Abstract
By providing a source of α-smooth muscle actin (α-SMA)-expressing myofibroblasts, microvascular pericytes contribute to the matrix remodeling that occurs during tissue repair. However, the extent to which pericytes may contribute to the fibroblast phenotype post-repair is unknown. In this report, we test whether pericytes isolated from human placenta can in principle become fibroblast-like. Pericytes were cultured in vitro for 11 passages. The Affymetrix mRNA expression profile of passage 2 and passage 11 pericytes was compared. The expression of type I collagen, thrombospondin and fibronectin mRNAs was induced by passaging pericytes in culture. This induction of a fibroblast phenotype was paralleled by induction of connective tissue growth factor (CTGF/CCN2) and type I collagen protein expression and the fibroblast marker ASO2. These results indicate that, in principle, pericytes have the capacity to become fibroblast-like and that pericytes may contribute to the population of fibroblasts in a healed wound.
Keywords: Perictytes, CTGF, CCN2
Introduction
During tissue repair, fibroblasts migrate into the wound and produce and remodel extracellular matrix (ECM). The fibroblasts mediating this process are called myofibroblasts as they express the highly contractile protein α-smooth muscle actin (α-SMA) (Hinz and Gabbiani 2003). Although the origin of the myofibroblasts during tissue repair remains controversial, microvascular pericytes play a key role in this process in skin, contributing to ~30% of the myofibroblasts post-wounding (Rajkumar et al. 2006; Abraham et al. 2007; Kapoor et al. 2008).
Pericytes begin migrating into the wound in a fashion requiring signaling in response to platelet derived growth factor (Rajkumar et al. 2006). Pericytes express α-SMA and CTGF (Connective tissue growth factor/CCN2) and thus are likely to contribute to the migratory and contractile phenotype of myofibroblasts (Rajkumar et al. 2006; Kennedy et al. 2007; Kapoor et al. 2008). However, the extent to which pericytes may contribute to the overall characteristics of tissue during and after wounding is unclear.
Pericytes passaged in cell culture increase their expression of prolyl-4-hydroxylase, type I procollagen, and collagen pro-alpha1(I) mRNA, suggesting that pericytes may develop into cells which highly express fibrotic markers (Ivarsson et al. 1996). In this report, we use Affymetrix gene profiling and Western blot analysis of pericytes passaged in cell culture to assess the extent to which pericytes may, in principle, become fibroblast-like. Our data provide new insights into the potential origin of the fibroblast during and after normal tissue repair.
Materials and methods
Cell culture
Pericytes were isolated from human placenta and cultured as previously described (Sundberg et al. 1996). Vascular fragments of human term placenta were isolated by enzymatic digestion and separation in Percoll, as previously described. Their microvascular origin was ascertained by confocal microscopy using antibodies specific for endothelial cells (PAL-E) and pericytes (high-molecular-weight-melanoma-associated antigen), as described. Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (Life Technologies), 100 U/ml penicillin, and 100 mg/ml streptomycin, and cultured in a humidified atmosphere of 5% CO2, and were passaged 1:4. Phase photography was taken on live cells using a Nikon microscope and camera.
Western blot analysis
Cells were cultured until 80% confluence in DMEM 10% FBS. Cells were cultured in DMEM, 0.5% FBS for an additional 24 h to avoid the confounding effects of added serum on protein expression. Cell layers were harvested using 2% SDS. Proteins were quantified (Bradford, Bio-Rad). Equal amounts of protein (25 μg) were subjected to SDS/PAGE using 4–12% polyacrylamide gels (Invitrogen). Gels were blotted onto nitrocellulose, and proteins were detected using anti-CCN2, anti-GAPDH (Santa Cruz), anti-type I collagen (Biodesign), anti-ASO2 or α-SMA (Sigma) antibodies followed by an appropriate HRP-conjugated secondary antibody (Jackson Immunoresearch). Proteins were detected using an enhanced chemiluminescence kit (Amersham).
RNA quality assessment, probe preparation and gene chip hybridization and analysis
Microarrays and analysis using human U133A v2.0 chips were performed essentially as previously described (Chen et al. 2005; Renzoni et al. 2004) by Imperial College, London. RNA was harvested (Trizol, Invitrogen) and quantified spectrophometrically. Biotinylated complimentary RNA (cRNA) was prepared from 10 µg of total RNA as per the Affymetrix GeneChip Technical Analysis Manual (Affymetrix, Santa Clara, CA). Double-stranded cDNA was synthesized using SuperScript II (Invitrogen, Carlsbad, CA) and oligo(dT) 24 primers. Biotin-labeled cRNA was prepared by using the Bizarre High-Yield RNA Transcript Labeling kit (Enzo Brioche, New York, NY). Fifteen µg of labeled cRNA was hybridized to Human Gene Chips and were processed as described in the Affymetrix Technical Analysis Manual (Affymetrix, Santa Clara, CA). Gene Chips were scanned with the Affymetrix GeneChip Scanner 3000 (Affymetrix, Santa Clara, CA). Signal intensities for genes were generated using GCOS1.2 (Affymetrix) using default values for the Statistical Expression algorithm parameters and a Target Signal of 150 for all probe sets and a Normalization Value of 1. Normalization (per chip to the 50th percentile, and per gene to baseline control) was performed in GeneSpring 7.2 (Agilent Technologies Inc., Palo Alto, CA). Experiments were performed twice, and fold changes were identified using the GeneSpring filter. Messages induced greater than 2.5-fold were selected.
Results
Passaging of pericytes induces a fibroblast-like phenotype
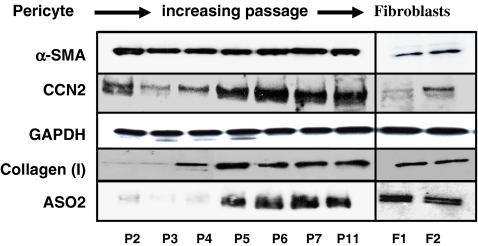
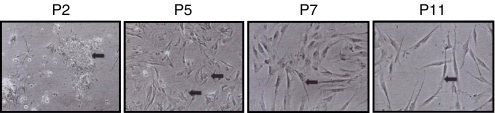
To determine whether placenta perictyes could become fibroblast-like, we passaged pericytes for 11 passages (with a 1:4 split per passage) on plastic. RNAs were extracted from cells at passage 2 and passage 11 and subjected to Affymetrix genome-wide profiling. Table 1 illustrates that genes involved with ECM and ECM remodeling were induced greater than 2.5 fold. These mRNAs included type I collagen, as expected (Ivarsson et al. 1996), thrombospondin, fibronectin and several matrix metalloprotenases (Table 1). Moreover, the angiogenic factor vascular endothelial growth factor, which is involved with tissue remodeling and repair, was also induced (Tonnesen et al. 2000). Western blot analysis confirmed that type I collagen was induced by passaging (Fig. 1). Moreover, CCN2 (Leask and Abraham 2006) and the fibroblast marker ASO2 (Saalbach et al. 1996) were also induced by passaging pericytes in culture (Fig. 1). Pericytes also acquired a fibroblast-like morphology (Fig. 2). These results suggest that pericytes, in addition to contributing to the α-SMA-expressing contractile myofibroblasts during cutaneous wounding (Rajkumar et al. 2006; Kapoor et al. 2008; Fig. 1), may also contribute to the ECM production by fibroblasts during and after wounding.
Table 1.
Affymetrix Gene expression profiling of pericytes at passage 2 and passage 11
| Gene name | Accession Number | Fold change |
|---|---|---|
| Col1A1 | Y15916 | 27.84 |
| GREM1 (Gremlin) | NM 013372 | 9.34 |
| THBS1(Thrombospondin-1) | AI812030 | 8.07 |
| FN1 (Fibronectin 1) | AJ276395 | 6.91 |
| PDGF-D | NM 0025208 | 5.22 |
| MMP7 | NM 002423 | 5.18 |
| IL-8 | AF043337 | 4.21 |
| SDC1 (Syndecan 1) | NM 002997 | 3.64 |
| VEGF | AF091352 | 3.63 |
| ACTB (Actin beta) | 3.49 | |
| MMP14 | ZA4981 | 2.98 |
| BMP1 | NM 001199 | 2.66 |
Messages induced greater than 2.5-fold at passage 11 are shown
Fig. 1.
Induction of CCN2, type I collagen and ASO2 protein production in pericytes passaged in vitro. Protein harvested from primary human placenta pericyes in culture for 2, 3, 4, 5, 6, 7,11 passages post-isolation (P2, P3, P4, P5, P6, P7 and P11, respectively) were subjected to Western blot analysis with anti-CCN2, anti-ASO2, anti-type I collagen, anti-α-SMA and anti-GAPDH antibodies are shown. Four different isolates of pericytes were tested. Westerns of protein extracts from human dermal fibroblasts from two different individuals are shown (F1 and F2)
Fig. 2.
Change in morphology of cultured pericytes upon passaging. Phase contrast images of pericytes were taken at passage (P) 2, 5, 7 and 11. Note that cells gradually acquire a linear, fibroblast-like morphology
Discussion
Microvascular pericytes contribute comprise about 1/3 of the total number of myofibroblasts in wound tissue (Rajkumar et al. 2006; Kapoor et al. 2008). Pericytes express α-SMA (Rajkumar et al. 2006; Kapoor et al. 2008) and thus are likely to contribute to ECM contraction during the wound healing process. Moreover, cell culture passaged pericytes begin to express elevated levels of type I collagen mRNA and protein (Ivarsson et al. 1996). In this report, we were able to show that Affymetrix mRNA expression profiling of pericytes passaged extensively in culture revealed that pericytes can become fibroblast like, by expressing an mRNA profile consistent with an ECM remodeling phenotype. Cell-culture passaged pericytes also responded by elevating CCN2 protein production, supporting the hypothesis that CCN2 expression is a faithful marker of fibroblast activation (Leask et al. 2009). This alteration in protein expression profile was paralleled by the induction of ASO2 (CD90) protein, a fibroblast-specific marker (Saalbach et al. 1996). These results collectively enhance the notion that pericytes may be a source of fibroblasts in vivo. Please note that although it is possible that cell culture conditions may have selected for myofibroblasts, the change in cell morphology upon passaging argues against this interpretation. Further studies (for example a three dimensional cell culture system) may permit a greater evaluation of the extent to which pericytes may contribute to matrix production and remodeling.
Acknowledgements
Our work is funded by the Arthritis Research Campaign, the Reynaud’s and Scleroderma Association, the Canadian Institute of Heath Research, the Canadian Foundation for Innovation, and the Scleroderma Society. A.L is an Arthritis Society (Scleroderma Society of Ontario) New Investigator and a recipient of Early Researcher Award.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Abraham DJ, Eckes B, Rajkumar V, Krieg T (2007) New developments in fibroblast and myofibroblast biology: implications for fibrosis and scleroderma. Curr Rheumatol Rep 9:136–143. doi:10.1007/s11926-007-0008-z [DOI] [PubMed]
- Chen Y, Shi-Wen X, van Beek J, Kennedy L, McLeod M, Renzoni EA, Bou-Gharios G, Wilcox-Adelman S, Goetinck PF, Eastwood M, Black CM, Abraham DJ, Leask A (2005) Matrix contraction by dermal fibroblasts requires transforming growth factor-beta/activin-linked kinase 5, heparan sulfate-containing proteoglycans, and MEK/ERK: insights into pathological scarring in chronic fibrotic disease. Am J Pathol 67:1699–1711 [DOI] [PMC free article] [PubMed]
- Hinz B, Gabbiani G (2003) Mechanisms of force generation and transmission by myofibroblasts. Curr Opin Biotechnol 14:538–546. doi:10.1016/j.copbio.2003.08.006 [DOI] [PubMed]
- Ivarsson M, Sundberg C, Farrokhnia N, Pertoft H, Rubin K, Gerdin B (1996) Recruitment of type I collagen producing cells from the microvasculature in vitro. Exp Cell Res 229:336–349. doi:10.1006/excr.1996.0379 [DOI] [PubMed]
- Kapoor M, Liu S, Huh K, Parapuram S, Kennedy L, Leask A (2008) Connective tissue growth factor promoter activity in normal and wounded skin. Fibrogenesis Tissue Repair 1:3. doi:10.1186/1755-1536-1-3 [DOI] [PMC free article] [PubMed]
- Kennedy L, Liu S, Shi-Wen X, Chen Y, Eastwood M, Carter DE, Lyons KM, Black CM, Abraham DJ, Leask A (2007) CCN2 is necessary for the function of mouse embryonic fibroblasts. Exp Cell Res 313:952–964. doi:10.1016/j.yexcr.2006.12.006 [DOI] [PubMed]
- Leask A, Abraham DJ (2006) All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci 119:4803–4810. doi:10.1242/jcs.03270 [DOI] [PubMed]
- Leask A, Parapuram SK, Shiwen X Abraham DJ (2009) Connective tissue growth factor (CTGF, CCN2) gene expression: a potent clinical marker of fibroproliferative disease. J. Cell Commun Signal., epub [DOI] [PMC free article] [PubMed]
- Rajkumar VS, Shiwen X, Bostrom M, Leoni P, Muddle J, Ivarsson M, Gerdin B, Denton CP, Bou-Gharios G, Black CM, Abraham DJ (2006) Platelet-derived growth factor-beta receptor activation is essential for fibroblast and pericyte recruitment during cutaneous wound healing. Am J Pathol 169:2254–2265. doi:10.2353/ajpath.2006.060196 [DOI] [PMC free article] [PubMed]
- Renzoni EA, Abraham DJ, Howat S, Shi-Wen X, Sestini P, Bou-Gharios G, Wells AU, Veeraraghavan S, Nicholson AG, Denton CP, Leask A, Pearson JD, Black CM, Welsh KI, du Bois RM (2004) Gene expression profiling reveals novel TGFbeta targets in adult lung fibroblasts. Respir Res 5:24. doi:10.1186/1465-9921-5-24 [DOI] [PMC free article] [PubMed]
- Saalbach A, Aneregg U, Bruns M, Schnabel E, Herrmann K, Haustein UF (1996) Novel fibroblast-specific monoclonal Abs: properties and specificities. J Invest Dermatol 106:1314. doi:10.1111/1523-1747.ep12349035 [DOI] [PubMed]
- Sundberg C, Ivarsson M, Gerdin B, Rubin K (1996) Pericytes as collagen-producing cells in excessive dermal scarring. Lab Invest 74:452–466 [PubMed]
- Tonnesen MG, Feng X, Clark RA (2000) Angiogenesis in wound healing. J Investig Dermatol Symp Proc 5:40–46. doi:10.1046/j.1087-0024.2000.00014.x [DOI] [PubMed]