Abstract
Background
The DExD/H domain containing RNA helicases such as retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5) are key cytosolic pattern recognition receptors (PRRs) for detecting nucleotide pathogen associated molecular patterns (PAMPs) of invading viruses. The RIG-I and MDA5 proteins differentially recognise conserved PAMPs in double stranded or single stranded viral RNA molecules, leading to activation of the interferon system in vertebrates. They share three core protein domains including a RNA helicase domain near the C terminus (HELICc), one or more caspase activation and recruitment domains (CARDs) and an ATP dependent DExD/H domain. The RIG-I/MDA5 directed interferon response is negatively regulated by laboratory of genetics and physiology 2 (LGP2) and is believed to be controlled by the mitochondria antiviral signalling protein (MAVS), a CARD containing protein associated with mitochondria.
Results
The DExD/H containing RNA helicases including RIG-I, MDA5 and LGP2 were analysed in silico in a wide spectrum of invertebrate and vertebrate genomes. The gene synteny of MDA5 and LGP2 is well conserved among vertebrates whilst conservation of the gene synteny of RIG-I is less apparent. Invertebrate homologues had a closer phylogenetic relationship with the vertebrate RIG-Is than the MDA5/LGP2 molecules, suggesting the RIG-I homologues may have emerged earlier in evolution, possibly prior to the appearance of vertebrates. Our data suggest that the RIG-I like helicases possibly originated from three distinct genes coding for the core domains including the HELICc, CARD and ATP dependent DExD/H domains through gene fusion and gene/domain duplication. Furthermore, presence of domains similar to a prokaryotic DNA restriction enzyme III domain (Res III), and a zinc finger domain of transcription factor (TF) IIS have been detected by bioinformatic analysis.
Conclusion
The RIG-I/MDA5 viral surveillance system is conserved in vertebrates. The RIG-I like helicase family appears to have evolved from a common ancestor that originated from genes encoding different core functional domains. Diversification of core functional domains might be fundamental to their functional divergence in terms of recognition of different viral PAMPs.
Background
Pattern recognition receptors (PRRs) are crucial to animal surveillance of pathogen invasion. The PRRs recognise conserved pathogen-associated molecular pattern (PAMP) motifs, including proteins, lipids and nucleotides, resulting in activation of host innate defences [1]. The PRRs comprise three major groups, toll like receptors (TLR), retinoic acid induced RIG-I like receptors and nucleotide oligomerization domain (NOD) containing proteins, sensing PAMPs extracellularly or within the cytoplasmic region.
The RIG-I like receptors are crucial to the RNA virus triggered interferon response. They consist of three members, retinoic acid-inducible gene I (RIG-I, also named DEAD (Asp-Glu-Ala-Asp) box polypeptide 58 (DDX58)) and melanoma differentiation-associated gene 5 (MDA5, also named interferon induced with helicase C domain 1 (IFIH1)), and laboratory of genetics and physiology 2 (LGP2, also named DExH (Asp-Glu-X-His) box polypeptide 58 (DHX58)), which share a common functional RNA helicase domain near the C terminus (HELICc) specifically binding to the RNA molecules with viral origin [2-4]. Two tandem arranged caspase activation and recruitment domains (CARDs) involved in protein-protein interactions are present at the N terminal region of the RIG-I and MDA5 proteins but not LGP2, triggering the interferon response via activation of interferon regulatory factor 3 and NFkB [3,5]. Another distinct core domain is the ATP dependent DExD/H domain containing a conserved motif Asp-Glu-X-Asp/His (DExD/H) which is involved in ATP-dependent RNA or DNA unwinding. RIG-I/MDA5 directed interferon signalling is now known to be controlled by the mitochondria antiviral signalling protein (MAVS), a CARD containing protein associated with mitochondria, and negatively regulated by LGP2 which lacks a CARD domain [4,6,7]. LGP2 has been shown to interfere with the binding process of RIG-I/MDA5 to viral RNAs [8].
Both RIG-I and MDA5 appear to have overlapping binding properties with viral PAMPs and share similar signalling pathways leading to activation of the interferon system. However, evidence of differential recognition of viral PAMPs by RIG-I has begun to emerge recently. It seems that MDA5 preferentially binds long, capped di- or mono-5' phosphate double stranded (ds) RNAs whilst RIG-I has high binding affinity with short dsRNAs or 5' ppp uncapped single stranded (ss) RNAs [9-11]. Interestingly, neither RIG-I nor MDA5 has a classic RNA binding motif. A zinc-binding domain located at the C terminal region (802–925 aa) of human has been shown to specifically bind to viral derived 5'ppp RNA [12,13]. RIG-I and MDA5 respond differently to infection with various viral strains, with RIG-I sensitive to paramyxoviruses, orthomyxoviruses, and the rhabdovirus vesicular stomatitis virus whilst MDA5 reacts to picornaviruses [11,14]. Some viral proteins, such as the V protein of paramyxoviruses, interact with MDA5, a mechanism possibly used by viruses as a means to escape host surveillance.
Whilst most studies are focused on the RIG-I like PRRs in mammals, little is known about such molecules in other living organisms. A recent study surveying the purple sea urchin genome has revealed multiple putative RIG-I like homologues that appear to be present in invertebrates [15]. More recently, it has been hypothesised that MDA5 might have emerged before RIG-I and their domain arrangement evolved independently by domain grafting rather than by a simple gene duplication event [16]. In this study, we took a comparative genomics approach by analysing RIG-I like PRRs in a number of invertebrate and vertebrate genomes, in order to elucidate the origin and evolution of the RIG-I like PRR family. Bioinformatic analysis of functional domains of RIG-I, MDA5 and LGP2 has identified two evolutionary conserved domains in MDA5 and LGP2 which may be critical to the recognition and processing of viral nucleotide PAMPs.
Results
Sequence identification
Extensive BLAST analysis of vertebrate genomes or expressed sequence tag databases using known RIG-I protein sequences identified a putative full length RIG-I homologue in Western Clawed Xenopus but not in chicken (Table 1). The putative Xenopus RIG-I is 945 aa in length, sharing 43.6% identity with human RIG-I and contains conserved domains such as a DExD/H domain in the middle region and a helicase domain at the C terminal region. A less homologous CARD domain at the N terminus is also apparent. In the zebrafish genome, a single RIG-I like gene encoding a protein of 628 aa was found in chromosome 23 (Ensemble prediction ID No., ENSDARG00000039785), some 300 aa shorter than the mammalian and amphibian RIG-I proteins. Although it contains a DExD/H domain and a CARD domain at the N terminus, it lacks a classic helicase c (HELICc) domain. It is uncertain whether zebrafish RIG-I is produced as a functional protein since no EST matching the predicted RIG-I exists. In Japanese pufferfish, tetraodon, medaka and stickleback, no RIG-I homologues were identified either in the EST databases or genome databases.
Table 1.
Sequence information of homologues of RIG-I, MDA5, LGP2, DICER and eIF4A in vertebrates and invertebrates
| Gene name | ENSEMBL prediction ID | GenBank GI EST |
Protein length | Identity/similarity to human homologue |
| RIG-I | ||||
| Human | ENSG00000107201 | NM_014314 | 925 | 100/100 |
| Xenopus | ENSXETG00000009200 | 945 | 43.6/64.2 | |
| Zebrafish | ENSDARG00000039785 | 628 | 25.9/41.3 | |
| MDA5 | ||||
| Human | ENSG00000115267 | NM_022168 | 1025 | 100/100 |
| Chicken | ENSGALG00000011089 | XM_422031 | 1285 | 47.8/58.8 |
| Xenopus | ENSXETG00000013176 | 1003 | 53.3/69.2 | |
| Zebrafish | ENSDARG00000018553 | XM_689032 | 1219 | 34.5/52.4 |
| Stickleback | ENSGACG00000005518 | 1008 | 47.1/64.3 | |
| Medaka | ENSORLG00000016902 | 987 | 47/65.9 | |
| Fugu | ENSTRUG00000001413 | 1000 | 44.7/63 | |
| Tetraodon | ENSTNIG00000016500 | 1038 | 42.6/63 | |
| LGP2 | ||||
| Human | ENSG00000108771 | NM_024119 | 678 | 100/100 |
| Chicken | ENSGALG00000023821 | 588 | 43.6/59.1 | |
| Xenopus | ENSXETG00000002302 | 588 | 46.8/61.8 | |
| Zebrafish | ENSDARG00000070935 | XM_001920601 | 682 | 40.8/62.5 |
| Stickleback | ENSGACG00000008740 | 681 | 47.2/68.9 | |
| Medaka | ENSORLG00000003825 | 673 | 47.4/66.1 | |
| Fugu | ENSTRUG00000015710 | 671 | 46.2/64.7 | |
| Tetraodon | ENSTNIG00000011713 | 680 | 47.7/65.9 | |
| DICER | ||||
| Human | ENSG00000100697 | NM_177438 | 1922 | 100/100 |
| Chicken | ENSGALG00000010999 | NM_001040465 | 1921 | 92.0/96.0 |
| Xenopus | ENSXETG00000023315 | 422 | 18.9/20.6 | |
| Zebrafish | ENSDARG00000001129 | XM_678382 | 1975 | 75.5/84.4 |
| Stickleback | ENSGACG00000020134 | 1901 | 75.9/84.5 | |
| Medaka | ENSORLG00000011022 | 1905 | 76.5/85.2 | |
| Fugu | ENSTRUG00000006156 | 1896 | 75.8/84.4 | |
| Tetraodon | ENSTNIG00000005441 | 1915 | 75.8/85.0 | |
| Sea urchin DICER | XM_785801 | 1850 | 41.0/60.1 | |
| Caenorhabditis elegans DRH1 | F15B10.2 | NM_068617 | 1037 | 14.2/27.5 |
| Caenorhabditis elegans DRH2 | C01B10.1 | 956 | 14.4/26.1 | |
| Caenorhabditis elegans DRH3 | D2005.5 | NM_059760 | 1119 | 15.8/28.7 |
| Caenorhabditis elegans DCR1 | K12H4.8 | NM_066360 | 1845 | 33.7/53.3 |
| Drosophila DCR1 | NM_079729 | 2249 | 32.0/50.1 | |
| Jewel wasp DICER1 | XM_001605237 | 1917 | 37.3/59.5 | |
| eIF4A | ||||
| Human eIF4A1 | ENSG00000161960 | BT019879 | 407 | 100/100 |
| Chicken eIF4A2 | NM_204549 | 407 | 90.4/96.3 | |
| Xenopus eIF4A | ENSXETG00000020123 | NM_001011139 | 406 | 94.8/98.5 |
| Zebrafish eIF4A1a | ENSDARG00000040268 | NM_198366 | 406 | 89.4/96.8 |
| Zebrafish eIF4A1b | ENSDARG00000003032 | NM_201510 | 406 | 89.2/97.1 |
| Stickleback eIF4A1 | ENSGACG00000020072 | 406 | 89.7/97.3 | |
| Medaka eIF4A1 | ENSORLG00000010659 | 405 | 89.7/97.3 | |
| Fugu eIF4A1 | ENSTRUG00000002188 | 406 | 78.9/90.4 | |
| ENSTRUG00000017170 | 408 | 63.9/81.6 | ||
| ENSTRUG00000011113 | 411 | 62.6/80.5 | ||
| Tetraodon eIF4A1 | ENSTNIG00000004408 | 292 | 55.6/64.9 | |
| ENSTNIG00000017079 | 352 | 60.7/73.5 | ||
| ENSTNIG00000006865 | 384 | 68.1/84.3 | ||
| ENSTNIG00000007405 | 411 | 69.2/87.1 | ||
| ENSTNIG00000004366 | 316 | 55.4/66.8 | ||
| ENSTNIG00000000303 | 339 | 47.7/64.6 | ||
| Drosophila eIF4A | FBgn0001942 | NM_164668 | 403 | 72.1/86.7 |
| NM_164669 | ||||
| NM_057247 | ||||
| NM_164670 | ||||
| Sclerotinia sclerotiorum eIF4A | XM_001594601 | 398 | 74.7/86.5 | |
| Botryotinia fuckeliana eIF4A | XM_001561331 | 398 | 74.4/86.5 | |
| Invertebrate RIG-I like genes | ||||
| Sea urchin RIG-I (LOC591972) | NW_001470282 | XM_791516 | 960 | 26.4/47.8 |
| Sea urchin RIG-I (LOC575036) | NW_001312424 | XM_001176480 | 823 | 23.8/43.2 |
| Sea urchin RIG-I (LOC767124) | NW_001297703 | XM_001203626 | 303 | 11.8/19.2 |
| Sea urchin RIG-I (LOC574972) | NW_001312424 | XM_775381 | 968 | 29.3/50.3 |
| Sea urchin RNA helicase (LOC593153) | XM_792644 | 996 | 21.4/41.0 | |
| Sea urchin RNA helicase (LOC583008) | XM_001198571 | 480 | 16.1/26.4 | |
| Sea urchin RIG-I | XM_778463 | 927 | 28.7/49.7 | |
| Sea urchin RNA helicase (LOC578749) | XM_778903 | 870 | 27.2/48.6 | |
| Sea urchin RIG-I (LOC582062) | XM_782035 | 209 | 10.7/14.5 | |
| Sea urchin RIG-I (LOC577076) | XM_777329 | 1051 | 19.9/39.7 | |
| Nematostella vectensis RIG-I/MDA5 like gene 1 |
XM_001636292 XP_001636342 |
672 | 26.3/41.0 | |
| Nematostella vectensis RIG-I/MDA5 like gene 2 |
XM_001639190 XP_001639240 |
689 | 25.5/41.7 |
Unlike RIG-I, whose presence in chicken and some fish species is uncertain, the MDA5 homologues can be found throughout vertebrate species including fish, amphibians, birds and mammals (Table 1). MDA5 is encoded by a single copy gene and the putative proteins have comparable length, ranging from 987 aa to 1285 aa (Table 1). In addition to the conserved DExD/H domain and HELICc domain, two tandem CARD domains at the N terminal regions are predicted and are well conserved among vertebrate MDA5 molecules except for the zebrafish MDA5 that lacks a clear CARD domain. The CARD motifs near the N-terminus (referred to as the first CARD) are more diverse than the second CARD motif (Table 2).
Table 2.
Key structural domains predicted in the Pfam database.
| GenBank Accession number |
CARD1 | CARD2 | Res III | DExD/H | HELICc | TFIIS-C | |
| RIG-I | |||||||
| Human | O95786 | 1–87 | 104–191 (0.69) |
244–420 (7.1e-14) |
655–734 (9.2e-15) |
||
| Xenopus | ENSXETP00000020202 | 103–189 (0.43) |
303–434 (1.6e-10) |
723–785 (1.3e-13) |
|||
| Zebrafish | ENSDARP00000058175 (partial) |
103–186 (3.7) |
246–268 (0.019) |
||||
| MDA5 | |||||||
| Human | Q9BYX4 | 7–97 | 115–200 (2.4e-18) |
305–493 (3.2e-22) |
743–826 (1.1e-20) |
||
| Chicken | XP_422031 | 37–90 (15) |
112–198 (7.8e-06) |
298–485 (4.8e-20) |
717–800 (5.6e-21) |
936–944 (0.51) |
|
| Xenopus | ENSXETP00000028841 | 12–97 (0.073) |
142–198 (0.002) |
299–487 (7.7e-20) |
719–802 (1.1e-21) |
938–946 (8.6) |
|
| Fugu | ENSTRUP00000003254 | 10–96 (0.072) |
110–196 (3.2e-05) |
300–488 (6.4e-15) |
711–794 (1.6e-19) |
930–939 (1.1) |
|
| LGP2 | |||||||
| Human | NP_077024 | 4–178 (1.3e-13) |
392–475 (3.3e-21) |
||||
| Chicken | ENSGALP00000005315 | 1–116 (4.3e-05) |
521–532 (3.7) |
||||
| Xenopus | ENSXETP00000004920 | 1–173 (1.5e-17) |
395–478 (7.8e-24) |
||||
| Fugu | ENSTRUP00000040150 | 3–172 (8.5e-19) |
396–477 (9.3e-20) |
||||
| DICER | |||||||
| Human | NP_803187 | 45–207 (1.9e-08) |
499–556 (7.7e-17) |
||||
| Chicken | NP_001035555 | 45–207 (5.1e-10) |
499–556 (7.7e-17) |
||||
| Xenopus | ENSXETP00000050382 (partial) |
35–209 (6.9e-06) |
|||||
| Fugu | ENSTRUP00000014961 | 46–217 (7.3e-06) |
499–556 (7.7e-17) |
||||
| eIF4A | |||||||
| Human | AAV38682 | 56–223 (2.5e-63) |
291–367 (1.3e-35) |
||||
| Chicken | NP_989880 | 57–224 (6.7e-62) |
292–368 (6.5e-36) |
||||
| Xenopus | NP_001085314 | 56–223 (1.1e-62) |
291–367 (6.5e-36) |
||||
| Fugu | ENSTRUP00000005018 | 56–223 (5.7e-59) |
291–367 (3.6e-36) |
Note: The amino acid position of each domain is listed and the E values against the Pfam database are indicated in parentheses.
LGP2 is an adaptor protein lacking CARD domains but containing a DExD/H domain and a HELICc domain homologous to their corresponding motifs in the RIG-I and MDA5 protein. It competes with RIG-I and MDA5 for the ligands, viral derived RNA PAMPs, but is unable to interact with down stream signalling proteins due to the absence of CARD domains. Thus it acts as a negative regulator of the RIG-I/MDA5 directed antiviral response. LGP2 appears to co-exist with MDA5 in vertebrates as a single copy gene. It is located in a different chromosome to MDA5 in every species analysed. The putative LGP2 proteins from non-mammalian species contain 588–682 aa, much shorter than the RIG-I and MDA5 proteins. The DExD/H domain and the HELICc domain in the LGP2 protein share higher homology with the corresponding regions in MDA5 than those in RIG-I. The LGP2 DExD/H domains are 33.4–55.3% identical to the MDA5 counterparts compared to 22.3–39.8% for the RIG-I proteins. Similarly, 47.6–66.7% identity is seen between the LGP2 HELICc domains and the MDA5 HELICc domains, in contrast to 31.7–48.7% between LGP2 HELICc domains and the RIG-I helicase domains.
Twelve genes coding for RNA helicase proteins homologous to RIG-I/MDA5/LGP2 have been reported in a recent survey of the sea urchin genome draft [15]. Some of the deduced proteins contain CARD domains in addition to DExD/H and HELICc domains. Using the human MDA5 protein sequence as a bait, a partial homologue sequence was obtained from the sea anemone Nematostella vectensis genome database http://www.stellabase.org/, http://blast.ncbi.nlm.nih.gov/. This partial sequence was then used to search the NCBI database and two contigs (NEMVEDRAFT_v1g95706 and NEMVEDRAFT_v1g87071) were retrieved, which encoded two putative RIG-I/MDA5/LGP2 homologues. The putative proteins are 672 aa and 689 aa in length, similar to that of LGP2. Further prediction of functional motifs revealed the presence of a DExD/H domain and a HELICc domain but not the N terminal CARD domain. The proteins share 17.4–26.3 identity with RIG-I, 21.7–32.4% with MDA5 and 25.3–36.3% with LGP2.
Gene synteny analysis
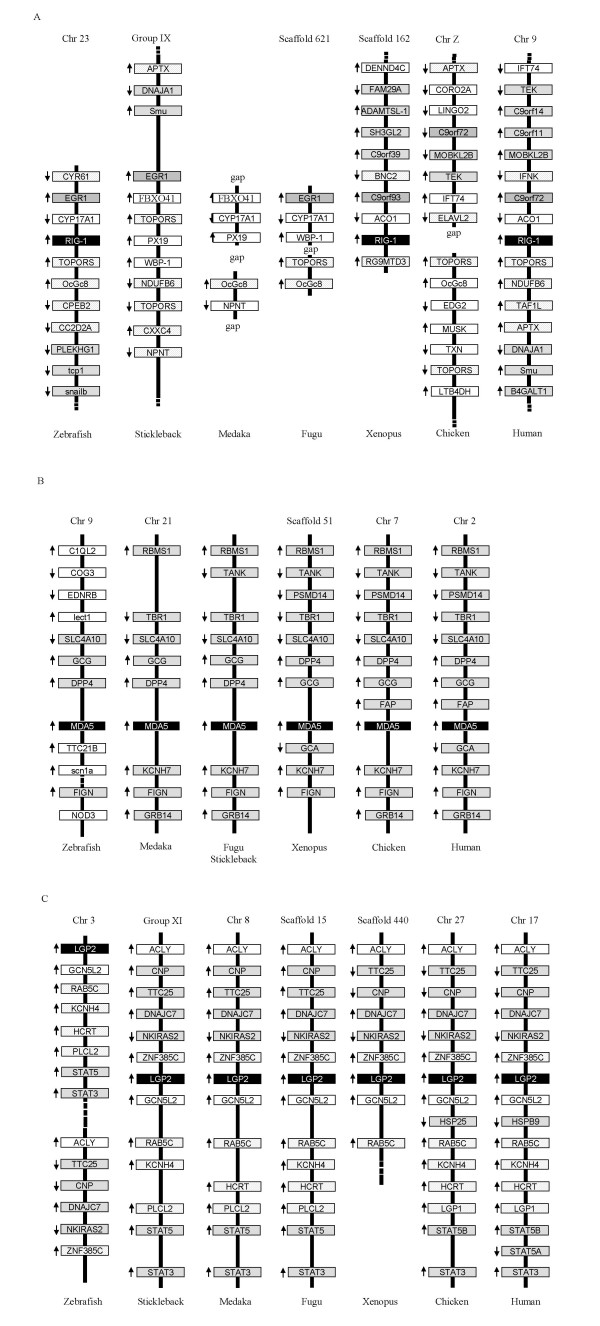
To gain an insight into whether the genes surrounding RIG-I, MDA5 and LGP2 are evolutionary conserved, we analysed draft genome sequences of invertebrates, fish, amphibians, birds and humans. The RIG-I locus was identifiable in Xenopus where the genes neighbouring RIG-I were different to those in humans except for the ACO1 gene (Fig. 1A). Since the upstream region of the RIG-I locus was not available in the Xenopus genome database, whether RIG-I clustered with TOPORS remains to be determined. In zebrafish, the RIG-I gene was immediately downstream of the TOPORS gene, which is adjacent to the RIG-I gene in humans. In contrast to the observation in zebrafish, the stickleback genome appears to lack RIG-I, which is not due to poor genome analysis since the sequence of the RIG-I locus is of good quality. In Fugu, medaka and chicken, the RIG-I gene could not be identified but was hindered by incomplete sequence data.
Figure 1.
Comparative analysis of gene synteny of RIG-I (A), MDA5 (B) and LGP2 (C) in vertebrate genomes. The gene symbols are used according to the human genome map (Build 36.3, http://www.ncbi.nlm.nih.gov/projects/mapview).
The gene synteny of MDA5/LGP2 is well conserved in vertebrates, from fish to humans (Fig. 1B and 1C). Eight genes surrounding MDA5 in stickleback, Fugu and medaka appear in the genomes of Xenopus, chicken and humans, in the same order and the same transcriptional orientation. Less conservation of gene synteny was noted in the zebrafish genome where only 4 conserved neighbouring genes were present in the MDA5 locus. Similarly, the gene composition and arrangement in the LGP2 gene locus shows remarkable conservation during vertebrate evolution.
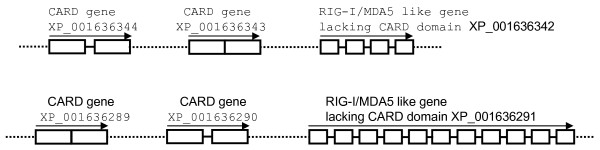
In invertebrates, two loci containing prototype homologues of RIG-I/MDA5 were found downstream of two independent genes coding for two CARD like molecules in Nematostella vectensis (Fig. 2). Some 12 RIG-I/MDA like genes have also been predicted from the purple sea urchin genome [15]. Whilst it was possible to find appropriate contigs containing RIG-I/MDA5 like genes from the sea urchin genome project, it was not possible to assess gene synteny due to a lack of genome assembly.
Figure 2.
Genomic location of predicted RIG-I/MDA5 homologues in sea anemone Nematostella vectensis. Exons and gene transcriptional orientation are indicated by blank boxes and arrows respectively. The accession numbers of predicted genes are given.
Phylogenetic analysis
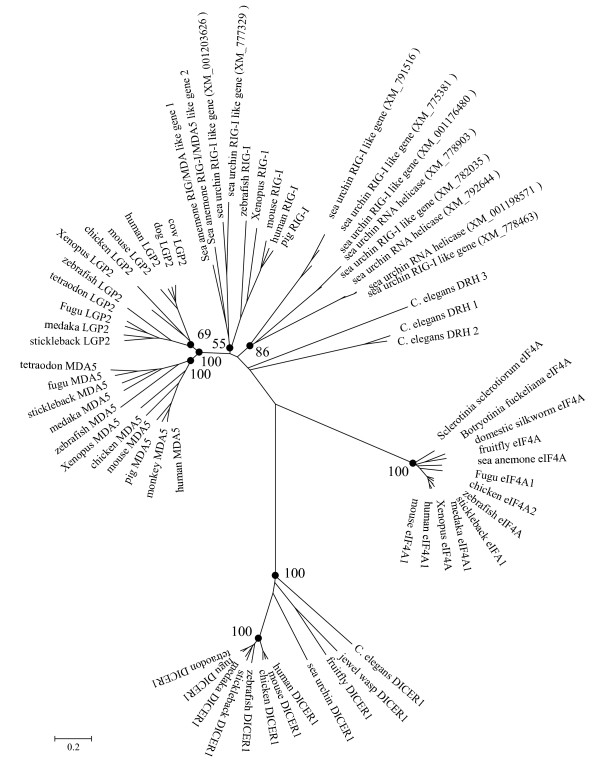
RIG-I, MDA5 and LGP2 are believed to bind RNA molecules through helicase-C domains. Closely related proteins containing a RNA binding helicase-C domain and a DExD/H helicase domain are the double-stranded RNA-specific endoribonuclease (DICER) and elongation initiation factor (eIF) 4A. DICER belongs to the RNase III family that cleaves double-stranded RNA (dsRNA) into short double-stranded RNA fragments, called small interfering RNA, that is required for specific cleavage of complementary viral RNAs, whilst eIF4A mainly participates in translation and other processes such as pre-mRNA splicing and ribosome biogenesis. Since homology analysis of the RIG-I and MDA5 proteins in the databases using the BLASTP programme gave top scores to DICER and eIF4A in addition to the RIG-I, MDA5 and LGP2 proteins, we included DICER and eIF4A in the phylogenetic tree analysis using the neighbour joining method within the Mega4 programme. As shown in Fig. 3, DICER and eIF4A formed two distinct groups with a long evolutionary distance to the branch that evolved into invertebrate DICER like helicase (DRH), RIG-I, MDA5 and LGP2. Apparently, DICERs are more distantly related to the ancestor of RIG-I, MDA5 and LGP2 than eIF4A. Comparing to DICERs and eIF4As, C. elegans DRHs were located close to some of the sea urchin RIG-I/MDA like proteins, the prototype of the vertebrate counterparts. Within the RIG-I branch are two of the RIG-I like homologues from Nematostella vectensis, two from the sea urchin (LOC767124 and LOC577076), and predicted or known RIG-I molecules from zebrafish, Xenopus, and mammals, suggesting RIG-I may have emerged in invertebrates. The fact that RIG-I homologues were absent in most fish species suggests that the RIG-I gene may have been lost in some fish lineages. The tree also indicates that LGP2 and MDA5 proteins from vertebrate species form two clear groups closely neighbouring each other, suggesting they possibly diverged from a common ancestor that originated from a RIG-I like molecule in invertebrates or early vertebrates. In addition, two phylogenetic trees were constructed using helicase C domains and DExD/H domains and a similar grouping of the major branches was observed (data not shown).
Figure 3.
Phylogenetic tree analysis of DExD/H box RNA helicases. Multiple alignment of the full length protein sequences of known or predicted RIG-I, MDA5, LGP2, eIF4A and DICER was generated by CLUSTALW http:///www.ebi.ac.uk and used for construction of a phylogenetic tree using the neighbour-joining method within the Mega3.1 programme. The bootstrap values of the branches were obtained by testing the tree 10,000 times and values over 50% percent marked. The sequences used for tree construction are listed in Table 1. Others are as follows: monkey MDA5, NP_001040588; mouse MDA5, EDL26991; pig_MDA5, NP_001093664; mouse LGP2, NP_084426; cow LGP2, NP_001015545; dog LGP2, XP_860567; mouse RIG-I, Q6Q899; pig RIG-I, Q9GLV6; mouse DICER1, EDL18787.
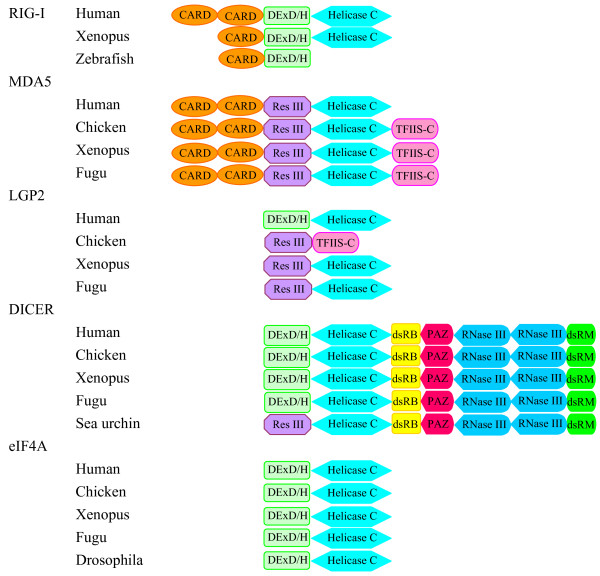
Domain analysis
The putative domains were analysed in the Pfam database using a cut-off E-value of 10.0 and selected domains listed in Table 2. A comprison of the domains is shown in Fig. 4. A classical helicase C domain is present in all 5 types of DExD/H helicases, including RIG-I, MDA5, LGP2, DICER and eIF4A. The DExD/H box responsible for ATP binding and hydrolysis was detected in the middle region of RIG-I, DICER and eIF4A by the Pfam HMM search. Similarly, the corresponding region in MDA5 and LGP2 was detected as a conserved restriction domain of bacterial type III restriction enzymes (Res III) (E-values between 4.3e-15 and 3.2e-22), sharing some degree of homology with the RIG-I DExD/H box. Two CARD domains were predicted in the N terminal regions of RIG-I and MDA5 except for Xenopus and zebrafish RIG-I, which contained a single CARD domain corresponding to the second CARD domain of the human molecule (CARD2). In general, the N-terminal CARD domain (CARD1) was less conserved than the CARD2 domain. The E-value of CARD2 in RIG-I ranges between 0.43 and 3.7, significantly higher than that in MDA5 (0.002–2.4e-18), indicating significant divergence of the CARD domains in different vertebrate groups. In addition, a fragment at the C-terminal region in most MDA5 proteins of chicken, Xenopus and fish, contained a putative domain with moderate E-values (0.51–8.60) to the 4 cysteine (C4) type transcription factor (TF) IIS central domain. The C terminal region of human RIG-I comprised a region distantly related to the C4 type zinc finger domain, which was shown to bind to dsRNA and 5'ppp viral RNA with the involvement of zinc ion [12,17]. The Pfam HMM analysis failed to identify this C4 TFIIS domain in RIG-I possibly due to low homology.
Figure 4.
Schematic of key functional domains of RIG-I, MDA5, LGP2, DICER and eIF4A predicted by the Pfam HMM programme.
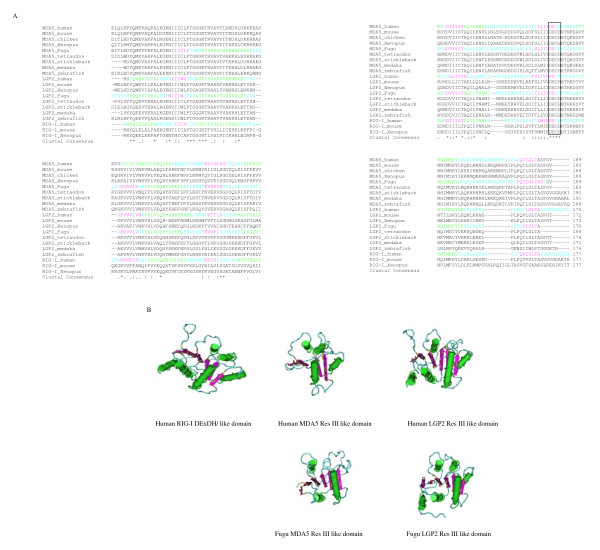
The domain sequences were further analysed by multiple alignment and their 3 dimensional structures modelled. It is apparent that the DExD/H and Res III domains comprised a well conserved DECH motif (Fig. 5A). The overall structures of DExD/H boxes and Res III domains were similar, with β sheets sandwiched by α-helices on each side (Fig. 5B). Six β strands were arranged in the same orientation in the human RIG-I DExD/H box and MDA5/LGP2 Res III domains except for the human MDA5 Res III domain, where 4 β strands were present. Conversely, numbers of predicted α-helices varied significantly among domains. Although homology analysis showed low sequence similarities between the MDA5 TFIIS domain and the C4 type zinc finger nucleotide binding motif within the human TFIIS central domain, 3D modelling displayed significant structural similarities (Fig. 6). Our modelling data indicated the C terminal region of RIG-I/MDA5 proteins across vertebrates possessed a conserved C4 type zinc finger nucleotide binding motif, in agreement with the studies in humans where a putative domain distantly related to the C4 type zinc finger protein was shown to bind to viral nucleotide PAMPs [12]. Remarkably, the 4 cysteines involved in capturing zinc ion were in close physical contact, forming a conserved pocket on the surface of all the domains analysed. However, arrangement of the cysteines differed in the human TFIIS-C domain and RIG-I/MDA5 domains (Fig. 6). In addition to the C4 type zinc finger nucleotide binding motif, the RIG-I C terminal region and the MDA5 TFIIS domain contained another noticeable β strand structure which appeared to support the C4 type pocket. In the human RIG-I, a single α helix was also detected.
Figure 5.
Multiple alignment (A) and structural modelling (B) of Res III. The domain sequences were predicted by the Pfam HMM programme and aligned using the CLUSTALW programme. The 3-dimensional structures of the domains were generated using the 3D-jigsaw comparative modelling programme and the VAST search programme and visualised by the Cn3D programme (Version 4.1). Identical (*) and similar (:, .) residues are shown below the alignment. The conserved signature "DECH" is boxed. Amino acid position of the domain position is listed in Table 2. Predicted α-helices (green), β-strands (purple or dark blue) and loops (light blue) are marked in both the alignment and the 3D structures respectively.
Figure 6.
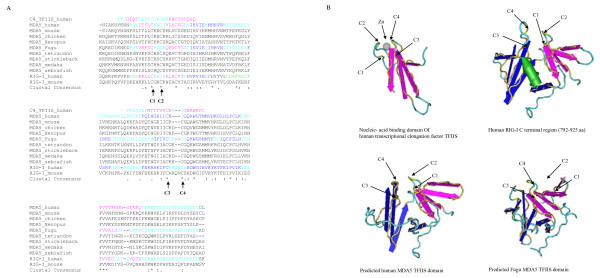
Multiple alignment (A) and structural modelling (B) of TFIIS domains. The domain sequences were predicted by the Pfam HMM programme and aligned using the CLUSTALW programme. The 3-dimensional structures of the domains were generated using the 3D-jigsaw comparative modelling programme and the VAST search programme and visualised by the Cn3D programme (Version 4.1). Identical (*) and similar (:, .) residues are shown below the alignment. Cysteines (C1–4) involved in zinc (Zn) binding are indicated by arrows in the alignment and the 3D structures. Amino acid position of the domain position is listed in Table 2. Predicted α-helices (green), β-strands (purple or dark blue) and loops (light blue) are marked in both the alignment and the 3D structures respectively.
Discussion
The RIG-I like helicase family members have recently been reported to play pivotal roles in recognising viral nucleotides in mammals. In this report, the RIG-like homologues have been identified in silico in the nucleotide databases of invertebrates and vertebrates and their evolutionary origin discussed.
Double stranded RNA is the genetic component of viruses with double stranded genomes and part of a single stranded RNA with secondary structures. It can be generated during viral replication and RNA metabolism. This nature of dsRNA makes it the prime target for host PRRs. Classical double stranded RNA binding domains are often used by some cytosolic PRRs, such as PKRs, as the detectors to sense viral presence. DICER proteins also contain two dsRNA binding domains (dsRBDs) for capturing dsRNA molecules. In the present study, a zinc finger domain similar to that of transcription factor (TF) IIS has been found in MDA5 but not in LGP2, with moderate homology to the RIG-I C terminal region. Furthermore, a well conserved type III restriction enzyme domain responsible for restriction in prokaryotic organisms is identified in the middle of both MDA5 and the N terminal region of LGP2. This domain was not detected in RIG-I molecules by the Pfam HMM programme although it shared some degree of homology. We speculate that these two domains may serve as potential binding domains to interact with viral PAMPs.
One striking finding is that a well conserved restriction enzyme III (Res III) domain is predicted in all MDA5 and LGP2 proteins (except human LGP2). The Res III domain is structurally similar to the DExD/H domain. Restriction enzymes are important components of prokaryotic DNA restriction-modification mechanisms in defence against foreign DNA [18]. They function in combination with one or two modification enzymes (DNA-methyltransferases) that protect the cell's own DNA from cleavage by the restriction enzymes. Restriction enzymes consist of four types depending on their recognition sequences and location of cleavage sites. Type III enzymes recognize short 5–6 bp long asymmetric DNA sequences and cleave 25–27 bp downstream to generate short, single-stranded 5' overhang ends. Type III enzymes contain two functional subunits Res (restriction) and Mod (modification), specifically for DNA cleavage of unmethylated double stranded foreign DNA (Res unit) and protection of self DNA from damage by methylation (Mod unit), respectively. Classic strand separation helicase activities have not been detected for type III restriction enzymes [19]. The Res III domain predicted in MDA5 and LGP2 have significant homology with bacterial Res III domains and multiple alignment reveals significant conservation (Fig. 5A). MDA5/LGP2 are also similar to the RNase III domains in the RNA endonuclease DICER and DICER like helicases which process dsRNA into 21–23 nt 3' overhang small RNAs, with 2 nt protrusions, and ATP-binding domains in bacterial and yeast DNA helicases [20,21]. Integrated nuclease domains with excision activities are seen in the DICER proteins where two ribonuclease III domains cut double stranded RNAs, releasing 2 nt 3' end overhang 21–23 nt RNA molecules which are essential for specific cleavage of viral RNAs [20,22].
Another putative important domain, a zinc finger motif similar to that of transcription factor (TF) IIS, was identified by homology analysis in the Pfam database. The zinc finger motif can bind a range of targets including DNAs, RNAs, proteins and even lipids. It is known that the zinc finger motif at the C-terminus of the TFIIS is essential for RNA binding and processing [23]. Integrated TFIIS zinc ribbon C-terminal domains are also found in some viral proteins [24,25]. The TFIIS motif located near the C terminus of the RIG-I/MDA5 proteins was detectable in the Pfam database although the E value (0.51–8.6) is moderate (Table 2). Structural modelling confirmed remarkable conservation of a C4 type zinc finger pocket and a β-strand structure compared to the C4 type zinc finger nucleic acid binding domain in the human TFIIS. Furthermore, a β-strand motif is also present within this domain in addition to the C4 type β-strand zinc finger structure. Whether it is involved in recognition of viral RNA PAMPs remains to be determined. A recent study has demonstrated that a C terminal domain in human RIG-I (792–925 aa) was involved in binding dsRNA or 5'ppp RNA, which was confirmed by magnetic resonance and X-ray crystallography [12,13]. This region was also shown to suppress RIG-I signalling [8]. Thus it is possible that viruses could interfere with this host recognition system by their own TFIIS-C containing proteins.
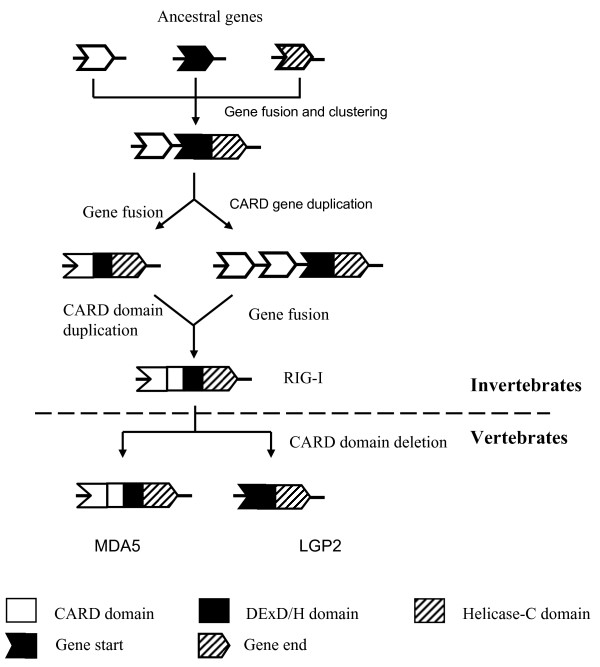
The origin and evolution of RIG-I, MDA5 and LGP2 were analysed in this study. Our data suggest they evolved from common invertebrate ancestors encoding distinct core domains (Fig. 7), which was supported by the presence of the RIG-I like genes in sea anemone Nematostella vectensis and sea urchin genomes [15]. Tandem clustering of two CARD genes with the RIG-I like genes without CARD domains in the Nematostella vectensis genome provides a strong clue as to how RIG-I, MDA5 and LGP2 could have evolved during evolution through gene fusion, domain duplication and domain deletion (Fig. 7), supporting recent analysis suggesting that CARD1 could have been grafted independently rather than duplicated from CARD2 during evolution [16]. In Deuterostome invertebrates, the RIG-I/MDA5 like genes appear to have expanded enormously, as seen in the sea urchin, with some differentiating into molecules with a closer phylogenetic relationship to the vertebrate RIG-I molecules (Fig. 3). We speculate that RIG-I emerged earlier than MDA5/LGP2 since vertebrate RIG-Is grouped with the invertebrate progenitors rather than the MDA5 and LGP2 proteins which are present uniquely in vertebrates, in stark contrast with the evolutionary model proposed by Sarkar et al [16], where LGP2 preceded both MDA5 and RIG-I in evolution. Moreover, the phylogenetic tree constructed in the present study shows that MDA5 has a closer relationship with LGP2 rather than RIG-I, suggesting MDA5 and LGP2 originated from a more recent gene duplication event, unlike the phylogenetic results obtained by Sarker et al [16]. If MDA5/LGP2 did diverge from RIG-I more recently, the order of their appearance is not clear. Also, it is uncertain from the present study why RIG-I was not found in all teleost fish. Although a putative gene coding for a partial RIG-I is predicted in the zebrafish genome, with conserved gene synteny to the Xenopus and human RIG-I locus (Fig. 1A), it is absent in the other fish genomes. Poor quality of the genome sequences makes conclusions difficult but as no RIG-I sequences were found in the vast number of fish EST sequences, perhaps functional RIG-I genes have been lost in some teleost fish species.
Figure 7.
Hypothetical evolutionary origin of RIG-I, MDA5 and LGP2.
Conclusion
The RIG-I/MDA5/LGP2 system is an ancient antiviral system well conserved in vertebrates. Our data suggest that these helicase PRRs have evolved from an ancient progenitor originated from genes coding for individual functional domains and expanded by multiple evolutionary events leading to gene and/or domain gain and loss. The present study provides important clues for further elucidation of RIG-I/MDA5 mediated antiviral defence in vertebrates.
Methods
Database mining
To identify MDA5, LGP2 and RIG-I genes in the available teleost genomes, the tblastn search using the human MDA5, LGP2 and RIG-I protein sequences as baits was performed against the genomes of zebrafish (Danio rerio), pufferfish (Takifugu rubripes and Tetraodon nigroviridis), medaka (Oryzias latipes), stickleback (Gasterosteus aculeatus), Western Clawed Xenopus (Xenopus tropicalis) and Chicken (Gallus gallus) in the Ensembl database http://www.ensembl.org. The obtained sequences were reciprocally searched against the other genomes to further verify their identity. The translated proteins from predicted transcripts were verified by BLASTP in the NCBI non-redundant protein sequence database and the SWISSPROT protein database http://www.ncbi.nlm.nih.gov. In addition, known MDA5, LGP2 and RIG-I genes were retrieved from the NCBI database for analysis.
For gene synteny analyis, human MDA-5, LGP2 and RIG-I were used as anchor sites. Orthologous comparisons of the genes in the regions of approximately 1 to 10 mb (million base pairs) flanking the human (NCBI 36) anchor site with medaka (HdrR), zebrafish (Zv7), stickleback (BROAD S1), pufferfish (FUGU 4.0, TETRAODON 7), Western Clawed Xenopus (JGI 4.1) or chicken (WASHUC2) genome were done within the Ensembl genome browser using the GeneView and MultiContigView options. Manual annotation of orthologous genes was also performed using FGENESH+ to predict structures based on homology with human genes: "fish" specific parameters were applied in this program.
Sequence analysis
The conserved domains were predicted using software at the ExPASy Molecular Biology Server http://pfam.sanger.ac.uk. Caspase recruitment domain, DExD/H box helicase, Type III restriction enzyme and helicase conserved C-terminal domains were predicted by a Pfam HMM search with a cutoff value of 10.0. The full-length amino acid sequences and the conserved functional domains were used in phylogenetic tree analysis. Multiple protein sequence alignments were performed using the ClustalW programme (version 1.83) [26]. A phylogenetic tree was constructed using the neighbour-joining method within the MEGA (4.0) package [27]. Data were analyzed using Poisson correction, and gaps were removed by pairwise deletion. The topological stability of the neighbour-joining trees was evaluated by 10,000 bootstrap replications. The three dimensional (3D) structures were predicted using the 3D JIGSAW protein comparative modelling programme [28] and compared to those in the MMDB/PDB database by VAST search analysis http://www.ncbi.nlm.nih.gov/Structure/VAST. The 3D structural images were displayed by the Cn3D programme (version 4.1).
Authors' contributions
JZ initiated the study, performed bioinformatic and phylogenetic analysis, and drafted the manuscript. MXC made substantial contributions to bioinformatic analysis. PN and CJS advised on data analysis and interpretation. CJS contributed significantly in editing the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This work was supported by the Royal Society of Edinburgh and National Natural Science Foundation of China (grant numbers: 30711130225 and 30830083).
Contributor Information
Jun Zou, Email: j.j.zou@abdn.ac.uk.
Mingxian Chang, Email: mingxianchang@ihb.ac.cn.
Pin Nie, Email: pinnie@ihb.ac.cn.
Chris J Secombes, Email: c.secombes@abdn.ac.uk.
References
- Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Venkataraman T, Valdes M, Elsby R, Kakuta S, Caceres G, Saijo S, Iwakura Y, Barber GN. Loss of DExD/H box RNA helicase LGP2 manifests disparate antiviral responses. J Immunol. 2007;178:6444–6455. doi: 10.4049/jimmunol.178.10.6444. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, Foy E, Loo YM, Gale M, Jr, Akira S, Yonehara S, Kato A, Fujita T. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175:2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- Holm GH, Zurney J, Tumilasci V, Leveille S, Danthi P, Hiscott J, Sherry B, Dermody TS. Retinoic acid-inducible gene-I and interferon-beta promoter stimulator-1 augment proapoptotic responses following mammalian reovirus infection via interferon regulatory factor-3. J Biol Chem. 2007;282:21953–21961. doi: 10.1074/jbc.M702112200. [DOI] [PubMed] [Google Scholar]
- Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- Rothenfusser S, Goutagny N, DiPerna G, Gong M, Monks BG, Schoenemeyer A, Yamamoto M, Akira S, Fitzgerald KA. The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J Immunol. 2005;175:5260–5268. doi: 10.4049/jimmunol.175.8.5260. [DOI] [PubMed] [Google Scholar]
- Saito T, Hirai R, Loo YM, Owen D, Johnson CL, Sinha SC, Akira S, Fujita T, Gale M., Jr Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc Natl Acad Sci USA. 2007;104:582–587. doi: 10.1073/pnas.0606699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T, Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, Hartmann G. 5'-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Cui S, Eisenacher K, Kirchhofer A, Brzozka K, Lammens A, Lammens K, Fujita T, Conzelmann KK, Krug A, Hopfner KP. The C-terminal regulatory domain is the RNA 5'-triphosphate sensor of RIG-I. Mol Cell. 2008;29:169–179. doi: 10.1016/j.molcel.2007.10.032. [DOI] [PubMed] [Google Scholar]
- Takahasi K, Yoneyama M, Nishihori T, Hirai R, Kumeta H, Narita R, Gale M, Jr, Inagaki F, Fujita T. Nonself RNA-sensing mechanism of RIG-I helicase and activation of antiviral immune responses. Mol Cell. 2008;29:428–440. doi: 10.1016/j.molcel.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Fujita T. Structural mechanism of RNA recognition by the RIG-I-like receptors. Immunity. 2008;29:178–181. doi: 10.1016/j.immuni.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Hibino T, Loza-Coll M, Messier C, Majeske AJ, Cohen AH, Terwilliger DP, Buckley KM, Brockton V, Nair SV, Berney K, Fugmann SD, Anderson MK, Pancer Z, Cameron RA, Smith LC, Rast JP. The immune gene repertoire encoded in the purple sea urchin genome. Dev Biol. 2006;300:349–365. doi: 10.1016/j.ydbio.2006.08.065. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Desalle R, Fisher PB. Evolution of MDA-5/RIG-I-dependent innate immunity: independent evolution by domain grafting. Proc Natl Acad Sci USA. 2008;105:17040–17045. doi: 10.1073/pnas.0804956105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Gale M., Jr Differential recognition of double-stranded RNA by RIG-I-like receptors in antiviral immunity. J Exp Med. 2008;205:1523–1527. doi: 10.1084/jem.20081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden DT, Murray NE, Rao DN. Nucleoside triphosphate-dependent restriction enzymes. Nucleic Acids Res. 2001;29:3728–3741. doi: 10.1093/nar/29.18.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Rao DN. Mutations in the Res subunit of the EcoPI restriction enzyme that affect ATP-dependent reactions. J Mol Biol. 1997;269:342–354. doi: 10.1006/jmbi.1997.1045. [DOI] [PubMed] [Google Scholar]
- Nicholson RH, Nicholson AW. Molecular characterization of a mouse cDNA encoding Dicer, a ribonuclease III ortholog involved in RNA interference. Mamm Genome. 2002;13:67–73. doi: 10.1007/s00335-001-2119-6. [DOI] [PubMed] [Google Scholar]
- Prakash R, Krejci L, Van Komen S, Anke Schurer K, Kramer W, Sung P. Saccharomyces cerevisiae MPH1 gene, required for homologous recombination-mediated mutation avoidance, encodes a 3' to 5' DNA helicase. J Biol Chem. 2005;280:7854–7860. doi: 10.1074/jbc.M413898200. [DOI] [PubMed] [Google Scholar]
- MacRae IJ, Zhou K, Doudna JA. Structural determinants of RNA recognition and cleavage by Dicer. Nat Struct Mol Biol. 2007;14:934–940. doi: 10.1038/nsmb1293. [DOI] [PubMed] [Google Scholar]
- Jeon C, Yoon H, Agarwal K. The transcription factor TFIIS zinc ribbon dipeptide Asp-Glu is critical for stimulation of elongation and RNA cleavage by RNA polymerase II. Proc Natl Acad Sci USA. 1994;91:9106–9110. doi: 10.1073/pnas.91.19.9106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn BY, Gershon PD, Jones EV, Moss B. Identification of rpo30, a vaccinia virus RNA polymerase gene with structural similarity to a eucaryotic transcription elongation factor. Mol Cell Biol. 1990;10:5433–5441. doi: 10.1128/mcb.10.10.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JM, Salas ML, Vinuela E. Genes homologous to ubiquitin-conjugating proteins and eukaryotic transcription factor SII in African swine fever virus. Virology. 1992;186:40–52. doi: 10.1016/0042-6822(92)90059-X. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Contreras-Moreira B, Bates PA. Domain fishing: a first step in protein comparative modelling. Bioinformatics. 2002;18:1141–1142. doi: 10.1093/bioinformatics/18.8.1141. [DOI] [PubMed] [Google Scholar]