Abstract
Macrophages are unique innate immune cells that play an integral role in the defense of the host by virtue of their ability to recognize, engulf, and kill pathogens while sending out danger signals via cytokines to recruit and activate inflammatory cells. It is becoming increasingly clear that purinergic signaling events are essential components of the macrophage response to pathogen challenges and disorders such as sepsis may be, at least in part, regulated by these important sensors. The activation of the P2X7 receptor is a powerful event in the regulation of the caspase-1 inflammasome. We provide evidence that the inflammasome activation requires “priming” of macrophages prior to ATP activation of the P2X7R. Inhibition of the inflammasome activation by the tyrosine kinase inhibitor, AG126, suggests regulation by phosphorylation. Finally, the P2X7R may also be activated by other elements of the host response such as the antimicrobial peptide LL-37, which adds a new, physiologically relevant agonist to the P2X7R pathway. Therapeutic approaches to inflammation and sepsis will certainly be enhanced by an increased understanding of how purinergic receptors modulate the inflammasomes.
Keywords: Macrophage, IL-18, LPS, Priming, AG126, Tyrosine kinase
Introduction
Purinergic receptors of the P2X7 form have revolutionized our concepts about the mechanisms involved in the activation of caspase-1. The ability of exogenous pathogen-associated molecular patterns, such as lipopolysaccharide (LPS), to induce the processing and release of interleukin (IL)-1β has been part of the IL-1β literature since IL-1’s discovery as endogenous pyrogen [1, 2]. However, the relatively recent recognition of the marked potentiation of the LPS effect by P2X7 receptor activation has transformed our understanding of host cells’ ability to sense danger and respond. In this review we will focus on the role of P2X7 receptor activation in the modulation of monocyte and macrophage caspase-1 activation.
Human monocytes express P2X7 receptors
Human monocytes express four- to fivefold higher levels of P2X7 receptors than lymphocytes when analyzed by flow cytometry [3]. Furthermore, monocytes further increase the numbers of surface receptors as they differentiate into macrophages [4]. Flow cytometry of fresh human monocytes, gating on the CD14 monocyte marker, reveals that both baseline and LPS-treated monocytes have similar levels of surface expressed P2X7 [5] confirming previous data showing expression on 1-day-old monocytes [6]. The size of monocyte P2X7R is 75 kDa by immunoblotting [7] but the detection is weak. In fact we have seen a 55-kDa P2X7 form when immunoblotting freshly harvested blood monocytes but this may represent a cleavage artifact from monocyte proteases [5].
Fresh monocytes are potent releasers of mature IL-1β upon endotoxin activation when given high-dose LPS. LPS at 10 μg/ml will induce the release of mature IL-1β within 2–4 h of stimulation. However, low-dose LPS (e.g., 1 ng/ml) releases only minimal amounts of mature IL-1β but produces near maximum quantities of the precursor form of IL-1β, the 31-kDa proIL-1β.
Monocytes induced to produce the 31-kDa proIL-1β can be induced to release large amounts of processed 17-kDa mature IL-1β with the addition of the P2X7 receptor agonist, ATP [8–11]. Monocytes primed for 3 h with 1 ng/ml of LPS and then given a 30-min pulse with 5 mM ATP release 10- to 20-fold higher amounts of processed, mature IL-1β than monocytes treated with high-dose LPS alone [5]. This ATP-mediated effect is blocked by oATP. Thus, it is clear that the monocyte P2X7 receptors are functional.
Priming required for ATP-induced caspase-1 activation
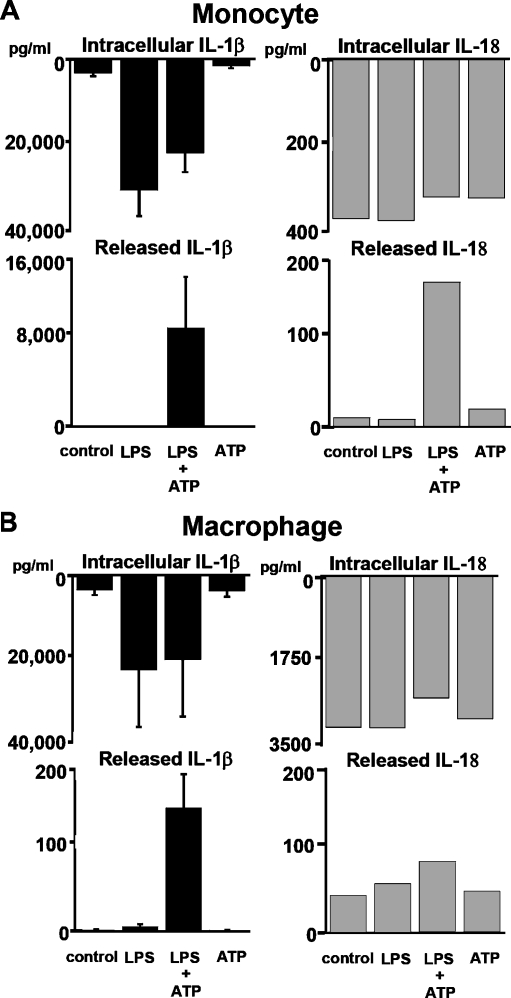
P2X7 activation: requirement for priming This LPS priming step is necessary as ATP stimulation alone will not induce IL-1β release. Indeed LPS priming is needed to induce sufficient intracellular proIL-1β substrate for the ATP-induced caspase-1 activation. However, substrate generation is not the only function of the priming step. This fact is derived from studies with the other well-recognized substrate of active caspase-1, IL-18 [12]. Fortunately for the study of priming’s role in the ATP-mediated activation of caspase-1, IL-18 is constitutively present in fresh monocytes. Thus, if priming were simply a means to generate substrate for the P2X7-mediated caspase-1 activation, then one would anticipate that ATP stimulation alone would generate active processed IL-18. This is not the case. As outlined in Fig. 1a, although fresh human monocytes contain preformed intracellular proIL-18, they release no processed IL-18 unless preactivated, in this case with LPS.
Fig. 1.
Caspase-1-dependent processing and release of IL-1β and IL-18. a “Priming” required of monocytes. Fresh human monocytes purified by CD14 magnetic beads (n = 3) cultured at 106 cells/ml induce abundant levels of intracellular proIL-1β (lysed at 106 cells/ml) in response to lipopolysaccharide (LPS) but require additional stimulation with ATP to process and release IL-1β. In contrast, these same monocytes constitutively contain IL-18, but they still require LPS priming for the ATP effect on processing and release. b “Priming” blunted in alveolar macrophages. Human lung alveolar macrophages from the same individuals in a obtained by bronchoalveolar lavage show blunted response to LPS priming for ATP-induced processing and release of both IL-1β and IL-18 (representative experiment). However, macrophages have similar levels of induced intracellular IL-1β and almost log-fold higher concentrations of intracellular IL-18
Time of priming event As early as 15–30 min after LPS priming, presumably too early for significant new proteins synthesis, monocytes respond to ATP inducing near maximum processing and release of mature IL-18. The exact processes involved in the priming event remain poorly understood.
P2X7 activation inhibited by tyrphostin AG126
Attempting to characterize the signaling events that are induced by ATP, we previously tested a battery of kinase inhibitors for their ability to block ATP-induced activation of IL-1β processing by primed monocytes [5]. As shown in Table 1, one tyrosine kinase inhibitor, AG126, a synthetic homologue of erbstatin, potently inhibits the ATP-induced caspase-1 activation event even when given after the LPS-induced priming [5, 13]. This finding is particularly intriguing since it had been shown that AG126 can prevent sepsis-induced death in a murine model of sepsis and can block chronic inflammation [14, 15]. In this same vein, we have recently noted that apoptosis of splenic B lymphocytes is characteristic of the murine response to sepsis and that this apoptosis is in part dependent upon caspase-1 activation since it is absent in caspase-1 knockout animals [16]. Consistent with the possibility that the sepsis protection of AG126 is due to its inhibition of ATP-induced caspase-1 activity, we have shown that intraperitoneal AG126 can also prevent splenic cell apoptosis in murine sepsis as well (Fig. 2). Although there is no evidence directly connecting AG126 to the P2X7 receptor, recent work by Shemon et al. further supports the notion that the P2X7 receptor functions via tyrosine kinase activation [17]. Furthermore, they show that B lymphocytes may be targeted via their own P2X7 receptors. Of note, however, Shemon et al.’s protein tyrosine kinase inhibitor works on the extracellular side of the P2X7 receptor and therefore may function by competing with ATP at the extracellular binding site. There is a highly conserved tyrosine residue in the carboxy terminus of the P2X7 receptor near the second transmembrane region which appears to affect its channel function [18]. The role of AG126 in this regard is still unknown. We have unpublished data that AG126 can slow the spontaneous activation of lysed THP-1 caspase-1 that was first described by Miller et al. [19]. However, this does not prove a connection to the P2X7R nor whether the AG126 effect is intracellular or extracellular. Since it is not known if this P2X7 tyrosine is the target of AG126, future investigations into the target(s) of AG126 will be of considerable interest for therapeutics.
Table 1.
Effect of selected protein kinase inhibitors on ATP-mediated release of IL-1β from LPS-primed monocytesa
| Stimulator/inhibitor | Concentration of inhibitors | % Inhibition |
|---|---|---|
| LPS + ATP | 0 | |
| Me2SO | 0.01% | 0 |
| Genistein | 50 μM | 7 |
| AG1296 | 1.0 μM | 0 |
| AG1478 | 10.0 μM | 4 |
| AG126 | 50.0 μM | 88 |
| PPI | 1.0 μM | 0 |
| Herbimycin A | 1.0 μg/ml | 35 |
aAdapted from [5]. Cells stimulated with 1 ng/ml of LPS for 3 h, treated with the inhibitors for 30 min, and then stimulated with ATP 5 mM for 30 min. Supernatants harvested and assayed for IL-1β. Date is percent inhibition relative to LPS + ATP
Fig. 2.
Tyrphostin AG126 protects mice from sepsis mortality and splenic cell apoptosis. Since AG126 blocks the ATP-induced (i.e., P2X7-mediated) processing of IL-1β and IL-18, we tested AG126 for its ability to inhibit E. coli-induced mortality (top) and splenic apoptosis at 24 h post challenge (bottom). In the survival experiment, mice were pretreated with AG126 or dimethyl sulfoxide (DMSO) control i.p. followed by live E. coli 108 cfu per animal and followed for survival
Macrophages less responsive than monocytes to P2X7 agonists
Alveolar macrophages are frontline pathogen sensors that live in the alveolar space where they encounter inhaled particulates and pathogens. These macrophages rapidly engulf pathogens and either kill them or send out cytokine responses that recruit assistance from neutrophils and lymphocytes. It is believed that each alveolus contains numerous macrophages [20]. It is remarkable that in general these macrophages are able to deal with the massive challenge of eliminating inhaled particulate and pathogens without causing untoward inflammation. Importantly, P2X7 receptor function has been linked to the ability of macrophages to handle pathogens. For example, P2X7 receptor activation induces macrophages to kill one of the most lung-adapted pathogens, i.e., intracellular Mycobacterium [21].
Macrophages derive predominantly from circulating blood monocytes which are short-lived descendants of bone marrow precursors that circulate for 24–48 h before taking up tissue residence and differentiating into macrophages or being eliminated by programmed cell death [22–24]. In this context, we have long been interested in the relative ability of lung macrophages versus blood monocytes to respond to pathogen stimulation. Alveolar macrophages do sense bacterial endotoxins. For example, normal human lung macrophages stimulated with 10 μg/ml of E. coli LPS release significant amounts of tumor necrosis factor (TNF) but surprisingly little IL-1β [25]. However, fresh blood monocytes given the same LPS challenge are excellent producers of IL-1β but release more limited quantities of TNF [25, 26]. Importantly, the relative ability of alveolar macrophages to produce IL-18 and IL-1β is intact. Alveolar macrophage intracellular proIL-1β is similar to that of monocytes. Intracellular proIL-18 is even more abundant in alveolar macrophages and is present constitutively, i.e., without priming (Fig. 1b). Nevertheless, macrophages seem to lack some of the upstream events necessary to activate the P2X7 receptor-mediated activation of the inflammasome as evidenced by the minimum response to priming and ATP. This monocyte/macrophage difference in IL-1β processing and release remains poorly understood despite more that two decades of recognition. It suggests additional regulatory elements that remain to be characterized.
Alternative means of activating P2X7: protegrin and cathelicidin
ATP’s effect on the P2X7 receptor is maximum at relatively high concentrations of extracellular ATP. Concentrations in the 1–5 mM range, i.e., equivalent to the intracellular concentration of ATP, are typically used experimentally for caspase-1 activation experiments [9, 10, 27]. These high concentration requirements for ATP raise the issue about whether these levels are achievable during physiological regulation of the P2X7 receptor. In the microenvironment of cell compartments it is possible that local release of ATP from adjacent cells (e.g., platelets [28]) or even autocrine release induced by activating agents such as LPS [29, 30] may provide ATP concentrations that approach the 1–5 mM range. However, much remains to be uncovered about how the P2X7 receptors work under normal physiological conditions.
In the context of IL-1β/IL-18 processing and release, ATP induces the release of potassium, an event that is dependent upon pore formation and loss of intracellular vs extracellular potassium gradients. In a yet to be fully understood mechanism, ATP-induced loss of intracellular potassium induces the activation of the NALP3 (NLRP3) inflammasome followed by the rapid activation of caspase-1 and the processing and release of IL-1β and IL-18 [31]. Blocking the potassium efflux by exogenous potassium, however, blocks the ability of ATP to induce IL-1β/IL-18 processing [9, 13]. Thus, a major outcome of the P2X7 receptor activation event appears to be the induction of pores that allow potassium escape. Recently, the identification of pannexin-1 as a P2X7 receptor-activated pore follows this theme [32]. Perregaux and Gabel have shown that several agents that are capable of inducing pores independently of P2X7 receptors can induce the activation of caspase-1 and IL-1β processing and release [9]. The antibiotic ionophore nigericin induces inflammasome activation by virtue of its ability to efflux potassium from cells [33]. More recently, the role of pannexin-1 in the activation of caspase-1 has further expanded. Pelegrin and Surprenant have shown that inhibition of pannexin-1 can block caspase-1 activation even in the presence of potassium efflux suggesting that it functions downstream of potassium’s efflux [34]. Interestingly, pannexin-1 may also function to bring PAMPs into the cytosol for pathogen sensing [35].
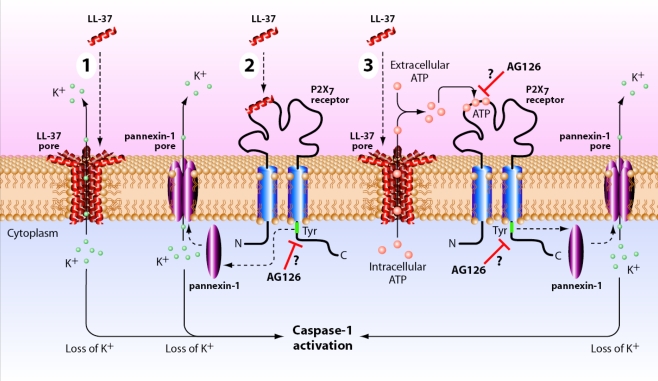
Cathelicidin as pore inducer If ionophores can induce inflammasome activation by inducing potassium efflux, then antimicrobial peptides might do the same. Antimicrobial peptides are particularly interesting since their release is a physiologic response of the host to pathogen-induced TLR and NLR signals [36, 37]. This relevance was confirmed with protegrins, which are neutrophil antimicrobial products that when applied to LPS-activated human monocytes induce the rapid processing and release of IL-1β [38]. Akin to the ionophore nigericin’s mechanism, the protegrin function was shown to be due to their pore-forming function because eliminating the efflux of potassium prevents the inflammasome activation event [38].In an effort to mimic the protegrin function, we recently applied the same reasoning to another important antimicrobial peptide cathelicidin (LL-37), which also functions at least in part by virtue of membrane permeabilization and has been shown to be produced in the lung by epithelial cells [30, 39–41]. However, to our surprise it appears that LL-37, while certainly capable of inducing pore formation, functions at least in part via its ability to modulate the P2X7 receptor.Although LL-37 is capable of enhancing IL-1β processing and release by LPS-primed monocytes, it actually inhibits LPS’s effect when added simultaneously. Presumably, this inhibition is due to the ability of LL-37 to bind to LPS and prevent its interaction with LBP and CD14 [42]. However, when added to preactivated monocytes, i.e., monocytes that are induced to express intracellular proIL-1β, LL-37 significantly augments the release of processed IL-1β, the signature of inflammasome activation. Importantly, LL-37 does not alter the release of IL-8, an event independent of inflammasome activation [30].Although LL-37 does induce pore formation as evidenced by the uptake of the fluorescent nucleic acid dye, YO-PRO-1, and the release of intracellular ATP, it appears that LL-37 is directly activating P2X7 receptors. Inhibitors of P2X7 receptor function were able to interfere with the LL-37-mediated inflammasome activation. KN04, KN62, and oxidized ATP all inhibited IL-1β processing and release induced by LL-37 in a dose-response fashion. This direct inhibition of the P2X7 receptor suggests that LL-37 works at least in part via activation of this purinergic receptor. Furthermore, oxidized ATP significantly inhibits the pore formation. However, this effect does not appear to be due to LL-37-mediated release of endogenous ATP. First of all, the concentration of ATP released in response to LL-37 is in the 100–200 nM range, well below the mM range needed for exogenously added ATP. Secondly, the addition of apyrase to the LL-37-stimulated cells does not block the IL-1β processing events but completely inhibits 5 mM ATP that has been exogenously added [30]. Thus, it appears that LL-37 can activate P2X7 receptors by a mechanism that is independent of the release of endogenous ATP (see Fig. 3 for review of potential mechanisms of the LL-37 effect). Regardless of the exact mechanism though, it appears that specific antimicrobial peptides may have a dual function by linking IL-1β release to its direct effects on bacterial killing.
Fig. 3.
Schematic of potential mechanism of LL-37 interaction with P2X7 receptor. Shown schematically are the potential mechanisms whereby the antimicrobial peptide, LL-37 (shown as an alpha helix), may activate the P2X7 receptor and induce caspase-1 activation (numbered 1–3). In process number 1, LL-37, via its cationic nature generates a transmembrane pore that allows potassium (K+) to efflux from the cytosol. Low intracellular potassium results in inflammasome assembly (not shown) and hence caspase-1 activation; in number 2, LL-37 directly interacts with the P2X7 receptor inducing a tyrosine phosphorylation event that is critical to the generation of a pannexin-1 pore that either allows potassium efflux (shown) or triggers a potassium-independent activation event [34]; finally possibility number 3 involves the P2X7 receptor indirectly by means of an LL-37 pore that allows the activation of the P2X7 receptor via autocrine ATP. AG126 is shown as a putative inhibitor either of the critical phosphorylation of the carboxy-terminal tyrosine 343 [18] of the P2X7 receptor or of its extracellular ATP binding site
Summary
Macrophages are unique innate immune cells that play an integral role in the defense of the host by virtue of their ability to recognize, engulf, and kill pathogens while sending out danger signals to recruit additional cells to the battle. It is becoming increasingly clear that purinergic signaling events are essential components of the macrophage response to pathogen challenges and that disorders such as sepsis may be, at least in part, regulated by these important sensors. The activation of the P2X7 receptor is a powerful event in the regulation of the caspase-1 inflammasome and this receptor may be activated by other elements of the host response such as the antimicrobial peptide LL-37. Our therapeutic possibilities will certainly be enhanced by increase understanding of the regulation of the purinergically regulated pores.
Acknowledgments
Timothy Eubank, Ph.D. for graphic design support.
References
- 1.Murphy PA, Simon PL, Willoughby WF (1980) Endogenous pyrogens made by rabbit peritoneal exudative cells are identical with lymphocyte-activating factors made by rabbit alveolar macrophages. J Immunol 124:2498–2501 [PubMed]
- 2.Rosenwasser LJ, Dinarello CA (1981) Ability of human leukocyte pyrogen to enhance phytohemagglutinin induced murine thymocyte proliferation. Cell Immunol 63:134–142 [DOI] [PubMed]
- 3.Gu BJ, Zhang WY, Bendall LJ, Chessell IP, Buell GN, Wiley JS (2000) Expression of P2X(7) purinoceptors on human lymphocytes and monocytes: evidence for nonfunctional P2X(7) receptors. Am J Physiol Cell Physiol 279:C1189–C1197 [DOI] [PubMed]
- 4.Gudipaty L, Humphreys BD, Buell G, Dubyak GR (2001) Regulation of P2X(7) nucleotide receptor function in human monocytes by extracellular ions and receptor density. Am J Physiol Cell Physiol 280:C943–C953 [DOI] [PubMed]
- 5.Mehta VB, Hart J, Wewers MD (2001) ATP-stimulated release of interleukin (IL)-1b and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem 276:3820–3826 [DOI] [PubMed]
- 6.Buell G, Chessell IP, Michel AD, Collo G, Salazzo M, Herren S, Gretener D, Grahames C, Kaur R, Kosco-Vilbois MH, Humphrey PP (1998) Blockade of human P2X7 receptor function with a monoclonal antibody. Blood 92:3521–3528 [PubMed]
- 7.Aga M, Johnson CJ, Hart AP, Guadarrama AG, Suresh M, Svaren J, Bertics PJ, Darien BJ (2002) Modulation of monocyte signaling and pore formation in response to agonists of the nucleotide receptor P2X(7). J Leukoc Biol 72:222–232 [PubMed]
- 8.Hogquist KA, Unanue ER, Chaplin DD (1991) Release of IL-1 from mononuclear phagocytes. J Immunol 147:2181–2186 [PubMed]
- 9.Perregaux D, Gabel CA (1994) Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem 269:15195–15203 [PubMed]
- 10.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, Di Virgilio F (1997) Extracellular ATP triggers IL-1b release by activating the purinergic P2Z receptor of human macrophages. J Immunol 159:1451–1458 [PubMed]
- 11.Griffiths RJ, Stam EJ, Downs JT, Otterness IG (1995) ATP induces the release of IL-1 from LPS-primed cells in vivo. J Immunol 154:2821–2828 [PubMed]
- 12.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T, Flavell RA, Sato V, Harding MW, Livingston DJ, Su MSS (1997) Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science 275:206–209 [DOI] [PubMed]
- 13.Kahlenberg JM, Dubyak GR (2004) Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Physiol Cell Physiol 286:C1100–C1108 [DOI] [PubMed]
- 14.Novogrodsky A, Vanichkin A, Patya M, Gazit A, Osherov N, Levitzki A (1994) Prevention of lipopolysaccharide-induced lethal toxicity by tyrosine kinase inhibitors. Science 264:1319–1322 [DOI] [PubMed]
- 15.Cuzzocrea S, McDonald MC, Mazzon E, Siriwardena D, Calabro G, Britti D, Mazzullo G, De Sarro A, Caputi AP, Thiemermann C (2000) The tyrosine kinase inhibitor tyrphostin AG126 reduces the development of acute and chronic inflammation. Am J Pathol 157:145–158 [DOI] [PMC free article] [PubMed]
- 16.Sarkar A, Hall MW, Exline M, Hart J, Knatz N, Gatson N, Wewers MD (2006) Caspase-1 regulates Escherichia coli sepsis and splenic B cell apoptosis independently of interleukin-1beta and interleukin-18. Am J Respir Crit Care Med 174:1003–1010 [DOI] [PMC free article] [PubMed]
- 17.Shemon AN, Sluyter R, Stokes L, Manley PW, Wiley JS (2008) Inhibition of the human P2X7 receptor by a novel protein tyrosine kinase antagonist. Biochem Biophys Res Commun 365:515–520 [DOI] [PubMed]
- 18.Kim M, Jiang LH, Wilson HL, North RA, Surprenant A (2001) Proteomic and functional evidence for a P2X7 receptor signalling complex. EMBO J 20:6347–6358 [DOI] [PMC free article] [PubMed]
- 19.Ayala JM, Yamin T-T, Egger LA, Chin J, Kostura MJ, Miller DK (1994) IL-1beta-converting enzyme is present in monocytic cells as an inactive 45-kDa precursor. J Immunol 153:2592–2599 [PubMed]
- 20.Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER (1982) Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis 126:332–337 [DOI] [PubMed]
- 21.Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, Kumararatne DS (1997) ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity 7:433–444 [DOI] [PubMed]
- 22.van Furth R, Cohn ZA (1968) The origin and kinetics of mononuclear phagocytes. J Exp Med 128:415–433 [DOI] [PMC free article] [PubMed]
- 23.van Furth R (1985) Monocyte production during inflammation. Comp Immunol Microbiol Infect Dis 8:205–211 [DOI] [PubMed]
- 24.Fahy RJ, Doseff AI, Wewers MD (1999) Spontaneous human monocyte apoptosis utilizes a caspase-3 dependent pathway which is blocked by endotoxin and is independent of caspase-1. J Immunol 163:1755–1762 [PubMed]
- 25.Wewers MD, Herzyk DJ (1989) Alveolar macrophages differ from blood monocytes in human IL-1 beta release. Quantitation by enzyme-linked immunoassay. J Immunol 143:1635–1641 [PubMed]
- 26.Wewers MD, Rennard SI, Hance AJ, Bitterman PB, Crystal RG (1984) Normal human alveolar macrophages obtained by bronchoalveolar lavage have a limited capacity to release interleukin-1. J Clin Invest 74:2208–2218 [DOI] [PMC free article] [PubMed]
- 27.Laliberte RE, Eggler J, Gabel CA (1999) ATP treatment of human monocytes promotes caspase-1 maturation and externalization. J Biol Chem 274:36944–36951 [DOI] [PubMed]
- 28.Beigi R, Kobatake E, Aizawa M, Dubyak GR (1999) Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am J Physiol Cell Physiol 276:C267–C278 [DOI] [PubMed]
- 29.Sperlagh B, Hasko G, Nemeth Z, Vizi ES (1998) ATP released by LPS increases nitric oxide production in raw 264.7 macrophage cell line via P2Z/P2X7 receptors. Neurochem Int 33:209–215 [DOI] [PubMed]
- 30.Elssner A, Duncan M, Gavrilin M, Wewers MD (2004) A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol 172:4987–4994 [DOI] [PubMed]
- 31.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228–232 [DOI] [PubMed]
- 32.Pelegrin P, Surprenant A (2006) Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 25:5071–5082 [DOI] [PMC free article] [PubMed]
- 33.Perregaux D, Barberia J, Lanzetti AJ, Geoghegan KF, Carty TJ, Gabel CA (1992) IL-1 beta maturation: evidence that mature cytokine formation can be induced specifically by nigericin. J Immunol 149:1294–1303 [PubMed]
- 34.Pelegrin P, Surprenant A (2007) Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem 282:2386–2394 [DOI] [PubMed]
- 35.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Núñez G (2007) Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 26:433–443 [DOI] [PubMed]
- 36.Thoma-Uszynski S, Stenger S, Takeuchi O, Ochoa MT, Engele M, Sieling PA, Barnes PF, Rollinghoff M, Bolcskei PL, Wagner M, Akira S, Norgard MV, Belisle JT, Godowski PJ, Bloom BR, Modlin RL (2001) Induction of direct antimicrobial activity through mammalian toll-like receptors. Science 291:1544–1547 [DOI] [PubMed]
- 37.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, Flavell RA (2005) Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307:731–734 [DOI] [PubMed]
- 38.Perregaux DG, Bhavsar K, Contillo L, Shi J, Gabel CA (2002) Antimicrobial peptides initiate IL-1 beta posttranslational processing: a novel role beyond innate immunity. J Immunol 168:3024–3032 [DOI] [PubMed]
- 39.Fahy RJ, Wewers MD (2005) Pulmonary defense and the human cathelicidin hCAP-18/LL-37. Immunol Res 31:75–89 [DOI] [PMC free article] [PubMed]
- 40.Bals R, Wang X, Zasloff M, Wilson JM (1998) The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA 95:9541–9546 [DOI] [PMC free article] [PubMed]
- 41.Johansson J, Gudmundsson GH, Rottenberg ME, Berndt KD, Agerberth B (1998) Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J Biol Chem 273:3718–3724 [DOI] [PubMed]
- 42.Nagaoka I, Hirota S, Niyonsaba F, Hirata M, Adachi Y, Tamura H, Heumann D (2001) Cathelicidin family of antibacterial peptides CAP18 and CAP11 inhibit the expression of TNF-alpha by blocking the binding of LPS to CD14(+) cells. J Immunol 167:3329–3338 [DOI] [PubMed]