Abstract
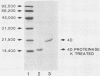
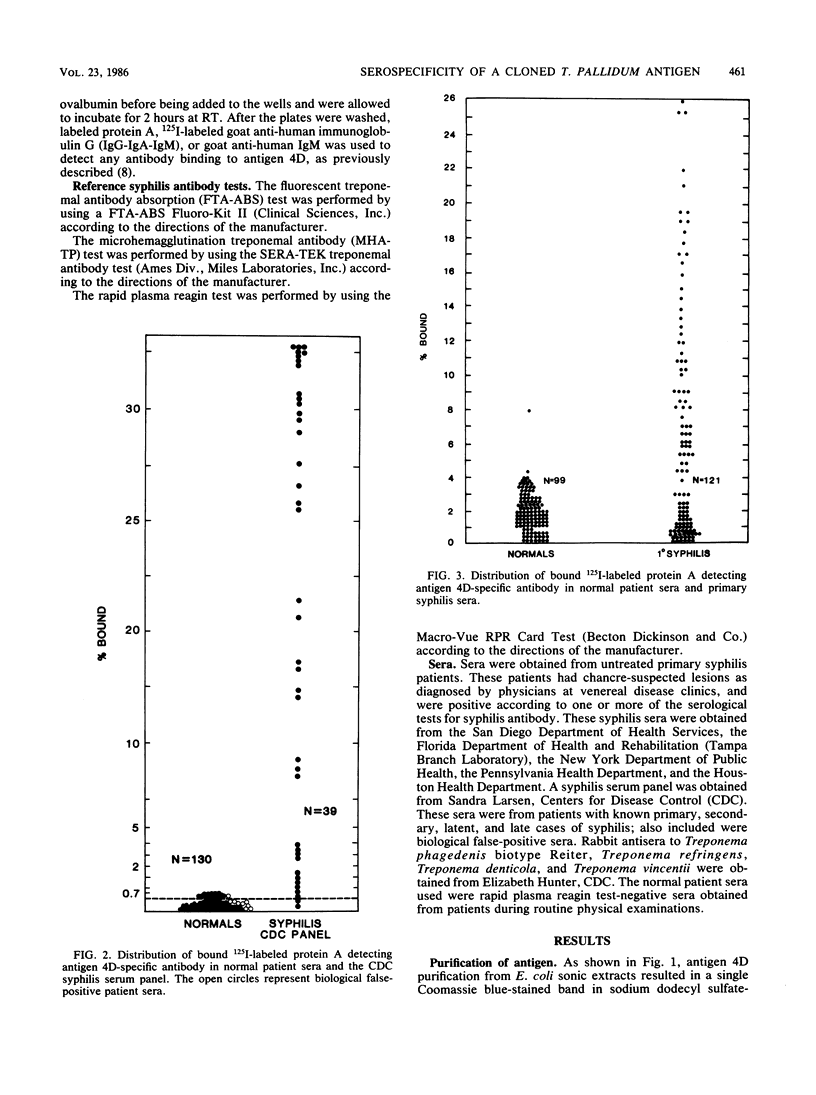
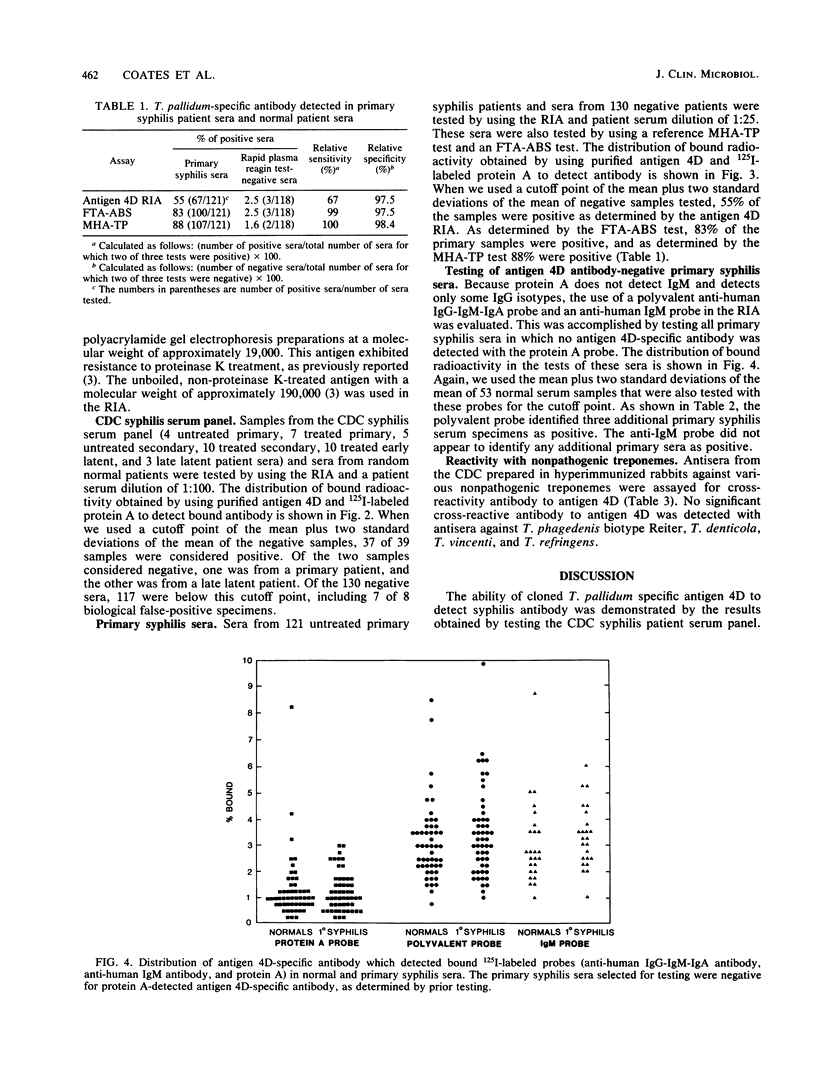
We evaluated the serological reactivity of a protease-resistant antigen designated 4D which was encoded by Treponema pallidum DNA and was expressed in Escherichia coli from recombinant plasmid pAW329. This 19,000-molecular-weight antigen was purified in its native, non-protease-treated form from E. coli sonic extracts by molecular sieving and ion-exchange chromatography. Antibody binding to antigen 4D was detected by a radioimmunoassay. Antigen 4D-specific antibody was detected in 95% of the sera in a Centers for Disease Control syphilis serum panel. It was also detected in 55% of 121 primary syphilis patients, whereas syphilis antibody was detected in 83% of the sera by a fluorescent treponemal antibody absorption test and in 88% of the sera by a T. pallidum microhemagglutination test. In tests of 118 normal sera, less than 3% demonstrated antibody to antigen 4D; these results are similar to microhemagglutination and fluorescent treponemal antibody absorption test results. Rabbit antisera against Treponema phagedenis, Treponema refringens, Treponema denticola, and Treponema vincentii did not react with antigen 4D.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dyckman J. D., Storms S., Huber T. W. Reactivity of microhemagglutination, fluorescent treponemal antibody absorption, and venereal disease research laboratory tests in primary syphilis. J Clin Microbiol. 1980 Oct;12(4):629–630. doi: 10.1128/jcm.12.4.629-630.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleberg N. C., Eisenstein B. I. The impact of new cloning techniques on the diagnosis and treatment of infectious diseases. N Engl J Med. 1984 Oct 4;311(14):892–901. doi: 10.1056/NEJM198410043111406. [DOI] [PubMed] [Google Scholar]
- Fehniger T. E., Walfield A. M., Cunningham T. M., Radolf J. D., Miller J. N., Lovett M. A. Purification and characterization of a cloned protease-resistant Treponema pallidum-specific antigen. Infect Immun. 1984 Nov;46(2):598–607. doi: 10.1128/iai.46.2.598-607.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieldsteel A. H., Cox D. L., Moeckli R. A. Cultivation of virulent Treponema pallidum in tissue culture. Infect Immun. 1981 May;32(2):908–915. doi: 10.1128/iai.32.2.908-915.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER E. F., DEACON W. E., MEYER P. E. AN IMPROVED FTA TEST FOR SYPHILIS, THE ABSORPTION PROCEDURE (FTA-ABS). Public Health Rep. 1964 May;79:410–412. [PMC free article] [PubMed] [Google Scholar]
- Hanff P. A., Fehniger T. E., Miller J. N., Lovett M. A. Humoral immune response in human syphilis to polypeptides of Treponema pallidum. J Immunol. 1982 Sep;129(3):1287–1291. [PubMed] [Google Scholar]
- Huber T. W., Storms S., Young P., Phillips L. E., Rogers T. E., Moore D. G., Williams R. P. Reactivity of microhemagglutination, fluorescent treponemal antibody absorption, Venereal Disease Research Laboratory, and rapid plasma reagin tests in primary syphilis. J Clin Microbiol. 1983 Mar;17(3):405–409. doi: 10.1128/jcm.17.3.405-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman N. R., Pickard A. R., Sigal N. H., Gearhart P. J., Metcalf E. S., Pierce S. K. Assessing B cell diversification by antigen receptor and precursor cell analysis. Ann Immunol (Paris) 1976 Jun-Jul;127(3-4):489–502. [PubMed] [Google Scholar]
- Moskophidis M., Müller F. Molecular analysis of immunoglobulins M and G immune response to protein antigens of Treponema pallidum in human syphilis. Infect Immun. 1984 Jan;43(1):127–132. doi: 10.1128/iai.43.1.127-132.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris S. J., Sell S. Antigenic complexity of Treponema pallidum: antigenicity and surface localization of major polypeptides. J Immunol. 1984 Nov;133(5):2686–2692. [PubMed] [Google Scholar]
- Stamm L. V., Kerner T. C., Jr, Bankaitis V. A., Bassford P. J., Jr Identification and preliminary characterization of Treponema pallidum protein antigens expressed in Escherichia coli. Infect Immun. 1983 Aug;41(2):709–721. doi: 10.1128/iai.41.2.709-721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walfield A. M., Hanff P. A., Lovett M. A. Expression of Treponema pallidum antigens in Escherichia coli. Science. 1982 Apr 30;216(4545):522–523. doi: 10.1126/science.7041257. [DOI] [PubMed] [Google Scholar]