Abstract
The CCN family of proteins is involved in diverse biological functions such as cell growth, adhesion, migration, angiogenesis, and regulation of extracellular matrix. We have investigated expression of CCN family genes and alternations induced by solar-simulated ultraviolet irradiation in human skin in vivo. Transcripts of all six CCN genes were expressed in human skin in vivo. CCN5 was most abundantly expressed followed by CCN2>CCN3>CCN1>CCN4>CCN6. Solar-simulated ultraviolet irradiation increased mRNA expression of CCN1 and CCN2. In contrast, mRNA levels of CCN3, CCN4, CCN5, and CCN6, were reduced. Knowledge gained from this study provides the foundation to explore the functional roles of CCN gene products in cutaneous biology and responses to solar ultraviolet irradiation.
Keywords: CCN, UV, Skin
Introduction
Human skin, the largest organ of the body, serves as a protective barrier to environmental damage. Skin is directly exposed to ultraviolet (UV) irradiation from the sun. Solar UV irradiation is a potent environmental factor that can deleteriously affect the structure and function of human skin. Acute exposure of human skin to UV irradiation causes sunburn, altered pigmentation, inflammation, immune suppression, and dermal connective tissue damage (Gilchrest and Yaar 1992; Kripke 1984; Matsumura and Ananthaswamy 2002; Pathak 1986). Chronic exposure to UV irradiation over many years disrupts normal skin and ultimately causes premature skin aging (photoaging) (Fisher et al.1996; Fisher et al.1997; Yaar and Gilchrest 2007) and cancer (Gloster and Brodland 1996; Green and Hedinger 2008; Katiyar et al. 2000; Miller and Mihm 2006).
CCN family proteins are secreted, matricellular signaling molecules. CCN proteins are capable of mediating diverse biological functions including cell growth, adhesion, migration, angiogenesis, and regulation of extracellular matrix (Chen et al. 2001; Kireeva et al. 1996; Perbal et al. 2003). CCN family of proteins currently consists of six highly conserved genes; CCN1/CYR61, CCN2/CTGF, CCN3/NOV, CCN4/WISP-1, CCN5/WISP-2, and CCN6/WISP-3 (Brigstock 1999; Brigstock et al. 2003). Altered expression of CCN genes is associated with numerous pathological states including fibrotic disorders and tumorigenesis (Brigstock et al. 2003; Leask and Abraham 2003; Perbal 2004; Planque and Perbal 2003). CCN proteins were discovered more than two decades ago (Bradham et al. 1991; Joliot et al. 1992; O’Brien et al. 1990), however, expression of CCN genes and their regulation by UV irradiation have not been studied in human skin in vivo. Here we quantified expression of the six CCN family genes, and further explored the impact of acute UV-irradiated on CCN gene expression in human skin in vivo.
Methods
UV irradiation and procurement of human tissue samples Human skin punch biopsies were obtained from healthy, adult human volunteers, as previously described (Fisher et al. 1998; Fisher et al. 1997; Fisher and Voorhees 1998; Fisher et al. 1997). For UV irradiation, sun-protected buttock skin was irradiated with 2 MED (minimum erythema dose) solar-simulated UV (SPEC 450 W xenon arc solar simulator) (Quan et al. 2004). Skin samples (4 mm diameter) were obtained at the indicated time points after UV irradiation exposure. All procedures involving human subjects were approved by the University of Michigan Institutional Review Board, and all subjects provided written informed consent.
RNA isolation and quantitative real-time reverse transcription polymerase chain reaction (RT-PCR) Total RNA was isolated from human skin samples using a commercial kit (RNeasy Mini Kit, Qiagen, Chatsworth CA) according to the manufacturer’s protocol. Total RNA was quantified using RiboGreen™ RNA Quantitation Reagent and Kit (Molecular Probes, Eugene, OR), and was reverse transcribed using Taqman Reverse Transcription kit (Applied Biosystems, Foster City, CA). Quantitiative real-time PCR was performed using Taqman Universal PCR Master Mix Reagents and ABI Prism 7700 Sequence Detector (Applied Biosystems). PCR primers and probes were designed using Primer Express software and produced by the custom oligonucleotide synthesis service (Applied Biosystems). CCN family genes PCR primers and probes are described in Table 1. PCR primers and probes for 36B4 (internal control) was published as previously described (Quan et al. 2002a; Quan et al. 2006).
Table 1.
Primer and probe nucleotide sequence for quantitative real-time RT-PCR analysis
| Gene identity | Primer sequence (5′–3′) | Probe sequence (5′–3′) | Size (bp) | Accession # |
|---|---|---|---|---|
| CCN1/CYR61 | Forward: TCAAAGACCTGTGGAACTGGTATC | CAATGACAACCCTGAGTGCCGCCT | 85 | AF307890 |
| Reverse: CACAAATCC-GGGTTTCTTTCA | ||||
| CCN2/CTGF | Forward: GTTTGGCCCAGACCCAACT | TGATTAGAGCCAACTGCCTGGTCCA | 70 | NM_001901 |
| Reverse: GGAACAGGCGCTCCACTCT | ||||
| CCN3/NOV | Forward: CCGCTGTCAGCTGGATGT | ACTGCCTGAGCCTAACTGCCCAGCT | 71 | AY082381 |
| Revers: CTCCAGGCACCTCAACTTTTCT | ||||
| CCN4/WISP1 | Forward: AGAGGCATCCATGAACTTCAC | CGGGCTGCATCAGCACACGCT | 75 | AF100779 |
| Reverse: CAAACTCCACAGTACTTGGGTTGA | ||||
| CCN5/WISP2 | Forward: ATGAGAGGCACACCGAAGAC | CACCTCCTGGCCTTCTCCCTCCT | 72 | NM_003881 |
| Reverse:CTGGGTACGCACCTTTGAGA | ||||
| CCN6/WISP3 | Forward: CATTATCATAATGGCCAAGTGTTTCA | CCCAACCCCTTGTTCAGCTGCCT | 70 | AF100781 |
| Reverse: CAATGGCCCCACTCACACA |
Multiplex PCRs were performed with each primer and probe set and 36B4 (internal control). Expression of each gene and 36B4 mRNA curves. cDNA plasmid standards for each individual genes were cloned using a pcRII® -TOPO® vector (Invitrogen, Carlsbad, CA). PCR cloning primers for CCN family genes are described in Table 2.
Table 2.
Primer nucleotide sequence for cloning CCN cDNA plasmid for quantitative real-time RT-PCR analysis
| Gene identity | Forward primer sequence (5′–3′) | Reverse primer sequence (5′–3′) | Size (bp) |
|---|---|---|---|
| CCN1/CYR61 | ACGGCTGCGGCTGCTGTAAGGTCT | AAGGCGGCACTCAGGGTTGTCAT | 638 |
| CCN2/CTGF | ACGAGCCCAAGGACCAAACCGTG | ACAGTTGTAATGGCAGGCACA | 488 |
| CCN3/NOV | TGCACGGCGGTAGAGGGAGATAAC | TGCAGGTGGATGGCTTTGAGTGAC | 527 |
| CCN4/WISP1 | GCGGCGCGTGAGCATACCT | CATAGGACCTGGCGGGAGAAGC | 498 |
| CCN5/WISP2 | ATGAGAGGCACACCGAAGACC | CTGGGCAGCCGCACATC | 425 |
| CCN6/WISP3 | GCCCCGTTGCCCTCCTG | GGCATTGTTTTGTAGCTTGTTGAA | 414 |
Statistical analysis
Comparisons between groups were determined with the Student’s t-test. All p values are two-tailed, and considered significant when p<0.05.
Results
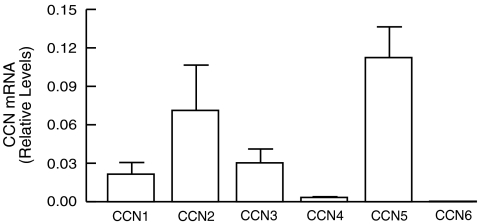
Expression of CCN genes in normal human skin in vivo To explore expression of CCN genes in human skin in vivo, total RNA was prepared from full-thickness punch biopsies of adult human buttock skin, and the mRNA levels were determined by quantitative real-time RT-PCR. Figure 1 shows that transcripts of all six CCN genes were detected in adult human skin. CCN5 was most abundantly expressed followed by CCN2>CCN3>CCN1>CCN4>CCN6. Expression of CCN5 transcript was several-fold higher than that observed for other CCN transcripts. mRNA expression of all six CCN genes was relatively low, compared to house keeping gene, 36B4, and among them CCN6 was near the limit of detection.
Fig. 1.
Expression of CCN genes in normal human skin in vivo. Full-thickness adult human skin samples from buttock were obtained, and total RNA was extracted as described in Methods. CCN members and 36B4 (internal control) mRNA levels were quantified using real-time RT-PCR. mRNA levels of CCN genes were normalized to mRNA levels of house keeping gene, 36B4. Data are expressed as mean±SEM, N = 6
Regulation of CCN gene expression in human skin by UV irradiation To investigate whether solar-simulated UV irradiation alters CCN gene expression, sun-protected human buttock skin was exposed to 2 MED (minimum erythema dose) solar-simulated UV irradiation. Skin samples were then taken at 4, 8, 16, 24, and 48 h following UV irradiation. Total RNA was prepared, and mRNA expression levels were determined by quantitative real-time RT-PCR. UV irradiation caused modest increases in both CCN1 and CCN2 at 4 and 8 h post UV irradiation (Fig. 2a, b). By 8 h, transcripts for both CCN1 and CCN2 were significantly increased approximately 15 and 4-fold, respectively. mRNA levels of both CCN1 and CCN2 returned to basal levels at 24 h following UV irradiation. In contrast, mRNA levels of CCN3, CCN4, CCN5, and CCN6 were modestly reduced following exposure of skin to UV irradiation. CCN3 mRNA levels decreased 40% at 24 h and returned to basal level at 48 h post UV irradiation (Fig. 2c). CCN4 mRNA levels were reduced 60% at 48 h following UV irradiation (Fig. 2d). CCN5 mRNA levels were decreased 50% at 24 h and returned to basal level at 48 h post UV irradiation (Fig. 2e). CCN6 mRNA levels were reduced 30% at 8 and 16 h and returned to basal level at 24 h post UV irradiation (Fig. 2f).
Fig. 2.
UV irradiation alters CCN gene expression in human skin in vivo. Adult human buttock skin was exposed to 2 MED solar-simulated UV irradiation. Skin samples were obtained at indicated time points and total RNA was extracted. mRNA levels of CCN family members and 36B4 were quantified using real-time RT-PCR. mRNA levels of CCN genes were normalized to mRNA levels of house keeping gene, 36B4 (internal control). a CCN1/CYR61 b CCN2/CTGF c CCN3/NOV d CCN4/WISP1 e CCN5/WISP5 f CCN6/WISP3. Results are expressed as mean ± SEM, N = 6 for each time point, *p < 0.05
Discussion
Human skin protects the body from the deleterious environmental factor, including effects of UV-irradiation from the sun. Chronic exposures to UV irradiation over many years causes accumulative skin damage and eventually causes skin to age prematurely (photoaging) and leads to skin cancer (Fisher et al. 1996; Fisher et al. 1997; Yaar and Gilchrest 2007). Chronic sun exposure impairs normal skin functions, such as wound healing, immune surveillance, and skin barrier (Gilchrest and Yaar 1992; Kripke 1984; Pathak 1986; Pathak et al. 1993). Photodamaged skin also provides a microenvironment that supports the development of skin cancers, the most common type of cancer in the United States (Hales et al. 1989; Matsumura and Ananthaswamy 2002; Pilcher et al. 1997; Stamp et al. 1988). More than a million people are diagnosed with skin cancer each year and current estimates are that 40–50% of Americans who live to age 65 will have skin cancer at least once.
We found that all six CCN genes are expressed in human skin in vivo. Among them, CCN5 is most highly expressed followed by CCN2>CCN3>CCN1>CCN4>CCN6. In general, CCN1 and CCN2 are immediate early genes and are associated with cellular proliferation, whereas CCN3-5 are associated with suppression of proliferation (Bleau et al. 2007; Brigstock 1999; Delmolino et al. 2001; Huang et al. 2008; Kleer et al. 2007; Kleer et al. 2002; Perbal 2001; Planque and Perbal 2003; Zhang et al. 2005). Solar-simulated UV irradiation increased mRNA expression of proliferation-associated CCN genes, CCN1 and CCN2, at early time points in human skin in vivo. In contrast, mRNA expression of growth arrest-associated CCN genes, CCN3 CCN4, CCN5, and CCN6, were reduced by solar-simulated UV irradiation at late time points in human skin in vivo. Taken together, these mRNA expression data suggest that CCN protein may be involved in the hyperproliferative response that follows exposure of human skin to acute UV irradiation (Fisher and Voorhees 1998; Fisher et al. 1997; Matsumura and Ananthaswamy 2002; Quan et al. 2002b). Further investigations are required to determine cell-type specificity of CCN gene expression and localization of CCN proteins in human skin.
Acknowledgments
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Supported by the National Institutes of Health (ES014697 [TQ])
References
- Bleau A, Planque N, Lazar N, Zambelli D, Ori A, Quan T et al (2007) Antiproliferative activity of CCN3: involvement of the C-terminal module and post-translational regulation. J Cell Biochem 101:1475–1491 [DOI] [PubMed]
- Bradham D, Igarashi A, Potter R, Grotendorst G (1991) Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol 114:1285–1294 [DOI] [PMC free article] [PubMed]
- Brigstock D (1999) The connective tissue growth factor/Cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocrine Rev 20:189–206 [DOI] [PubMed]
- Brigstock D, Goldschmeding R, Katsube K-I, Lam S-T, Lau L, Lyons K et al (2003) Proposal of a unified CCN nomenclature. Mol Pathol 56:127–128 [DOI] [PMC free article] [PubMed]
- Chen C-C, Mo F-E, Lau L (2001) The angiogenic factor Cyr61 activates a genetic program for wound healing in human skin fibroblasts. J Biol Chem 276:47329–47337 [DOI] [PubMed]
- Delmolino L, Stearns N, Castellot J Jr (2001) COP-1, a member of the CCN family, is a heparin-induced growth arrest specific gene in vascular smooth muscle cells. J Cell Physiol 188:45–55 [DOI] [PubMed]
- Fisher GJ, Voorhees JJ (1998) Molecular mechanisms of photoaging and its prevention by retinoic acid: Ultraviolet irradiation induces MAP kinase signal transduction cascades that induce AP-1-regulated matrix metalloproteinases that degrade human skin in vivo. J Invest Dermatol 3:61–68 [PubMed]
- Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S et al (1996) Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 379:335–339 [DOI] [PubMed]
- Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ (1997) Pathophysiology of premature skin aging induced by ultraviolet light. New Eng J Med 337:1419–1428 [DOI] [PubMed]
- Fisher G, Talwar H, Lin P, McPhillips F, Wang Z, Li X et al (1998) Retinoic acid inhibits induction of c-Jun protein by ultraviolet irradiation that occurs subsequent to activation of mitogen-activated protein kinase pathways in human skin in vivo. J Clin Invest 101:1432–1440 [DOI] [PMC free article] [PubMed]
- Gilchrest BA, Yaar M (1992) Ageing and photoageing of the skin: observations and the cellular and molecular level. Brit J Dermatol 127:25–30 [DOI] [PubMed]
- Gloster H, Brodland D (1996) The epidemiology of skin cancer. Dermatol Surg 22:217–226 [DOI] [PubMed]
- Green A, Hedinger R (2008) Models relating ultraviolet light and non-melanoma skin cancer incidence. Photochem Photobiol 28:283–291 [DOI] [PubMed]
- Hales S, Stamp G, Evans M, Fleming K (1989) Identification of the orgin of cells in human basal cell carcinoma xenocrafts in mice using in situ hybridization. Brit J Dermatol 120:351–357 [DOI] [PubMed]
- Huang W, Zhang Y, Varambally S, Chinnaiyan A, Banerjee M, Merajver S et al (2008) Inhibition of CCN6 (Wnt-1-induced signaling protein 3) down-regulates E-Cadherin in the breast epithelium through induction of Snail and ZEB1. Amer J Pathol 172:893–904 [DOI] [PMC free article] [PubMed]
- Joliot V, Martinerie C, Dambrine G, Plassiart G, Brisac M, Crachet J et al (1992) Proviral rearrangements and overexpression of a new cellular gene (nov) in myeloblastosis-associated virus type 1-induced mephroblastomas. Molecular Cellular Biol 12:10–21 [DOI] [PMC free article] [PubMed]
- Katiyar S, Matsui M, Mukhtar H (2000) Kinetics of UV light-induced cyclobutane pyrimidine dimers in human skin in vivo: An immunohistochemical analysis of both epidermis and dermis. Photochem Photobiol 72:788–793 [DOI] [PubMed]
- Kireeva M, Mo F-E, Yang G, Lau L (1996) Cyr61, a product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Mol Cell Biol 16:1326–1334 [DOI] [PMC free article] [PubMed]
- Kleer C, Zhang Y, Pan Q, van Golen K, Wu Z, Livant D et al (2002) WISP3 is a novel tumor suppressor gene of inflammatory breast cancer. Oncogene 21:3172–3180 [DOI] [PubMed]
- Kleer C, Zhang Y, Merajver S (2007) CCN6 (WISP6) as a new regulator of the epithelial phenotype in breast cancer. Cells tissues Organs 185:95–99 [DOI] [PubMed]
- Kripke ML (1984) Immunological unresponsiveness induced by ultraviolet radiation. Immunol Rev 80:87 [DOI] [PubMed]
- Leask A, Abraham D (2003) The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem Cell Biol 81:355–363 [DOI] [PubMed]
- Matsumura Y, Ananthaswamy H (2002) Molecular mechanisms of photocarcinogenesis. Front Biosci 7:d765–783 [DOI] [PubMed]
- Miller A, Mihm M Jr (2006) Melanoma. New Eng J Med 355:51–65 [DOI] [PubMed]
- O’Brien T, Yang G, Sanders LC, Lau L (1990) Expression of cyr61, a growth factor-inducible immediate-early gene. Mol Cell Biol 10:3569–3577 [DOI] [PMC free article] [PubMed]
- Pathak MA (1986) Immediate and delayed pigmentary and other cutaneous response to solar UVA radiation (320–400 nm). In: Urbach RWG F (ed) The Biological Effects of UVA Radiation. Praeger, New York, pp 156–167
- Pathak MA, Nghiem P, Fitzpatrick TB (1993) Acute and chronic effects of sun. In: Fitzpatrick TBEAZ, Wolff K, Freedberg IM, Austen KF (eds) ermatology in general medicine, 4th edn. McGraw-Hill, New York, pp 1598–1607
- Perbal B (2001) NOV (nephroblastoma overexpressed) and the CCN family of genes: structural and functional issues. J Clin Pathol: Mol Pathol 54:57–79 [DOI] [PMC free article] [PubMed]
- Perbal B (2004) CCN proteins: multifunctional signalling regulators. Lancet 363:62–64 [DOI] [PubMed]
- Perbal B, Brigstock D, Lau L (2003) Report on the second international workshop in the CCN family of genes. Mol Pathol 56:80–85 [DOI] [PMC free article] [PubMed]
- Pilcher BK, Dumin J, Sudbeck BD, Krane SM, Welgus HG, Parks WC (1997) The activity of collagenase I is required for keratinocyte migration on a type I collagen matrix. J Cell Biol 137:1445–1457 [DOI] [PMC free article] [PubMed]
- Planque N, Perbal B (2003) A structural approach to the role of CCN (CYR61/CTGF/NOV) proteins in tumourigenesis. Cancer Cell Intl 3:15 [DOI] [PMC free article] [PubMed]
- Quan T, He T, Kang S, Voorhees J, Fisher G (2002a) Connective tissue growth factor: Expression in human skin in vivo and inhibition by ultraviolet irradiation. J Invest Dermatol 118:402–408 [DOI] [PubMed]
- Quan T, He T, Kang S, Voorhees J, Fisher G (2002b) Ultraviolet irradiation alters transforming growth factor ß/Smad pathway in human skin in vivo. J Invest Dermatol 119:499–506 [DOI] [PubMed]
- Quan T, He T, Kang S, Voorhees J, Fisher G (2004) Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-ß type II receptor/Smad signaling. Amer J Pathol 165:741–751 [DOI] [PMC free article] [PubMed]
- Quan T, He T, Shao Y, Lin L, Kang S, Voorhees JJ et al (2006) Elevated cysteine-rich 61 mediates aberrant collagen homeostasis in chronologically aged and photoaged human skin. Am J Pathol 169:482–490 [DOI] [PMC free article] [PubMed]
- Stamp G, Quaba A, Braithwaite A, Wright N (1988) Basal cell carcinoma xenografts in nude mice: studies on epithelial differentiation and stromal relationship. J Pathol 156:213–225 [DOI] [PubMed]
- Yaar M, Gilchrest B (2007) Photoageing: mechanism, prevention and therapy. Brit J Dermatol 157:874–887 [DOI] [PubMed]
- Zhang Y, Pan Q, Zhong H, Merajver S, Kleer C (2005) Inhibition of CCN6 (WISP3) expression promotes neoplastic progression and enhances the effects of insulin-like growth factor-1 on breast epithelial cells. Breast Cancer Res 7:R1080–R1089 [DOI] [PMC free article] [PubMed]