Abstract
Exceptional fossil specimens with preserved soft parts from the Maotianshan Shale (ca 520 Myr ago) and the Burgess Shale (505 Myr ago) biotas indicate that the worldwide distributed bivalved arthropod Isoxys was probably a non-benthic visual predator. New lines of evidence come from the functional morphology of its powerful prehensile frontal appendages that, combined with large spherical eyes, are thought to have played a key role in the recognition and capture of swimming or epibenthic prey. The swimming and steering of this arthropod was achieved by the beating of multiple setose exopods and a flap-like telson. The appendage morphology of Isoxys indicates possible phylogenetical relationships with the megacheirans, a widespread group of assumed predator arthropods characterized by a pre-oral ‘great appendage’. Evidence from functional morphology and taphonomy suggests that Isoxys was able to migrate through the water column and was possibly exploiting hyperbenthic niches for food. Although certainly not unique, the case of Isoxys supports the idea that off-bottom animal interactions such as predation, associated with complex feeding strategies and behaviours (e.g. vertical migration and hunting) were established by the Early Cambrian. It also suggests that a prototype of a pelagic food chain had already started to build-up at least in the lower levels of the water column.
Keywords: Arthropoda, Cambrian, Chengjiang, Burgess Shale, predation, food chain
1. Introduction
Present-day pelagic ecosystems are inhabited by an extraordinary variety of organisms from picoplankton to large fishes and mammals that interact via complex food chains throughout the water column. The build-up process of the pelagic ecosystem, crucial to the functioning of the biosphere, raises the fundamental question of when and how animals started to colonize off-bottom niches (Rigby & Milsom 1996). Most exceptional Cambrian biotas (e.g. Chengjiang, Burgess Shale, Emu Bay Shale, Sirius Passet, Orsten) include potential nektonic swimmers and zooplankton among various groups such as arthropods (Butterfield 1994, 2001; Harvey & Butterfield 2008), chaetognaths (Chen & Huang 2002; Vannier 2007; Vannier et al. 2007b), ctenophores (Conway Morris & Collins 1996; Hu et al. 2007), eldoniids (Zhu et al. 2002), vetulicolids (Aldridge et al. 2007), and possibly chordates (Shu et al. 1999a, 2003). However, major uncertainties remain as to the exact lifestyle, habitat, bathymetry and dynamics of these animals in the water-column, thus questioning the actual occupancy of the non-benthic Cambrian ecospace. Isoxys is a bivalved arthropod known essentially from its exoskeletal parts (bivalved shields) that occur in the Lower and Middle Cambrian rocks of North America, South China, Siberia, South Australia and North Greenland (Williams et al. 1996). The tentative reconstruction of this assumed pelagic animal by Vannier & Chen (2000) is largely hypothetical. It is based on fragmentary information obtained from a few incomplete specimens from the Chengjiang fauna. Similarly, the idea that Isoxys might have been a predator (Hu 2005; Hu et al. 2007) lacks strong evidence. It is supported by a single incomplete juvenile specimen with a pointed frontal appendage. By contrast, the new excellently preserved fossil material described here from both the Lower Cambrian Chengjiang and the Middle Cambrian Burgess Shale Lagerstätten provide conclusive morphological evidence, reveal vital aspects of the biology of Isoxys such as feeding, vision and locomotion, and enlighten the colonization process of off-bottom niches by early animal life.
2. Material and methods
(a) South China
Fourteen specimens collected by S.-X. H. and A.-L. C. were examined. They were recovered from the Yu'anshan Formation, Maotianshan Shale Member, Eoredlichia-Wutingaspis biozone, Meishucunian (Lower Cambrian, Shergold & Cooper 2004) at Ercaicun and Xiaolantan (map in Hu 2005), all near Chengjiang, Yunnan Province, China. General information and complete references on the Chengjiang biota, its taphonomy and depositional environment were published in a series of recent papers (Chen et al. 2002, 2007; Chen 2004; Hou et al. 2004; Steiner et al. 2005; Hu 2005). Approximately 10 specimens show well-preserved eyes and the first pair of appendages. They belong to Isoxys auritus Jiang in Luo et al. (1982) and Isoxys curvirostratus (Vannier & Chen 2000), characterized by a reticulated and lineated ornament, respectively.
(b) Canada
The studied specimens were collected between 1975 and 2000 by Royal Ontario Museum (ROM) parties near Field, British Columbia, from various levels within the Burgess Shale Formation (mainly Walcott Quarry Shale, Raymond Quarry Shale Member and Emerald Lake Oncolite Member, all within the Bathyuriscus-Elrathina Zone, Middle Cambrian; for geology and stratigraphy, see Caron (2005) and Vannier et al. (2007a)). Preserved anatomical features such as eyes, head and trunk appendages, and midgut glands have been recognized in 41 specimens of Isoxys acutangulus (Walcott 1908) and one of Isoxys longissimus (Simonetta & delle Cave 1975) all housed in the ROM collections. The new systematic descriptions of these two species will be published separately (García-Bellido et al. in press b).
(c) Methods and terminology
Digital photographs were taken under different light conditions (low and high angles, non-polarized and polarized light, with specimens immersed in water or dry). ‘Carapace’ has long been used in a practical sense to designate the exoskeleton of numerous fossil and recent organisms, including Isoxys (Williams et al. 1996; Vannier & Chen 2000). We prefer to use here the term ‘shield’ that more specifically applies to arthropods (e.g. Walossek 1993; Maas et al. 2003) and, for the same reason, ‘pleural folds’ instead of ‘valves’.
The cladistic analysis of the great-appendage or megacheiran arthropods (Hou & Bergström 1997; Chen et al. 2004; Kühl et al. 2009) was performed using PAUP v. 4 (Swofford 2002).
(d) Repositories
CFM=The Chengjiang Museum, Chengjiang, Yunnan Province; ROM=Royal Ontario Museum, Toronto, Canada; YDSK=Yunnan Institute of Geological Sciences, Kunming, Yunnan Province.
(e) Abbreviations in figures
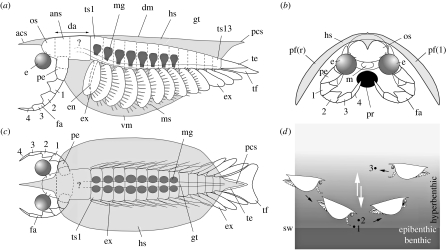
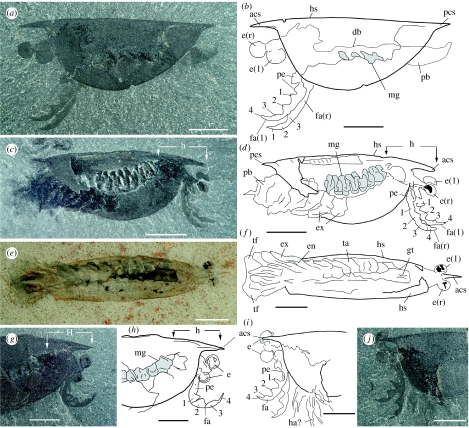
ans, antennular segment; acs, anterior cardinal spine; co, cornea; da, dorsal attachment of body; db, dorsal part of body; dm, dorsal margin of head shield; e, eye; e?, possible eye; en, endopod; es, eye stalk; ex, exopod; fa, frontal appendage; fa?, possible frontal appendage; gt, gut; h, presumed head; ha?, possible head appendage; hs, head shield; l, left; mg, midgut glands; ms, marginal setae; os, ocular segment; pb, posterior part of body; pe, peduncle; m, mouth; mu?, possible muscles (eye stalk); pcs, posterior cardinal spine; pf, pleural fold; r, right; re?, possible retinula; sw, sediment-water interface; ta, trunk appendage; te, telson; te?, possible telson; tf, telson flap; ts1–ts13, 1st–13th assumed trunk segment; vm, ventral margin of head shield; 1–4, distal podomeres of frontal appendage.
3. Functional anatomy of Isoxys
Isoxys is unique among Cambrian arthropods by its external shape with two prominent spines extending antero- and posterodorsally and a lack of strong lateral sculpture. Its head shield was attached to the rest of the body by a relatively short dorsal area (figures 1a and 2c,d). Trunk appendages often imprint their shape on the lateral surface of the head shield. This, added to frequent wrinkled preservation (Williams et al. 1996), suggests that Isoxys had a thin, flexible, non-mineralized and possibly translucent exoskeleton. Comparable light, streamlined shields with long dorsal spines are known in pelagic crustaceans such as Gnathophausia zoea (Lophograstrida; see the electronic supplementary material, figure 1c), planktotrophic larvae of malacostracans and halocypridid ostracods (Vannier & Chen 2000). These pointed shields are interpreted as exoskeletal adaptations to a non-benthic lifestyle and play a role in predator deterrence (Morgan 1989). Similarly, the head shield of Isoxys is assumed to have had a hydrodynamic and, possibly, a protective function against predation (Vannier & Chen 2005).
Figure 1.
Reconstruction of Isoxys based chiefly on specimens of I. acutangulus with preserved soft parts from the Middle Cambrian Burgess Shale. (a) Lateral view (left pleural fold of head shield removed, frontal appendage in assumed resting position). (b) Frontal view (frontal appendage grasping prey represented by black dot). (c) Dorsal view (translucent), respectively. (d) Presumed habitat and raptorial behaviour of Isoxys, targeting and capturing hypothetical prey (e.g. small soft-bodied or lightly sclerotized invertebrates) at/near the water-sediment interface (1, 2) or within the water column (3). White arrows indicate possible vertical migrations through the water column. Detailed morphology of head and endopod of trunk appendage unknown; long natatory setae of exopods not represented.
Figure 2.
Isoxys acutangulus (Walcott) from the Middle Cambrian Burgess Shale Formation, near Field, British Columbia, Canada. (a,b) Left lateral view (ROM 57898A). (c,d) Right lateral view (ROM 57912). (e,f) Dorsoventrally compressed specimen (ROM 57907). (g–j) Eyes and ‘great appendage’, right and left lateral view (ROM 57900 and ROM 57914A, respectively). All light photographs taken with polarized light. Midgut glands are in light grey. Scale bar 5 mm.
The anteriormost appendage of Isoxys was uniramous, curved and protruded beyond the anteroventral margin of the head shield. Since no other appendage occurs in front of it, it is likely to represent the antennula (the protocerebral appendage of all arthropods sensu stricto; see Waloszek et al. 2005). It was divided into a possibly two-segmented, proximal peduncle followed by a four-segmented claw-like unit. In I. acutangulus, three podomeres of the claw bore a conical tooth-like outgrowth that gave the inner margin of the appendage its serrated profile (figure 2a–d,i,j). The distal segment was pointed and subchelate. This frontal, pre-oral appendage could flex inwards and fit under the head shield, even though joints between podomeres are often unclear. It was inserted immediately behind the ocular segment. Additional head segments (figures 1a and 2c,d) may have filled the gap between the segment that bore the frontal appendage and the assumed first trunk segment (with biramous appendages and first pair of midgut glands). However, our material does not allow us to determine their number and the presence of possible appendages. The frontal appendage had an obvious prehensile function and is likely to have been used for both capturing prey and carrying food to the mouth region (figure 1c,d). The pedipalps of Recent and fossil arachnids (second pair of head appendages), such as the whip scorpions (e.g. Fox 2006; Tetlie & Dunlop 2008, fig. 6) are possible functional analogues of these structures. A single juvenile specimen of possibly I. auritus from the Lower Cambrian of China (Hu 2005) differs markedly from that of I. acutangulus in having a straight frontal appendage bearing numerous tiny spines (figure 3i,j).
Figure 3.
Isoxys from the Lower Cambrian Maotianshan Shale, Yunnan Province, South China. (a–d) Left and right lateral view (CFM 00047 and CFM 00169, respectively; both from Xiaolantan). (e–h) Fragment of anterior part with eye and part of the frontal appendage, left lateral view and detail of eye (CFM 00168; from Xiaoliantan). (i,j) Possible juvenile of I. auritus Jian in Luo et al. (1982) from Ercaicun, Lower Cambrian, Yunnan Province, China, right lateral view (YDKS 43). All light photographs. Midgut glands are in light grey. Scale bar 5 mm in (a,b,g–l), 1 mm in (c–f).
All trunk appendages had a short endopod and a long paddle-like exopod fringed with approximately 12–15 supporting setae. The detailed morphology of the proximal part of the limbs (e.g. basipod) and of the endopod (e.g. number of podomeres) is unknown. Isoxys probably swam by the metachronal beating of its flap-like exopods while the setae probably increased the effectiveness of the power stroke. The four last trunk appendages had a slightly different shape and orientation, with more slender exopods possibly lacking marginal setae. These posterior exopods formed, together with the telson flaps, a symmetrical fan-like structure resembling the uropods of recent crustaceans. By fanning out as the result of the vertical stroke of the posterior trunk, this tail fan is likely to have played a key role in the propulsion and steering of the animal through the water column.
Isoxys had a bulbous ocular segment from which a pair of large spherical eyes projected forwards and slightly downwards (figures 1a and 2). With Odaraia (Briggs 1981; Briggs et al. 1994), Isoxys is among those Cambrian arthropods with the largest eyes in relation to the body size (average diameter approx. 10% of body length). The eye of Isoxys exemplified by I. acutangulus had a short stalk and an external cornea-like cuticular layer. No hexagonal or square pattern is preserved. Some internal features are preserved, for example a black hemispherical body (figure 3e–h; possibly photoreceptive part of the eye) and a basal area (possibly photosensitive retinular units). The lack of information concerning key elements such as ommatidia, crystalline cones and retinula do not allow an estimation of the optical design and visual properties of the eyes of Isoxys, either apposition (light-adapted) or superposition (dark-adapted) type (e.g. Nilsson 1990). However, the co-occurence of large eyes and powerful prehensile appendages strongly suggest that Isoxys was a visual predator. Its hunting behaviour would have required great efficiency in the visual recognition of small and possibly semitransparent prey, and accurate depth perception. Its large, forwardly projecting spherical ‘panoramic’ eyes may have been an adaptive response to such requirements, especially in targeting moving prey (figure 1d).
Paired, lobate-to-subrectangular features were present along the midgut of Isoxys (figure 2c,d). They are often three-dimensionally preserved (apatite) and belong to the first eight, possibly 10, trunk segments. Identical features occur in numerous Cambrian arthropods such as Leanchoilia, Sidneyia, naraoiids and trilobites, and have exact counterparts in recent crustaceans (e.g. branchiurans, remipedes and copepods) and chelicerates. These features have been convincingly interpreted as serial digestive midgut glands (Butterfield 2002; Vannier & Chen 2002). They are considered as a characteristic feature of the ground pattern of Arthropoda sensu stricto (Waloszek et al. 2005). In recent arthropods, these glands are particularly well developed in non-regular feeders, especially predators (e.g. arachnids), parasites (e.g. branchiurans) and omnivores with predatory habits such as Limulus. The relationship of predation with a well-developed serial glandular system is exemplified by an arthropod predator from the Middle Cambrian of the Kaili Lagerstätte that preserves tiny eodiscoid trilobites within its gut (Zhu et al. 2004). The series of glands of Isoxys would point to comparable feeding strategies, i.e. intermittent feeding by predation and storage of soft food. No gut contents are preserved in our Isoxys specimens.
The frontal appendages of Isoxys bear no fine setae and were neither suited for particle feeding (Harvey & Butterfield 2008) nor for the capture of mesoplankton or microplankton. Instead, their size (approx. 15 mm long), recurved outline and stout spines make them efficient tools for the capture of small soft-bodied or lightly sclerotized invertebrates living in the water column or at/near the water-sediment interface (figure 1d) and possibly ranging between 5 and 20 mm. Comparable specialized frontal appendages with an assumed raptorial function occur in other arthropods from the Chengjiang and Burgess Shale faunas (Maas et al. 2004).
4. Discussion
(a) A new great-appendage arthropod ?
Great-appendage or megacheiran arthropods (Hou & Bergström 1997) encompass a variety of small epibenthic predators that do not exceed 10 cm long to, possibly, much larger anomalocaridids (Chen et al. 2004). They are characterized by a prominent pre-oral great appendage that displays various shapes in relation with mostly prehensile functions (e.g. spiny and claw-like in Haikoucaris ercaiensis, Chen et al. 2004; multi-flagellate in Leanchoilia superlata, Walcott 1912; multi-segmented in Anomalocaris saron, Hou et al. 1995; and Amplectobelua symbrachiata, Hou et al. 1995). The stratigraphic range of the great-appendage arthropods extends into the Devonian as shown by Schinderhannes bartelsi (Kühl et al. 2009), which displays an unusual combination of anomalocaridid and euarthropod characters.
By its location, orientation, size and overall structure (two-segmented peduncle followed by four podomeres bearing pointed outgrowths), the frontal appendage of Isoxys closely resembles the great-appendage of numerous non-anomalocaridid (e.g. Leanchoilia, Alalcomenaeus, Yohoia, Jianfengia; Whittington 1974; Bruton & Whittington 1983; Hou 1987; Briggs & Collins 1999; Chen 2004; Hou et al. 2004; García-Bellido & Collins 2007) and anomalocaridid (e.g. Parapeytoia; Hou et al. 1995) megacheirans. These resemblances and a tentative cladistic analysis based on 29 characters and 14 species (see the electronic supplementary material 2) would support the placement of Isoxys within the great-appendage arthropods and the grouping of Isoxys with other ‘bivalved’ arthropods such as Occacaris and Forfexicaris (Hou et al. 2004). Although the mode of locomotion of these two forms is uncertain, their egg-shaped exoskeleton and tail fan (Occacaris) would suggest a nektobenthic lifestyle. However, the relatively low values of robustness metrics of the tree, added to uncertainties concerning the number of head segments of Isoxys, limit our phylogenetic interpretations. The status of the great-appendage arthropods as a whole remains uncertain. They have been considered basal to the crown-group euarthropods (e.g. Budd 2002). Chen et al. (2004) placed them in the stem-lineage Chelicerata on the basis of presumed homologies between the great-appendage and the chelicera of the crown-group Chelicerata (e.g. Recent spiders, scorpions and horseshoe crabs). A recent cladistic analysis (Kühl et al. 2009) suggests that the group may be paraphyletic. These hypotheses require confirmation.
(b) Isoxys in the Cambrian marine ecosystem
In the Maotianshan Shale, Burgess Shale, Emu Bay Shale (South Australia; García-Bellido et al. in press a) localities and the Buen Formation of Greenland (Williams et al. 1996) Isoxys occurs predominantly as empty shields preserved in lateral or dorsoventral compression (see the electronic supplementary material, figure 1a,b). Although specimens with soft body preservation are rare, they show no important disarticulation (e.g. trunk tergites, appendages, attachment of the head shield to the rest of the body) and often preserve very fragile organs such as digestive glands (figure 2). This excludes a slow post-mortem sinking or drifting in the water column that, in present-day zooplankton, induces rapid physical fragmentation by autolysis, microbial and protozoan activity, turbulence and scavenging (Stemmann et al. 2004). Experiments with Recent shrimps have shown that the slightest disturbance provokes major disarticulation (Allison 1986; Briggs & Kear 1994) as soon as decay commences. A scenario consistent with taphonomy is that the clouds of mud that entombed most of the epibenthic fauna also trapped non-benthic animals such as Isoxys (see Hu (2005) and Caron & Jackson (2006) for Maotianshan and Burgess Shale taphonomy, respectively). Individuals that were relatively close to the bottom at the time of the turbidity event met the conditions for an optimal preservation of their anatomy. By contrast, those positioned higher in the water column, that were not entrained in turbiditic inputs, most probably died, decayed and eventually disarticulated before being buried, allowing the preservation of the most decay-resistant parts only, i.e. the exoskeletal shields. Part of the empty shields found in sediment may actually be shed exoskeletons that in Recent environments constitute one component of the vertical flux of particulate matter (Nicol & Stolp (1989) for Recent krill).
Our hypothesis is that Isoxys could migrate through the water column (e.g. via daily rhythms with alternating ascensions and passive sinking as in recent pelagic ostracods; Angel 1994) and was exploiting hyperbenthic niches for food. Recent hyperbenthic environments (approx. 1–10 m above bottom; Mees & Jones 1997) have a high biomass, and shelter a variety of planktonic and benthic species that migrate into the water column at some stage of their reproductive or daily cycles. Such environments are intensively exploited by pelagic predators (e.g. chaetognaths; Choe & Deibel 2000). Potential hyperbenthic prey for Isoxys are numerous and may have consisted of small adult invertebrates, for example, bradoriids (Hou et al. 1996; Shu et al. 1999b), crustaceans (Zhang et al. 2007), other arthropods such as Ercaia (Chen et al. 2001) or chaetognaths (Vannier et al. 2007b). Part of the diet of Isoxys may have also been composed of the swimming larvae (e.g. Müller & Waloszek 1986; Waloszek & Müller 1989) of arthropods and other groups, that were probably present in the lower levels of the water column (e.g. meroplankton).
The habitat and functional morphological features interpreted for Isoxys would support the idea that off-bottom animal interactions such as predation, associated with complex feeding strategies and behaviours (e.g. vertical migrations and hunting) already existed in the Early Cambrian, leading to trophic links between pelagic and benthic life, possibly via hyperbenthic communities. Possible ecological scenarios are worth briefly mentioning here. A ‘prototype’ of the pelagic food chain is likely to have been established at a relatively early stage of the Cambrian radiation, that probably involved arthropod (e.g. Isoxys, Tuzoia and anomalocaridids; Vannier et al. 2007a) and non-arthropod predators such as ctenophores and chaetognaths (Hu et al. 2007). We see it as a key step that possibly opened the way to a more extensive migration of animal life into the water column and, eventually, to the building-up of more complex, modern-type pelagic ecosystems. By contrast with the present-day situation, it is possible that the pelagic ecospace remained largely uninhabited by animals during the Cambrian period and that non-benthic animal life thrived essentially in the lowermost levels of the water column where sufficient food was available.
Acknowledgements
The authors thank D. Collins, J.-B. Caron and Parks Canada for access to the Burgess Shale collections of the ROM, L. Villier and J. Martin for cladistic analysis and G. Grellet-Tinner, M. Williams and the anonymous reviewers for their constructive criticism. Funding was provided by ANR (to J.V.; ORECO project BLAN06-3-136294), the Spanish Ministry of Education and Science (to D.C.G.-B.; Project CGL 2006-12245BTE), the National Natural Science Foundation of China (No. 40772020) and the National ‘973’ Project of China (grant no. 2006CB806401), both to S.-X. H.
Supplementary Material
Head shield in Isoxys from the Middle Cambrian Burgess Shale, and Recent pelagic lophogastrid crustacean
Cladistic analysis of 14 species of Cambrian ‘great appendage’ (megacheiran) arthropods, including Isoxys
References
- Aldridge R.J., Hou X.-G., Siveter D.J., Siveter D.J., Gabbott S.E. The systematics and phylogenetic relationships of vetulicolians. Palaeontology. 2007;50:131–168. doi:10.1111/j.1475-4983.2006.00606.x [Google Scholar]
- Allison P.A. Soft bodied animals in the fossil record: the role of decay in fragmentation during transport. Geology. 1986;14:979–981. doi:10.1130/0091-7613(1986)14<979:SAITFR>2.0.CO;2 [Google Scholar]
- Angel, M. V. 1994 Marine planktonic ostracods Synopses of the British Fauna (New Series). Shrewsbury, UK: the Linnean Society of London and the Estuarine and Coastal Sciences Association by Field Studies Council.
- Briggs D.E.G. The arthropod Odaraia alata Walcott, Middle Cambrian, Burgess Shale, British Columbia. Phil. Trans. R. Soc. Lond. B. 1981;291:541–585. doi:10.1098/rstb.1981.0007 [Google Scholar]
- Briggs D.E.G., Collins D. The arthropod Alalcomenaeus cambricus Simonetta, from the Middle Cambrian Burgess Shale of British Columbia. Palaeontology. 1999;42:953–977. doi:10.1111/1475-4983.00104 [Google Scholar]
- Briggs D.E.G., Kear A.J. Decay and mineralization of shrimps. Palaios. 1994;9:431–456. doi:10.2307/3515135 [Google Scholar]
- Briggs D.E.G., Erwin D.H., Collier F.J. Smithsonian Institution Press; Washington, DC: 1994. The fossils of the Burgess Shale. [Google Scholar]
- Bruton D.L., Whittington H.B. Emeraldella and Leanchoilia, two arthropods from the Burgess Shale, British Columbia. Phil. Trans. R. Soc. Lond. B. 1983;300:553–585. doi:10.1098/rstb.1983.0020 [Google Scholar]
- Budd G.E. A palaeontological solution to the arthropod head problem. Nature. 2002;417:271–275. doi: 10.1038/417271a. [DOI] [PubMed] [Google Scholar]
- Butterfield N.J. Burgess Shale-type fossils from a Lower Cambrian shallow shelf sequence in northwestern Canada. Nature. 1994;369:477–479. doi:10.1038/369477a0 [Google Scholar]
- Butterfield N.J. Ecology and evolution of Cambrian plankton. In: Zhuravlev A.Y., Riding R., editors. The ecology of the Cambrian radiation. Columbia University Press; New York, NY: 2001. pp. 200–216. [Google Scholar]
- Butterfield N.J. Leanchoilia guts and the interpretation of three-dimensional structures in Burgess Shale-type fossils. Paleobiology. 2002;28:155–171. doi:10.1666/0094-8373(2002)028<0155:LGATIO>2.0.CO;2 [Google Scholar]
- Caron, J.-B. 2005 Taphonomy and community analysis of the Middle Cambrian Greater Phyllopod Bed, Burgess Shale. PhD thesis, University of Toronto, Canada.
- Caron J.-B., Jackson D.A. Taphonomy of the Middle Cambrian, Greater Phyllopod Bed community, Burgess Shale. Palaios. 2006;21:451–465. doi:10.2110/palo.2003.P05-070R [Google Scholar]
- Chen J.-Y. Jiangsu Science and Technology Press; Nanjing, China: 2004. The dawn of animal world. [Google Scholar]
- Chen J.-Y., Huang D.-Y. A possible Lower Cambrian chaetognath (arrow worm) Science. 2002;298:187. doi: 10.1126/science.1075059. doi:10.1126/science.1075059 [DOI] [PubMed] [Google Scholar]
- Chen J.-Y., Vannier J., Huang D.-Y. The origin of crustaceans: new evidence from the Early Cambrian of China. Proc. R. Soc. Lond. B. 2001;268:2181–2187. doi: 10.1098/rspb.2001.1779. doi:10.1098/rspb.2001.1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.-Y., Waloszek D., Maas A. A new ‘great-appendage’ arthropod from the Lower Cambrian of China and homology of chelicerate chelicerae and raptorial antero-ventral appendages. Lethaia. 2004;37:3–20. [Google Scholar]
- Chen J.-Y., Waloszek D., Maas A., Braun A., Huang D.-Y., Wang X.-Q., Stein M. Early Cambrian Yangtze Plate Maotianshan Shale macrofauna biodiversity and the evolution of predation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007;254:250–272. doi:10.1016/j.palaeo.2007.03.018 [Google Scholar]
- Chen L.-Z., Luo H.-L., Hu S.-X., Yin J.-Y., Jiang Z.-W., Wu Z.-L., Li F., Chen A.-L. Yunnan Science and Technology Press; Kunming, China: 2002. Early Cambrian Chengjiang fauna in eastern Yunnan, China. [Google Scholar]
- Choe N., Deibel D. Seasonal vertical distribution and population dynamics of the chaetognath Parasagitta elegans in the water column and hyperbenthic zone of Conception Bay, Newfoundland. Mar. Biol. 2000;137:847–885. doi:10.1007/s002270000413 [Google Scholar]
- Conway Morris S., Collins D.H. Middle Cambrian ctenophores from the Stephen Formation, British Columbia, Canada. Phil. Trans. R. Soc. Lond. B. 1996;351:279–308. doi:10.1098/rstb.1996.0024 [Google Scholar]
- Fox, R. 2006 Thelyphonus, whip scorpion. In Invertebrate anatomy online Greenwood, SC: Lander University. See http://webs.lander.edu/rsfox/invertebrates/thelyphonus.html
- García-Bellido D.C., Collins D.H. Reassessment of the genus Leanchoilia (Arthropoda, Arachnomorpha) from the Middle Cambrian Burgess Shale, British Columbia, Canada. Palaeontology. 2007;50:693–709. doi:10.1111/j.1475-4983.2007.00649.x [Google Scholar]
- García-Bellido, D. C., Paterson, J. R., Edgecombe, G. D., Jago, J. B., Gehling, J. G. & Lee, M. S. Y. In press a The bivalved arthropods Isoxys and Tuzoia with soft-part preservation from the lower Cambrian Emu Bay Shale Lagerstätte (Kangaroo Island, Australia). Palaeontology
- García-Bellido D.C., Vannier J., Collins D. Soft-part preservation in two species of the arthropod Isoxys from the middle Cambrian Burgess Shale of British Columbia, Canada. Acta Palaentologica Polononica. In press [Google Scholar]
- Harvey T.H.P., Butterfield N.J. Sophisticated particle-feeding in a large Early Cambrian crustacean. Nature. 2008;452:868–871. doi: 10.1038/nature06724. doi:10.1038/nature06724 [DOI] [PubMed] [Google Scholar]
- Hou X.-G. Two new arthropods from the Lower Cambrian, Chengjiang, eastern Yunnan. Acta Palaeontol. Sin. 1987;26:236–256. [Google Scholar]
- Hou X.-G., Bergström J. Arthropods of the Lower Cambrian Chengjiang fauna, southwest China. Fossils Strata. 1997;45:1–116. [Google Scholar]
- Hou X.-G., Bergström J., Ahlberg P. Anomalocaris and other large animals in the Lower Cambrian Chengjiang founa of southwest China. Geologiska Föreningens i Stockholm Förhandlinger. 1995;117:163–183. [Google Scholar]
- Hou X.-G., Siveter D.J., Williams M., Waloszek D., Bergström J. Appendages of the arthropod Kunmingella from the early Cambrian of China: its bearing on the systematic position of the Bradoriida and the fossil record of the Ostracoda. Phil. Trans. R. Soc. Lond. B. 1996;351:1131–1145. doi:10.1098/rstb.1996.0098 [Google Scholar]
- Hou X.-G., Aldridge R.J., Bergström J., Siveter D.J., Feng X.-H. Blackwell; Oxford, UK: 2004. The Cambrian fossils of Chengjiang, China. [Google Scholar]
- Hu S.-X. Taphonomy and palaeoecology of the Early Cambrian Chengjiang biota from eastern Yunnan, China. Berliner Paläobiologische Abhandlungen. 2005;7:1–197. [Google Scholar]
- Hu S.-X., Steiner M., Zhu M.-Y., Erdtmann B.-D., Luo H.-L., Chen L.-Z., Weber B. Diverse pelagic predators from the Chengjiang Lagerstätte and the establishment of modern-style pelagic ecosystems in the early Cambrian. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007;254:307–316. doi:10.1016/j.palaeo.2007.03.044 [Google Scholar]
- Kühl G., Briggs D.E.G., Rust J. A great-appendage arthropod with a radial mouth from the Lower Devonian Hunsrück Slate, Germany. Nature. 2009;323:771–773. doi: 10.1126/science.1166586. [DOI] [PubMed] [Google Scholar]
- Luo H.-L., Jiang Z.-W., Wu X.-C., Song X.-L., Ouyang L. People's Publishing House of Yunnan; Kunming, China: 1982. The Sinian–Cambrian boundary in eastern Yunnan, China. [Google Scholar]
- Maas A., Waloszek D., Müller K. Morphology, ontogeny and phylogeny of the Phosphatocopina (Crustacea) from the Upper Cambrian ‘Orsten’ of Sweden. Fossils Strata. 2003;49:1–238. [Google Scholar]
- Maas, A., Waloszek, D., Chen, J.-Y., Braun, A., Wang, X.-Q. & Huang, D.-Y. 2004 Phylogeny and life habits of early arthropods: predation in the Early Cambrian Sea. Prog. Nat. Sci. (Special Issue 2004) 14, 158–166. (doi:10.1080/10020070412331343301)
- Mees J., Jones M.B. The hyperbenthos. Oceanogr. Mar. Biol. Annu. Rev. 1997;35:221–255. [Google Scholar]
- Morgan S.G. Adaptive significance of spination in estuarine crab zoeae. Ecology. 1989;70:462–482. doi:10.2307/1937551 [Google Scholar]
- Müller K.J., Waloszek D. Arthropod larvae from the Upper Cambrian of Sweden. Trans. R. Soc. Edinb. Earth Sci. 1986;77:157–179. [Google Scholar]
- Nicol S., Stolp M. Sinking rates of cast exoskeletons of Antarctic krill (Euphausia superba Dana) and their role in the vertical flux of particulate matter and fluoride in the Southern Ocean. Deep Sea Res. A. Oceanogr. Res. Papers. 1989;36:1753–1762. doi:10.1016/0198-0149(89)90070-8 [Google Scholar]
- Nilsson D.E. From cornea to retinal image in invertebrate eyes. Trends Neurosci. 1990;13:55–64. doi: 10.1016/0166-2236(90)90069-m. doi:10.1016/0166-2236(90)90069-M [DOI] [PubMed] [Google Scholar]
- Rigby S., Milsom C. Benthic origins of zooplankton: an environmentally determined macroevolutionary effect. Geology. 1996;24:52–54. doi:10.1130/0091-7613(1996)024<0052:BOOZAE>2.3.CO;2 [Google Scholar]
- Shergold J.H., Cooper R.A. The Cambrian period. In: Gradstein F.M., Ogg J.G., Smith A.G., editors. A geological time scale 2004. Cambridge University Press; Cambridge, UK: 2004. pp. 147–164. [Google Scholar]
- Shu D.-G., et al. Lower Cambrian vertebrates. Nature. 1999a;402:42–46. doi:10.1038/46965 [Google Scholar]
- Shu D.-G., Vannier J., Huo H.-L., Chen L., Zhang X.-L., Hu S.-X. Anatomy and lifestyle of Kunmingella (Arthropoda, Bradoriida) from the Chengjiang fossil Lagerstätte (Lower Cambrian; southwest China) Lethaia. 1999b;32:279–298. [Google Scholar]
- Shu D.-G., et al. Head and backbone of the Early Cambrian vertebrate Haikouichthys. Nature. 2003;421:526–529. doi: 10.1038/nature01264. doi:10.1038/nature01264 [DOI] [PubMed] [Google Scholar]
- Steiner M., Zhu M.-Y., Zhao Y.-L., Erdtmann B.-D. Lower Cambrian Burgess Shale-type fossil associations of South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005;220:129–152. doi:10.1016/j.palaeo.2003.06.001 [Google Scholar]
- Stemmann L., Jackson G.A., Ianson D. A vertical model of particle size distribution and fluxes in the midwater column that includes biological and physical processes. Part I: model formulation. Deep-Sea Res. I. 2004;51:865–884. doi:10.1016/j.dsr.2004.03.001 [Google Scholar]
- Swofford, D. L. 2002 PAUP*: phylogenetic analysis using parsimony (*and other methods), v. 4.0.b2a. Sunderland MA: Sinauer Associates.
- Tetlie O.E., Dunlop J.A. Geralinura carbonaria (Arachnida; Uropygi) from Mazon Creek, Illinois, USA, and the origin of subchelate pedipalps in whip scorpions. J. Paleontol. 2008;82:299–312. doi:10.1666/06-138.1 [Google Scholar]
- Vannier J. Early Cambrian origin of complex marine ecosystems. In: Williams M., Haywood A., Gregory F.J., Schmidt D.N., editors. Deep time perspectives on climate change. The Micropalaeontological Society and the Geological Society Publishing House; London, UK: 2007. pp. 81–100. [Google Scholar]
- Vannier J., Chen J.-Y. The Early Cambrian colonization of pelagic niches exemplified by Isoxys (Arthropoda) Lethaia. 2000;33:295–311. doi:10.1080/002411600750053862 [Google Scholar]
- Vannier J., Chen J.-Y. Digestive system and feeding mode in Cambrian naraoiid arthropods. Lethaia. 2002;35:107–120. doi:10.1080/002411602320183971 [Google Scholar]
- Vannier J., Chen J.-Y. Early Cambrian food chain: new evidence from fossil aggregates in the Maotianshan Shale biota, SW China. Palaios. 2005;20:3–26. doi:10.2110/palo.2003.p03-40 [Google Scholar]
- Vannier J., Caron J.-B., Yuan J.-L., Briggs D.E.G., Collins D., Zhao Y.-L., Zhu M.-Y. Tuzoia: morphology and lifestyle of a large bivalved arthropod of the Cambrian seas. J. Paleontol. 2007a;81:445–471. doi:10.1666/pleo05070.1 [Google Scholar]
- Vannier J., Steiner M., Renvoisé E., Hu S., Casanova J.-P. Early Cambrian origin of modern food webs: evidence from predator arrow worms. Proc. R. Soc. B. 2007b;274:627–633. doi: 10.1098/rspb.2006.3761. doi:10.1098/rspb.2006.3761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcott C.D. Cambrian geology and paleontology II, no. 6. Middle Cambrian Branchiopoda, Malacostraca, Trilobita, and Merostomata. Smithson. Miscellaneous Collect. 1912;57:145–227. [Google Scholar]
- Walossek D. The Upper Cambrian Rehbachiella and the phylogeny of Branchiopoda and Crustacea. Fossils Strata. 1993;32:1–202. [Google Scholar]
- Waloszek D., Müller K.J. A second type A-nauplius from the Upper Cambrian ‘Orsten’ of Sweden. Lethaia. 1989;22:301–306. doi:10.1111/j.1502-3931.1989.tb01345.x [Google Scholar]
- Waloszek D., Chen J.-Y., Maas A., Wang X.-Q. Early Cambrian arthropods: new insights into arthropod head and structural evolution. Arthropod Struct. Dev. 2005;34:189–205. doi:10.1016/j.asd.2005.01.005 [Google Scholar]
- Whittington H.B. Yohoia Walcott and Plenocaris n. gen., arthropods from the Burgess Shale, British Columbia. Geol. Survey Canada Bull. 1974;231:1–21. [Google Scholar]
- Williams M., Siveter D.J., Peel J.S. Isoxys (Arthropoda) from the Early Cambrian Sirius Passet Lagerstätte, North Greenland. J. Paleontol. 1996;70:947–954. [Google Scholar]
- Zhang X.-G., Siveter D.J., Waloszek D., Maas A. An epipodite-bearing crown-group crustacean from the Lower Cambrian. Nature. 2007;449:595–598. doi: 10.1038/nature06138. doi:10.1038/nature06138 [DOI] [PubMed] [Google Scholar]
- Zhu M.-Y., Zhao Y.-L., Chen J.-Y. Revision of the Cambrian discoidal animals Stellostomites eumorphus and Pararotadiscus guizhouensis from South China. Geobios. 2002;35:165–185. doi:10.1016/S0016-6995(02)00025-6 [Google Scholar]
- Zhu M.-Y., Vannier J., Van Iten H., Zhao Y.-L. Direct evidence for predation on trilobites in the Cambrian. Proc. R. Soc. Lond. B. 2004;271:277–280. doi: 10.1098/rsbl.2004.0194. doi:10.1098/rsbl.2004.0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Head shield in Isoxys from the Middle Cambrian Burgess Shale, and Recent pelagic lophogastrid crustacean
Cladistic analysis of 14 species of Cambrian ‘great appendage’ (megacheiran) arthropods, including Isoxys