007;17:1520–1526. doi: 10.1016/j.cub.2007.08.032. doi:10.1016/j.cub.2007.08.032 [DOI] [PubMed] [Google Scholar]
- Robinson E.J.H., Richardson T.O., Sendova-Franks A.B., Feinerman O., Franks N.R. Radio-tagging reveals the roles of corpulence, experience and social information in ant decision making. Behav. Ecol. Sociobiol. 2009;63:627–636. doi:10.1007/s00265-008-0696-z [Google Scholar]
- Seeley T.D., Buhrman S.C. Group decision making in swarms of honey bees. Behav. Ecol. Sociobiol. 1999;45:19–31. doi:10.1007/s002650050536 [Google Scholar]
- Seeley T.D., Visscher P.K. Group decision making in nest-site selection by honey bees. Apidologie. 2004;35:101–116. doi:10.1051/apido:2004004 [Google Scholar]
- Seeley T.D., Mikheyev A.S., Pagano G.J. Dancing bees tune both duration and rate of waggle-run production in relation to nectar-source profitability. J. Comp. Physiol. A. 2000;186:813–819. doi: 10.1007/s003590000134. doi:10.1007/s003590000134 [DOI] [PubMed] [Google Scholar]
- Todd P.M., Miller G.F. From pride and prejudice to persuasion: satisficing in mate search. In: Gigerenzer G., Todd P.M., editors. Simple heuristics that make us smart. Oxford University Press; Oxford, UK: 1999. pp. 287–308. [Google Scholar]
- Visscher P.K., Camazine S. Collective decisions and cognition in bees. Nature. 1999;397:400. doi: 10.1038/17047. doi:10.1038/17047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Further details of RFID-tagging methods and recruitment latency analysis