ABSTRACT
BACKGROUND
Process of care failures may contribute to diagnostic errors in breast cancer care.
OBJECTIVE
To identify patient- and provider-related process of care failures in breast cancer screening and follow-up in a non-claims-based cohort.
DESIGN
Retrospective chart review of a cohort of patients referred to two Boston cancer centers with new breast cancer diagnoses between January 1, 1999 and December 31, 2004.
PARTICIPANTS
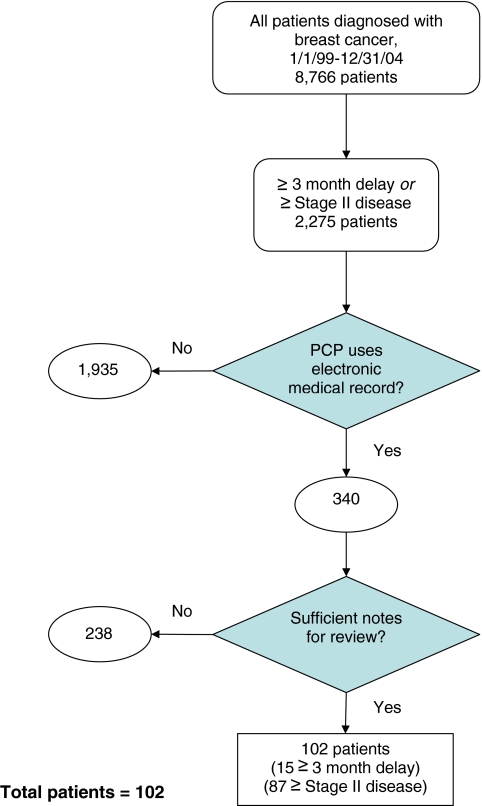
We identified 2,275 women who reported ≥90 days between symptom onset and breast cancer diagnosis or presentation with at least stage II disease. We then selected the 340 (14.9%) whose physicians shared an electronic medical record. We excluded 238 subjects whose records were insufficient for review, yielding a final cohort of 102 patients.
INTERVENTIONS
None
MEASUREMENTS
We tabulated the number and types of process of care failures and examined risk factors using bivariate analyses and multivariable Poisson regression.
MAIN RESULTS
Twenty-six of 102 patients experienced ≥1 process of care failure. The most common failures occurred when physicians failed to perform an adequate physical examination, when patients failed to seek care, and when diagnostic or laboratory tests were ordered but patients failed to complete them. Failures were attributed in similar numbers to provider- and patient-related factors (n = 30 vs. n = 25, respectively). Process of care failures were more likely when the patient’s primary care physician was male (IRR 2.8, 95% CI 1.2 to 6.5) and when the patient was non-white (IRR 2.8, 95% CI 1.4 to 5.7).
CONCLUSIONS
Process failures were common in this patient cohort, with both clinicians and patients contributing to breakdowns in the diagnostic process.
KEY WORDS: breast cancer, diagnostic errors, medical errors, quality of health care
Although diagnostic errors may lead to significant morbidity and liability,1–3 there are few data to characterize the severity or extent of this problem, its causes, and associated risk factors. The number of malpractice claims for diagnostic errors suggests their scope: aggregate data from multiple studies show that from 1985 to 2003, diagnostic errors represented the largest category of medical malpractice claims in the United States1,4,5. Missed and delayed cancer diagnoses accounted for 37 percent of asserted and closed diagnosis-related claims in one series, with more than a quarter of those claims attributable to delays in the diagnosis of breast cancer1. In another study, missed breast cancer diagnoses were cited in 24% of all claims2.
Recently, investigators have begun to examine process of care failures, specific points in the diagnostic process where breakdowns occur2,6,7. In the most comprehensive and detailed study completed to date, Gandhi and colleagues reviewed diagnostic errors in closed claims for four large malpractice insurers2. They found that 59% of diagnostic errors had three or more contributing process breakdowns, delaying diagnosis by more than 1 year on average. Systems for guaranteeing an adequate medical history, physical examination, and follow-up plan potentially could have prevented many of these injuries.
Unfortunately, studies based on claims data have several inherent limitations. The claims that are litigated and the plaintiffs who bring them forward may not represent the population of patients who suffer medical errors and injuries8,9. In addition, claims are often asserted long after incidents occur, creating a lag between past and present systems and standards of care. Finally, because studies utilizing claims data have no natural comparison group, they are unable to draw conclusions about the patient and provider characteristics most associated with process of care failures.
To avoid these limitations, we investigated process of care failures in a patient population not derived from a malpractice claims cohort. The goal of the study was to use chart reviews to identify process of care failures in breast care and their associated risk factors among a retrospective cohort of patients who may be at high risk of such failures. We hypothesized that these failures would be prevalent, that they would span the breast care process, and that certain patient and provider characteristics would be associated with increased risk.
METHODS
Study Subjects
We identified subjects via the Dana-Farber Cancer Institute Clinical Research Information System (CRIS), a clinical and administrative data repository created in 1997 as a longitudinal resource for the study of patients with breast cancer. The database includes information on over 18,500 patients, including up to 90% of all patients who have undergone an initial oncology consultation or new patient visit at two Boston cancer hospitals since 1997. It contains data on presenting symptoms derived from a questionnaire completed by all new cancer patients, as well as clinical information abstracted from patients’ medical records.
We screened the CRIS database for patients who presented with a new diagnosis of breast cancer between January 1, 1999 and December 31, 2004 (8,766 patients). We selected a sub-sample of 2,275 (26.0%) patients we suspected to be at increased risk of process of care failures because they met one of the following criteria: (1) at least a 90-day interval between patient-reported symptom onset (recorded on a written intake survey) and cancer diagnosis (based on date of pathological diagnosis), irrespective of disease stage at presentation, or (2) presentation with at least stage II disease. In order to facilitate access to necessary diagnostic information and the details of patient visits, we then selected the 340 patients whose current primary care providers within a large, New England, integrated health-care system used a common electronic medical record (EMR)—14.9% of the 2,275 patients at increased risk for a missed or delayed diagnosis. Finally, we excluded 238 subjects (70.0% of 340) because the number or quality of primary care clinician notes in the EMR prior to the date of diagnosis was insufficient to perform the intended record review. This occurred when the patients’ primary care provider at the time of diagnosis had no EMR, or when there were no office notes or test results in the medical record. As shown in Figure 1, the final subject population comprised 102 patients, 15 with at least a 90-day interval between symptom onset and diagnosis and 87 with at least stage II breast cancer. These 102 cases served as the unit of analysis in this study, each with the potential for multiple process of care failures.
Figure 1.
Flowchart of cohort selection.
Data Collection
We modified a data collection instrument created by Gandhi and colleagues for use with malpractice claims records,2 adapting it for the local practice environment and the limitations of EMR review. The tool was further refined based on the results of three focus groups with nurses, primary care physicians, and breast oncologists. A physician investigator (M.G.S.) used the instrument to abstract data from the subjects’ EMRs, focusing on steps in the process of care from symptom onset, exam, or diagnostic abnormality through diagnosis.
The principle outcome of interest was the presence of a lapse in the process of care. The chart reviewer identified possible breakdowns in the process of care, including the adequacy of the clinician’s medical history, physical examination, ordering and interpretation of laboratory tests, transmission of results to the patient, and formulation of an appropriate follow-up plan. The reviewer also identified cases where tests or follow-up plans were ordered but not performed, and when the patient’s behavior contributed to a delay. These cases were reviewed by a two-member physician panel. Reviewers recorded their judgments about the likelihood of ten potential process of care failures (see Table 4), using a 5-point Likert scale ranging from “highly unlikely” to “highly likely.” Panelists judged each event independently before reconciling their consensus judgment by discussion. A consensus rating above the Likert scale midpoint was used as the lower boundary for the determination of a process of care failure. Interrater agreement was satisfactory (65–94% agreement).
Table 4.
Process of Care Failures
| Number with process failure/number eligible for analysis | ||
|---|---|---|
| n/N* | % | |
| Patient-related failures | ||
| Patient did not report symptoms or seek care in a timely fashion | 12/92 | 13.0 |
| Diagnostic/laboratory tests ordered but not performed due to patient-related factors | 10/78 | 12.8 |
| Patient did not adhere to follow-up plans | 3/95 | 3.2 |
| Provider-related failures | ||
| Clinician failed to obtain adequate medical history | 4/94 | 4.3 |
| Clinician failed to perform adequate physical exam | 13/93 | 14.0 |
| Appropriate diagnostic/laboratory tests not ordered | 10/90 | 11.1 |
| Diagnostic/laboratory tests ordered but not performed due to provider-related factors | 0/77 | 0 |
| Provider did not receive diagnostic/laboratory test results | 1/99 | 1.0 |
| Patient did not receive diagnostic/laboratory test results | 0/99 | 0 |
| Provider did not set up appropriate follow-up plans | 2/95 | 2.1 |
| Number of patients with at least one process failure (N = 102) | 26 | 25.5 |
| Patient-related process failure only | 6 | |
| Provider-related process failure only | 6 | |
| Patient and provider-related failure | 14 | |
| Number of process failures per patient (N = 102) | ||
| 0 | 76 | 74.5 |
| 1 | 7 | 6.9 |
| 2 | 11 | 10.8 |
| 3 | 6 | 5.9 |
| 4 | 2 | 2.0 |
| Total number of process failures | 55 | |
| Number of provider-related process failures | 30 | |
| Number of patient-related process failures | 25 | |
| Number of process failures per 100 patients | 54 | |
*N varies due to incomplete data
We also examined factors that we hypothesized to be associated with process of care failures based on previous research and focus group discussions. We abstracted from CRIS patient age, race, ethnicity, Ashkenazi descent, primary language, education, insurance type, employment status, number of household members, functional status, and the presence of previous cancer– information originally collected on the patient intake survey. The chart reviewer abstracted the following information from the medical record: cancer diagnosis, primary care physician (PCP) training status at the time of diagnosis, number of years the patient had been cared for by the PCP and at the PCP’s practice site, whether the patient had been followed by an obstetrician/gynecologist (OB/GYN), the patient’s initial presentation of breast cancer (e.g., abnormal mammogram, lump, etc.), the provider who first became aware of the breast problem, and the mechanism by which they became aware (e.g., patient report, abnormal study, etc.). We ascertained PCP gender and years since medical school graduation by searching the online physician profiles of the Massachusetts Board of Registration in Medicine.
We used chart review data to corroborate information provided by the patient regarding date of symptom onset. We identified discrepancies in two cases involving patients who presented with advanced disease. In one case, the date of symptom onset was found to be 2 weeks later than the patient-reported date; in the other case, symptom onset was 4 months earlier.
Data Analysis
We tabulated patients’ sociodemographic characteristics, their primary care providers’ age, gender, and level of training, and information about disease presentation. Characteristics of cases with one or more process failures were compared to characteristics of cases with none, using Fisher’s exact test for categorical variables and the Wilcoxon-Mann-Whitney test for continuous variables.
Multivariate analysis using the total number of process failures per patient as the outcome measure was performed using stepwise Poisson regression with forward selection (p < 0.05), clustered by primary care provider. Independent variables included in the model were those risk factors found to be significantly related to presence or absence of process breakdowns in bivariate analyses, and included age, racial minority (vs. white), Hispanic (vs. not), education (no college vs. some), insurance (Medicaid/self-insured vs. other), number of household members, presence of a second-degree relative with postmenopausal breast cancer, PCP gender, PCP training level (resident physician vs. attending), and presence or absence of care by an OB-GYN physician. Due to missing data elements, the number of observations in the stepwise regression was 88 of a possible 102.
The study was approved in advance by the institutional review board of the Dana-Farber Harvard Cancer Center. Analyses were performed using Stata 9.2 (StataCorp, College Station, TX).
RESULTS
Patient Characteristics and Disease Presentation
Table 1 shows the characteristics of the 102 study patients. Patients were referred to the cancer centers by 57 providers from 16 primary care practices. Subjects’ median age was 55.3 years, and approximately one-third were non-white. Most patients spoke English as their first language, and about half had a college-level education. Patients with Medicaid or self-insurance made up 10% of the cohort.
Table 1.
Characteristics of Patients, by Presence or Absence of Process of Care Failures
| Total (N = 102) | No process failures (N = 76) | Process failures (N = 26) | P-value | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Age (median ± SD) | 55.4 ± 13.1 | 54.2 ± 12.8 | 61.5 ± 13.4 | 0.096 |
| Race | 0.044 | |||
| White | 71 (69.6) | 58 (76.3) | 13 (50) | |
| Black | 15 (14.7) | 9 (11.8) | 6 (23.1) | |
| Asian | 2 (2.0) | 1 (1.3) | 1 (3.9) | |
| Other/ unknown | 14 (13.8) | 8 (10.5) | 6 (23.1) | |
| Hispanic descent | 0.094 | |||
| Yes | 8 (7.8) | 4 (5.3) | 4 (15.4) | |
| No | 87 (85.3) | 68 (89.5) | 19 (73.1) | |
| Unknown | 7 (6.7) | 4 (5.3) | 3 (11.5) | |
| Ashkenazi descent | 0.016 | |||
| Yes | 13 (12.8) | 13 (17.1) | 0 (0) | |
| No | 78 (76.5) | 55 (72.4) | 23 (88.5) | |
| Unknown | 11 (10.8) | 8 (10.5) | 3 (11.5) | |
| Primary language | 0.279 | |||
| English | 90 (88.2) | 69 (90.8) | 21 (80.8) | |
| Spanish | 4 (3.9) | 2 (2.6) | 2 (7.7) | |
| Other | 3 (2.9) | 2 (2.6) | 1 (3.9) | |
| Unknown | 5 (4.9) | 3 (4.0) | 2 (7.7) | |
| Education | 0.008 | |||
| Graduate or professional | 30 (29.4) | 26 (34.2) | 4 (15.4) | |
| College | 25 (24.5) | 21 (27.6) | 4 (15.4) | |
| Vocational/technical | 7 (6.9) | 7 (9.2) | 0 (0) | |
| High school | 24 (23.5) | 12 (15.8) | 12 (46.2) | |
| Grade school | 4 (3.9) | 2 (2.6) | 2 (7.7) | |
| Other/unknown | 12 (11.8) | 8 (10.5) | 4 (15.4) | |
| Insurance type | 0.003 | |||
| Managed care | 43 (42.2) | 35 (46.1) | 8 (30.8) | |
| Indemnity | 17 (16.7) | 15 (19.7) | 2 (7.7) | |
| Self pay | 3 (2.9) | 3 (4.0) | 0 (0) | |
| Medicaid | 7 (6.9) | 1 (1.3) | 6 (23.1) | |
| Medicare | 32 (31.4) | 22 (29) | 10 (38.5) | |
| Employment status | 0.493 | |||
| Employed full/part time | 40 (39.2) | 31 (40.8) | 9 (34.6) | |
| Unemployed | 9 (8.8) | 8 (10.5) | 1 (3.9) | |
| Retired | 22 (21.6) | 14 (18.4) | 8 (30.8) | |
| Other | 23 (22.6) | 18 (23.7) | 5 (19.2) | |
| Unknown | 8 (7.8) | 5 (6.6) | 3 (11.5) | |
| Household members | 0.018 | |||
| 0 | 49 (48.0) | 36 (47.4) | 13 (50.0) | |
| 1 | 25 (24.5) | 15 (19.7) | 10 (38.5) | |
| 2 or more | 27 (26.5) | 25 (32.9) | 2 (7.7) | |
| Unknown | 1 (0.98) | 0 (0) | 1 (3.9) | |
| Physical status | 0.119 | |||
| Fully active | 55 (53.9) | 44 (57.9) | 11 (42.3) | |
| Can walk and take care of self | 8 (7.8) | 6 (7.9) | 2 (7.7) | |
| Needs some help | 4 (3.9) | 1 (1.3) | 3 (11.5) | |
| Restricted in strenuous activity | 26 (25.50) | 19 (25) | 7 (26.9) | |
| Unknown | 9 (8.8) | 6 (7.9) | 3 (11.5) | |
| Previous cancer (any type) | 0.481 | |||
| Yes | 6 (5.9) | 4 (5.3) | 2 (7.7) | |
| No | 96 (94.1) | 72 (94.7) | 24 (92.3) |
As shown in Table 2, two-thirds of patients had a female primary care provider, and most were cared for by attending (rather than resident) physicians. In most cases, a single PCP cared for a given patient for at least 1 year, but 11 patients had been with their providers for less than 1 year prior to diagnosis. Thirty-eight patients had an identifiable OB/GYN in addition to their PCP, but few of those specialists used an EMR accessible to study investigators.
Table 2.
Provider-related Patient Characteristics, by Process of Care Failures
| Total (N = 102) | No process failures (N = 76) | Process failures (N = 26) | P-value | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| PCP* gender | 0.001 | |||
| Female | 68 (66.7) | 58 (76.3) | 10 (38.5) | |
| Male | 33 (32.4) | 18 (23.7) | 15 (57.7) | |
| Unknown | 1 (1.0) | 0 (0) | 1 (3.9) | |
| PCP training status | 0.041 | |||
| Attending | 91 (89.2) | 71 (93.4) | 20 (76.9) | |
| Resident | 9 (8.8) | 4 (5.3) | 5 (19.2) | |
| Unknown | 2 (2.0) | 1 (1.3) | 1 (3.9) | |
| Years since PCP medical school graduation (median ± SD) | 18 ± 11.0 | 19 ± 11.2 | 14 ± 10.1 | 0.231 |
| Number of years cared for at PCP’s practice site | 0.282 | |||
| <1 year | 6 (5.9) | 4 (5.3) | 2 (7.7) | |
| 1–5 years | 44 (43.1) | 36 (47.4) | 8 (30.8) | |
| 6+ years | 27 (26.5) | 18 (23.7) | 9 (34.6) | |
| Unknown | 25 (24.5) | 18 (23.7) | 7 (26.9) | |
| Number of years cared for by PCP | 0.292 | |||
| <1 year | 11 (10.8) | 7 (9.2) | 4 (15.4) | |
| 1–5 years | 49 (48.0) | 39 (51.3) | 10 (38.5) | |
| 6+ years | 23 (22.6) | 15 (19.7) | 8 (30.8) | |
| Unknown | 19 (18.6) | 15 (19.7) | 4 (15.4) | |
| Does patient have an OB/GYN†? | 0.016 | |||
| Yes | 38 (37.3) | 33 (43.4) | 5 (19.2) | |
| No | 51 (50.0) | 33 (43.4) | 18 (69.2) | |
| Unknown | 13 (12.8) | 10 (13.2) | 3 (11.5) | |
| If patient has an OB/GYN, are OB/GYN’s records in EMR? (n = 38) | 0.120 | |||
| Yes | 5 (13.2) | 3 (9.1) | 2 (40.0) | |
| No | 33 (86.8) | 30 (90.9) | 3 (60.0) | |
| Are patient’s radiology records in EMR? | 0.190 | |||
| Yes | 84 (82.4) | 63 (82.9) | 21 (80.8) | |
| No | 12 (11.8) | 7 (9.2) | 5 (19.2) | |
| Unknown | 6 (5.9) | 6 (7.9) | 0 (0) |
*Primary care physician
†Obstetrician/gynecologist
The median time from symptom onset to diagnosis was 27 days (range 0–77) in the ≥ stage II disease group and 148 days (range 91–802) in the delayed diagnosis group. The median time from diagnosis to treatment was 26 days (range 0–238). As show in Table 3, the most common initial presentations were a mass found on self-exam or clinical breast exam, or as the result of a screening mammogram.
Table 3.
Presentation of Breast Problem Leading to Cancer Diagnosis
| Total (N = 102) | No process failures (N = 76) | Process failures (N = 26) | P-value | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Initial presentation | 0.806 | |||
| Abnormal mammogram | 38 (37.3) | 27 (35.5) | 11 (42.3) | |
| Lump in breast found by self | 41 (40.2) | 31 (40.8) | 10 (38.5) | |
| Lump in breast found by clinician | 11 (10.8) | 9 (11.8) | 2 (7.7) | |
| Auxiliary mass | 2 (2.0) | 2 (2.6) | 0 (0) | |
| Inverted nipple | 2 (2.0) | 1 (1.3) | 1 (3.9) | |
| Nipple discharge | 1 (1.0) | 1 (1.3) | 0 (0) | |
| Breast pain | 2 (2.0) | 2 (2.6) | 0 (0) | |
| Other | 4 (3.9) | 2 (2.6) | 2 (7.7) | |
| Unknown | 1 (1.0) | 1 (1.3) | 0 (0) | |
| Provider who first became aware of breast problem | 0.906 | |||
| PCP* (including covering PCP, NP† at PCP’s office) | 37 (36.3) | 27 (35.5) | 10 (38.5) | |
| OB/GYN‡ (including covering OB/GYN and NP at OB/GYN’s office) | 18 (17.7) | 15 (19.7) | 3 (11.5) | |
| Surgeon | 3 (2.9) | 2 (2.6) | 1 (3.9) | |
| Radiologist | 37 (36.3) | 27 (35.5) | 10 (38.5) | |
| Other | 4 (3.9) | 3 (4.0) | 1 (3.9) | |
| Unknown | 3 (2.9) | 2 (2.6) | 1 (3.9) | |
| How did provider first become aware of breast problem? | 0.074 | |||
| Patient reported problem | 48 (47.1) | 38 (50.0) | 10 (38.5) | |
| Provider noted problem | 10 (9.8) | 8 (10.5) | 2 (7.7) | |
| Abnormal mammogram | 39 (38.2) | 29 (38.2) | 10 (38.5) | |
| Abnormal ultrasound | 1 (1.0) | 0 (0) | 1 (3.9) | |
| Other | 4 (3.9) | 1 (1.3) | 3 (11.5) | |
| Stage at cancer diagnosis | 0.019 | |||
| I | 11 (10.8) | 7 (9.2) | 4 (15.4) | |
| II | 72 (70.6) | 58 (76.3) | 14 (53.9) | |
| III | 8 (7.8) | 6 (7.9) | 2 (7.7) | |
| IV | 7 (6.9) | 2 (2.6) | 5 (19.2) | |
| Unknown | 4 (3.9) | 3 (4.0) | 1 (3.9) |
*Primary care physician
†Nurse practitioner
‡Obstetrician/gynecologist
Process of Care Failures
Twenty-six (25%) of the 102 study patients had at least one process of care failure; 19 (18%) patients had two or more. Table 4 shows that the most common process breakdowns occurred when physicians failed to perform an adequate physical examination, when patients failed to report symptoms or seek care in a timely fashion, and when patients failed to complete diagnostic or laboratory tests. Overall, process failures were attributed in similar numbers to providers and to patients (n = 30 vs. n = 25, respectively). Longer time intervals between diagnosis and treatment were associated with an increased likelihood of experiencing at least one process of care failure (median 25 vs. 33.5 days, p = 0.005). Patients with only provider-related process breakdowns had a median interval of 21.5 days between symptom onset and diagnosis compared to patients with only patient-related process breakdowns, for whom the median interval was 128.5 days (p = 0.002). Table 5 provides case examples that illustrate these process of care failures.
Table 5.
Examples of Process Failures in Breast Cancer Care
| Process failure | Example |
|---|---|
| Patient-related failures | |
| Patient did not report symptoms or seek care in a timely fashion | The patient noticed a lump in her right breast, but because she was self-employed and without health insurance, she had no regular physician. She waited 4 months to see an obstetrician/gynecologist, at which time a 2-cm right breast mass was detected and later confirmed as invasive carcinoma |
| Diagnostic/laboratory tests ordered but not performed due to patient-related factors | The patient underwent a screening mammogram, which was abnormal. She was referred to a breast center, but over the next 5 months failed to show up for numerous appointments. The patient eventually had a biopsy showing stage I disease |
| Patient did not adhere to follow-up plans | The patient’s routine screening mammogram was abnormal, but, according to primary care physician, she “did not follow through” with her appointment with a breast surgeon. The primary care physician noted 6 months later that the patient was “not worried” about the findings. A biopsy was performed almost 2 years after the initial mammogram, showing breast cancer |
| Provider-related failures | |
| Clinician failed to obtain adequate medical history | The patient noted a lump in her breast and sought attention at an outside institution (records not in electronic medical record), but was unable to schedule a mammogram for 6 months (reasons unknown). During this interval, the patient did see her primary care physician, but the chart note does not mention either the breast problem or a clinical breast exam |
| Clinician failed to perform adequate physical exam | The patient lost approximately 40 lbs over a 10-month span, at the end of which a mammogram was done (BIRADS 5). During this period, she had several primary care physician appointments, but a clinical breast exam was not documented. When a core needle biopsy was finally performed, it revealed invasive, metastatic carcinoma (stage IV) |
| Appropriate diagnostic tests not ordered | Although this elderly patient received two breast exams in the year prior to her cancer diagnosis, there is no record of a mammogram or ultrasound being ordered or performed in that year or the two preceding ones. When a routine mammogram was finally performed, it uncovered a spiculated mass, confirmed to be cancerous upon biopsy (stage II) |
| Provider did not receive test results | The elderly patient had an abnormal screening mammogram and a core biopsy a few weeks later, which revealed invasive carcinoma. The radiologist was not able to contact the PCP or other physician coverage at the time of the mammogram, and it is unclear when the patient’s PCP received this information. Although the patient underwent yearly mammograms prior to her diagnosis, her last documented clinical breast exam was more than 5 years prior to diagnosis |
| Provider did not set up appropriate follow-up plans | After an abnormal mammogram, the patient was referred to a breast surgeon, who scheduled a wire localization biopsy for the following month. The patient cancelled that appointment and the following one. The obstetrician/gynecologist wrote in the patient’s chart that “pt tried to reschedule but unable to.” The breast surgeon notes that the patient “was previously scheduled for wire localization biopsy, but canceled because of her discomfort. She was allowed to wait for an extended period without pain medication.” When finally performed, the biopsy revealed invasive carcinoma with mixed lobular and ductal features |
Risk Factors
As shown in Table 1, process of care failures were more likely among non-white patients, those without a college degree, and patients whose primary care provider was male or a resident physician of either gender. Process failures were particularly common among patients on Medicaid compared to those with other types of insurance, and among patients presenting with stage IV disease versus less advanced stage cancers. Patients without an OB/GYN were also more likely to experience breakdowns in the breast care process. In the multivariate Poisson regression, process of care failures were more likely when the patient’s PCP was male [incident rate ratio (IRR) 2.8, 95% CI 1.2 to 6.5] and when the patient was non-white (IRR 2.8, 95% CI 1.4 to 5.7), controlling also for Hispanic ethnicity (IRR 2.7, 95% CI 1.0 to 7.3).
DISCUSSION
In this study of 102 women who presented with a diagnostic delay of at least 90 days or at least stage II breast cancer, we found that process of care failures affected about one in four patients. Process breakdowns spanned all phases of the breast care process. About half were attributable to clinicians and their practices, delaying diagnosis by approximately 3 weeks; the remaining breakdowns were related to patients’ failure to seek care for initial symptoms in a timely manner or to follow through on recommended screenings or diagnostic tests, resulting in a median delay of more than 4 months. In the multivariate analysis, process failures were more likely when the primary care clinician was male and when the patient was non-white. Since this study was not based on malpractice claims data, it provides insight into how often patient factors, as well as failures in the medical system, contribute to a delayed diagnosis of breast cancer.
Our results inform and extend malpractice claims-based studies that document multiple deficiencies in the diagnostic process. Process failures in our study affected about one-quarter of patients, similar to the 29 percent reported in a malpractice claims study10. We also found that patients often experienced multiple process failures of varying types, corroborating findings from emergency department and ambulatory studies suggesting that the majority of medical errors involve two or more process breakdowns2,6.
Our study also substantiated findings demonstrating disparities in the breast care process associated with clinician gender11–13. While the benefits of self breast exams and clinical breast exams are controversial14, Lurie and colleagues showed that female physicians are more likely to talk with patients about breast cancer prevention, to believe in the effectiveness of mammography, and to feel comfortable performing clinical breast examinations13. Males, on the other hand, may have a tendency to defer breast examinations to nurse practitioners or OB/GYN physicians.
In contrast to previous work, our study identified patient behavior as a prominent component of process failures2,6. In 20 of 26 cases (77%), patients’ behavior contributed, at least in part, to suboptimal breast cancer care. This occurred when patients discovered findings at home but did not report them in a timely manner, or when they failed to complete recommended mammograms or adhere to their providers’ suggested follow-up plan.
How should we understand patients’ contributions to diagnostic errors in breast care? Denial may cause some patients to delay seeking treatment for suspicious symptoms or to fail to follow up on physician recommendations15,16. In addition, process failures in our study were more common among non-whites and Medicaid recipients, indicating a sociodemographic linkage that may be mediated by poor health literacy or practical obstacles17–24. For some patients, failure to report a breast lump or to adhere to a physician’s recommendation may indicate a lack of understanding of the significance of the finding or the urgency of the recommendation. Other patients may face financial constraints, transportation difficulties, or language barriers that could affect a patient’s decision to delay seeking care, as suggested by a study of low income women in Los Angeles23.
Do diagnostic delays affect clinical outcomes? The evidence seems to suggest that patient delay (defined in most studies as the duration between onset of symptoms and the first medical consultation) is associated with worse prognosis, whereas provider delay (defined as duration between first consultation and biopsy or start of treatment) is either neutral25–27 or favorable28. Afzelius and colleagues found that patient delays of >60 days resulted in poorer outcomes compared to short delays (0–14 days)28, and in a population-based study of 287 German women, Arndt and colleagues found that patient delay (categorized as <1 month, 1–3 months, and >3 months) was positively correlated with stage at diagnosis for poorly differentiated tumors (p = 0.03)29. In contrast, provider delays of 3 months or more were not associated with decreased survival in several studies25–28, perhaps because indolent tumors were more likely to be diagnosed late.
Our findings therefore offer several implications for clinical care. To address practice-level process failures, clinicians and practice administrators must ensure that mechanisms are in place to coordinate diagnostic screenings among multiple providers, to facilitate the follow-up and communication of critical test results, and to direct the implementation of appropriate care plans. To that end, several “best practices” have been developed encouraging the use of information technology to help streamline the communication process30.
To address patient-related process failures, clinicians and health-care organizations should create a more robust infrastructure to support the patients’ role in breast care. More effective outreach programs are needed to educate patients about findings suggestive of a breast abnormality, the need for regular mammography, and the urgency of adhering to follow-up plans. These educational interventions must be tailored to at-risk populations, targeting women whose languages, cultures, or social situations make it difficult for them to understand or implement optimal breast care18. A number of such interventions, including patient navigator programs and community-based outreach efforts, have shown some success,31,32 although further research is needed to determine which combination of initiatives is most efficacious for a given population.
This study has several limitations. First, the sample was selected from two Boston teaching hospitals and therefore may not be representative of a general population of women with breast cancer. However, the study hospitals treat almost half of all Boston residents with cancer, including one-third of the city’s racial minorities with cancer. Additionally, patients in this study were referred from within the hospitals’ wide primary care network, potentially mitigating the referral bias associated with cancer centers that provide primarily second opinion consultations. Second, the study is limited by the selection of cases from primary care practices with an EMR. While this approach facilitated data abstraction, it may limit the generalizability of the results beyond sites with electronic records. The EMR offers access to electronic reminders that may improve the quality of breast care, as well as easy access to electronic notes of providers who share the system. These features would presumably reduce the rate of missed and delayed diagnoses and underestimate the rate of process failures in breast care. Similarly, exclusion of cases with limited clinician notes in the EMR may have resulted in selection bias that underestimated the rate of process failures if the clinicians most meticulous about their notes were also more conscientious caregivers. Third, the rate of process failures derives from a study cohort of women with self-reported delays or presentation with at least stage II disease. These rates cannot be readily generalized to other patient populations. Therefore, future studies are needed to create population-based estimates of the true incidence and prevalence of diagnostic errors. Nevertheless, these results identify vulnerabilities that may affect the care of patients in high-risk groups, and they corroborate results from malpractice claims-based studies, which suffer from additional biases8,33. Finally, the study is limited by the challenge of ascertaining lapses in care. We relied on implicit judgments by physician reviewers using medical record review abstractions and on the quality of documentation in the medical record itself. Recognizing the difficulty of these judgments, reviewers generally presumed that care was adequate, absent evidence of a significant error or omission8,34,35.
In spite of these potential limitations, our study demonstrates a high rate of process failures among patients with self-reported delays in cancer diagnosis and among those who presented with at least stage II disease. While many process failures were attributable to lapses in care rendered by frontline clinicians, patients themselves played a prominent role in about half of the failures. Improving breast cancer care, therefore, is a two-pronged challenge. It requires targeted interventions that educate and empower patients to monitor the symptoms that should trigger a visit to the physician, and it demands that clinicians be prepared to deliver high quality, coordinated care when those patients arrive.
Acknowledgements
Funding for this project was provided by a grant from the Risk Management Foundation of the Harvard Medical Institutions.
Conflict of interest None disclosed.
Footnotes
This study was presented as an abstract at the National Patient Safety Foundation 2007 Annual Patient Safety Congress, Washington, DC, May 2–4, 2007. Funding was provided by a grant from the Risk Management Foundation of the Harvard Medical Institutions.
References
- 1.Schaefer M. Overview of CRICO cancer-related diagnosis claims 1992–2001. RMF Forum. 2002;22:4–9.
- 2.Gandhi TK, Kachalia A, Thomas EJ, et al. Missed and delayed diagnoses in the ambulatory setting: a study of closed malpractice claims. Ann Intern Med. 2006;145:488–96. [DOI] [PubMed]
- 3.Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353:1119–26. [DOI] [PubMed]
- 4.Phillips RL Jr, Bartholomew LA, Dovey SM, Fryer GE Jr, Miyoshi TJ, Green LA. Learning from malpractice claims about negligent, adverse events in primary care in the United States. Qual Saf Health Care. 2004;13:121–6. [DOI] [PMC free article] [PubMed]
- 5.Chandra A, Nundy S, Seabury SA. The growth of physician medical malpractice payments: evidence from the national practitioner data bank. Health Aff (Millwood). 2005;Suppl Web Exclusives: W5-40–W5-49. [DOI] [PubMed]
- 6.Kachalia A, Gandhi TK, Puopolo AL, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from four liability insurers. Ann Emerg Med. 2007;49:196–205. [DOI] [PubMed]
- 7.Rogers SO Jr, Gawande AA, Kwaan M, et al. Analysis of surgical errors in closed malpractice claims at four liability insurers. Surgery. 2006;140:25–33. [DOI] [PubMed]
- 8.Studdert DM, Thomas EJ, Burstin HR, Zbar BI, Orav EJ, Brennan TA. Negligent care and malpractice claiming behavior in Utah and Colorado. Med Care. 2000;38:250–60. [DOI] [PubMed]
- 9.Burstin HR, Johnson WG, Lipsitz SR, Brennan TA. Do the poor sue more? A case-control study of malpractice claims and socioeconomic status. JAMA. 1993;270:1697–701. [DOI] [PubMed]
- 10.Thomas EJ, Studdert DM, Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38:261–71. [DOI] [PubMed]
- 11.Kreuter MW, Strecher VJ, Harris R, Kobrin SC, Skinner CS. Are patients of women physicians screened more aggressively? J Gen Intern Med. 1995;10:119–25. [DOI] [PubMed]
- 12.Lurie N, Slater J, McGovern P, Ekstrum J, Quam L, Margolis K. Preventative care for women—does the sex of the physician matter? N Engl J Med. 1993;329:478–82. [DOI] [PubMed]
- 13.Lurie N, Margolis KL, McGovern PG, Mink PJ, Slater JS. Why do patients of female physicians have higher rates of breast and cervical cancer screening? J Gen Intern Med. 1997;12:34–43. [DOI] [PMC free article] [PubMed]
- 14.Kösters JP, Gøtzsche PC. Regular self-examination or clinical examination for early detection of breast cancer. Cochrane Database of Systematic Reviews 2003, Issue 2. Art. No.: CD003373. doi:10.1002/14651858.CD003373. [DOI] [PMC free article] [PubMed]
- 15.Weinmann S, Taplin SH, Gilbert J, et al. Characteristics of women refusing follow-up for tests or symptoms suggestive of breast cancer. J Natl Cancer Inst. 2005;35:33–8. [DOI] [PubMed]
- 16.Remennick L. The challenge of early breast cancer detection among immigrant and minority women in multicultural societies. Breast J. 2006;12:103–10. [DOI] [PubMed]
- 17.Gorin SS, Heck JE, Cheng B, Smith SJ. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Arch Intern Med. 2006;166:2244–52. [DOI] [PubMed]
- 18.Taplin SH, Ichikawa L, Yood Mu, Manos MM, Geiger AM, Weinmann S, et al. Reason for late-stage breast cancer: absence of screening or detection, or breakdown in follow-up? J Natl Cancer Inst. 2004;96:1518–27. [DOI] [PubMed]
- 19.Halliday T, Taira DA, Davis J, Chan H. Socioeconomic disparities in breast cancer screening in Hawaii. Prev Chronic Dis. 2007;4:1–9. [PMC free article] [PubMed]
- 20.Ives DG, Lave JR, Traven ND, Schulz R, Kuller LH. Mammography and pap smear use by older rural women. Public Health Rep. 1996;111:244–50. [PMC free article] [PubMed]
- 21.Centers for Disease Control and Prevention. Use of mammography services by women aged ≥65 years in Medicare—United States, 1991–1993. MMWR Morb Mortal Wkly Rep. 1995;44:777–81. [PubMed]
- 22.Wells K, Roetzheim RG. Health disparities in receipt of screening mammography in Latinas: a critical review of recent literature. Cancer Control. 2007;14:369–79. [DOI] [PubMed]
- 23.Kaplan CP, Crane LA, Stewart S, Juarez-Reyes M. Factors affecting follow-up among low-income women with breast abnormalities. J Womens Health. 2004;13:195–206. [DOI] [PubMed]
- 24.Caplan LS, May DS, Richardson LC. Time to diagnosis and treatment of breast cancer: results from the National Breast and Cervical Cancer Early Detection Program, 1991–1995. Am J Public Health. 2000;90:130–4. [DOI] [PMC free article] [PubMed]
- 25.Tartter P, Pace D, Frost M, Bernstein JL. Delay in diagnosis of breast cancer. Ann Surg. 1999;229:91–6. [DOI] [PMC free article] [PubMed]
- 26.Sainsbury R, Johnston C, Haward B. Effect on survival of delays in referral of patients with breast-cancer symptoms: a retrospective analysis. Lancet. 1999;353:1132–35. [DOI] [PubMed]
- 27.Hardin C, Pommier S, Pommier RF. The relationships among clinician delay of diagnosis of breast cancer and tumor size, nodal status, and stage. Am J Surg. 2006;192:506–8. [DOI] [PubMed]
- 28.Afzelius P, Zedeler K, Sommer H, Mouridsen HT, Blichert-Toft M. Patient’s and doctor’s delay in primary breast cancer: Prognostic implications. Acta Oncol. 1994;334:345–51. [DOI] [PubMed]
- 29.Arndt V, Sturmer T, Stegmaier C, Ziegler H, Dhom G, Brenner H. Patient delay and stage of diagnosis among breast cancer patients in Germany—a population-based study. Br J Cancer. 2002;86:1034–40. [DOI] [PMC free article] [PubMed]
- 30.Hanna D, Griswold P, Leape LL, Bates DW. Communicating critical test results: safe practice recommendations. Jt Comm J Qual Patient Saf. 2005;31:68–80. [DOI] [PubMed]
- 31.Ell K, Vourlekis B, Lee PJ, Xie B. Patient navigation and case management following an abnormal mammogram: a randomized clinical trial. Prev Med. 2007;44:26–33. [DOI] [PubMed]
- 32.Heyding RK, Cheung AM, Mocarski EJ, Moineddin R, Hwang SW. A community-based intervention to increase screening mammography among disadvantaged women at an inner-city drop-in center. Women Health. 2005;41:21–31. [DOI] [PubMed]
- 33.Localio AR, Lawthers AG, Brennan TA, Laird NM, Hebert LE, Peterson LM, Newhouse JP, Weiler PC, Hiatt HH. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325:245–51. [DOI] [PubMed]
- 34.Brennan TA, Localio AR, Leape LL, et al. Identification of adverse events occurring during hospitalization. A cross-sectional study of litigation, quality assurance, and medical records at two teaching hospitals. Ann Intern Med. 1990;112:221–6. [DOI] [PubMed]
- 35.Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377–84. [DOI] [PubMed]