Abstract
P2X7 receptor is a ligand-gated ion channel, which can induce the opening of large membrane pores. Here, we provide evidence that the receptor induces pore formation in astrocytes cultured from cortex, but not from the hippocampus. Furthermore, P2X7 receptor activation promptly induces p38 mitogen-activated protein kinase (MAPK) phosphorylation in cortical but not in hippocampal astrocytes. Given the role of p38 MAPK activation in pore opening, these data suggest that defective coupling of the receptor to the enzyme could occur in hippocampal cultures. The different capabilities of the receptor to open membrane pores cause relevant functional consequences. Upon pore formation, caspase-1 is activated and pro-IL1-β is cleaved and released extracellularly. The receptor stimulation does not result in interleukin-1beta secretion from hippocampal astrocytes, although the pro-cytokine is present in the cytosol of lipopolysaccharide-primed cultures. These results open the possibility that activation of P2X7 receptors differently influences the neuroinflammatory processes in distinct brain regions.
Keywords: P2X7, Hippocampus, Cortex, Astrocytes, Pore formation, IL-1β
Introduction
The P2X7 receptor is an ATP-gated cation channel that is widely expressed in cells of the immune system. This receptor differs from other members of the P2X family in its relatively low affinity for adenosine triphosphate (ATP), the presence of a long cytoplasmic C-terminus that contains several protein–protein interaction motifs, and the activation of two membrane conductance states depending on the exposure time of the agonist. The primary intracellular signal triggered by P2X7 receptor stimulation consists of a fast influx of Ca2+ and Na+ and efflux of K+. Upon repeated or prolonged ATP application, P2X7 stimulation also induces the opening of nonspecific pores, permeable to high molecular weight dyes, such as Yo-Pro-1. Recently, it has been suggested that pore formation does not depend on recruitment and clustering of P2X7 receptor subunits [1–3], but it rather involves the opening of a distinct membrane protein, pannexin-1, which can form hemichannels. P2X7-dependent pore opening has been implicated in the processing and release of cytokines such as interleukin-1beta (IL-1β), and in the initiation of cell death via both apoptotic and necrotic pathways [4]. Deletion of the cytoplasmic tail of the receptor and mutagenesis studies demonstrated a crucial role for the C-terminal region in pore formation [5–7] but not channel function. In the human population, both polymorphisms and splice variants have been recently discovered [8], including one that lacks the cytoplasmic tail. The high expression of this variant in several tissue may explain the lack of pore opening and apoptotic induction in some cell types [9], as recently described for astrocytes. In these glial cells, P2X7 receptor activation usually does not lead to an increase in membrane permeability and cytolysis ([10, 11], but see [12]), while the receptor stimulation induces apoptotic death in microglial cells [13].
Sustained activation of P2X7 receptor, besides favoring large pore opening, also results in activation of downstream protein kinases and other effector enzymes, which lead to second messengers generation and rearrangement of cytoskeletal elements associated with the C-terminus. In astrocytes and other glial cells, accumulating evidence indicate that the receptor is coupled to both ERK and p38 mitogen-activated protein kinase (MAPK) phosphorylation [14–17].
The presence of an ATP receptor with the functional properties of P2X7 was initially identified in astrocytes by Ballerini et al. [18]. Following the receptor cloning [5], RT-PCR studies confirmed the presence of mRNA encoding P2X7 receptors in these cells, and immunocytochemical studies showed P2X7 receptor immunoreactivity in acutely isolated and cultured astrocytes (for a review see [19]). A wide array of normal and pathological functions of the receptor, including release of various neurotransmitters, have been associated to P2X7 receptor activation in cultured glial cells. However, much less data are available on the role of P2X7 receptors in astroglial cells in situ and even on the presence of functional receptors in situ. While earlier studies described P2X7-like receptor currents in hippocampal astrocytes [20], recent results indicate that astrocytes of the CA1 subfield of mouse, rat, and human do not express functional P2X receptors, including the P2X7 receptors [21]. Given astrocytes are a heterogeneous cell population [22, 23] and distinct astrocyte subtypes, endowed with different voltage-dependent current patterns [24] and responsivity to glutamate [25] can coexist in a same brain region, the possibility opens that the properties and functions of P2X7 receptors may differ in astrocyte populations of distinct brain areas.
In this study, we provide evidence for a different behavior of the P2X7 receptor in cultured astrocytes from two rat brain regions, cortex and hippocampus. While P2X7 receptor stimulation induces large pore opening in cortical astrocytes, the receptor does not form a pore in hippocampal astrocytes. The different capabilities to induce pore formation affect the processing and release of IL-1β, thus possibly influencing neuroinflammatory processes during neurodegeneration in the two distinct brain regions.
Materials and methods
Primary cultures of hippocampal/cortical astrocytes Hippocampal/cortical astrocytic cultures from embryonic rat pups (E18) were obtained using previously described methods [26]. Briefly, after dissection, the hippocampi/cortices were dissociated by treatment with trypsin (0.25% for 10 min at 37°C) followed by fragmentation with a fire-polished Pasteur pipette. The dissociated cells were plated onto glass coverslips at a density of 0.5 × 106 cells/ml, and the cultures were grown in minimum essential medium (Invitrogen, Italy) supplemented with 20% fetal bovine serum (cortical astrocytes) or 10% horse serum (hippocampal astrocytes; Euroclone Ltd, UK) and glucose at a final concentration of 5.5 g/l.
Western blotting (P2X7, p38) After being grown on Petri dishes until confluence, the astrocytes were scraped, loaded onto an SDS-PAGE followed by Western blotting as previously described [26]. Briefly, after electrophoresis, the proteins were transferred to nitrocellulose filters which, after being incubated in blocking buffer (5% milk, 25 mM Tris–HCl, pH 7.5, 150 mM NaCl), were labeled with primary antibodies followed by the appropriate secondary antibodies conjugated to peroxidase diluted in blocking buffer containing 0.1–0.3% Tween 20. After extensive washing, the immunodecoration pattern was revealed using an enhanced chemiluminescence system (SuperSignal from Pierce, UK) following the manufacturer’s protocol.
Yo-Pro-1 uptake assay Cells were plated on 96 multi-wells at equal density (2,000 cells/ml) and cultured for 4–5 days. Cells were then exposed to 1 μM Yo-Pro-1 iodide dye (Invitrogen, Italy) in different experimental conditions and rinsed with phosphate-buffered solution, and dye uptake was quantified by spectrofluorimetric readouts at 485/535 nm and 10 Hz with a spectrophotometric system (1420 Multilabel Counter Victor 2- Wallac, Finland). To favor P2X7 opening, experiments were performed in the absence of extracellular calcium ions [27].
Interleukin-1beta enzyme-linked immunosorbent assay A mouse IL-1β enzyme-linked immunosorbent assay (ELISA) kit (Pierce Endogen, Italy) was used to quantify the presence of IL-1β in the supernatant of cortical/hippocampal astrocytes. Approximately 1,000,000 cells were pre-activated with 100 ng/ml lipopolysaccharide (LPS) for 6 h and stimulated at different experimental conditions in 1 ml of Krebs–Ringer solution. Conditioned media were then collected, and the assay was completed following the manufacturer’s protocol. Sample absorbance was measured with a spectrophotometric system (1420 Multilabel Counter Victor 2- Wallac, Finland) at 450 nm at 10 Hz. The actual IL-1β concentration was estimated on the basis of a standard curve at known concentration of IL-1β.
Single cell calcium imaging Cultures were loaded for 35–40 min at 37°C with 2 μM Fura-2-AM in Krebs–Ringer solution buffered with 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES; 125 mM NaCl, 5 mM KCl, 1.2 mM MgSO4, 2 mM CaCl2, 10 mM glucose, and 25 mM HEPES/NaOH, pH 7.4) and transferred to the recording chamber of an inverted microscope (Axiovert 100, Zeiss, Germany) equipped with a Ca2+ imaging unit. Polychrome IV (TILL Photonics, Germany) was used as a light source. Fura-2 fluorescence images were collected with a PCO Super VGA SensiCam (Axon Instruments, CA, USA) and analyzed with TILL Vision Software (TILL Photonics, Germany). Single cell 340/380 nm fluorescence ratios, acquired at 1–4/s, were analyzed with an Origin 6.0 software (Microcal Software Inc., MA, USA).
Chemicals 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate triethylammonium salt (BzATP), Fura-2-AM, KN-62, TNP-ATP, SB203582, tumor necrosis factor alpha (TNF-α), and apyrase were from SIGMA (Sigma-Aldrich, Italy).
Antibodies Rabbit Abs against ribophorin were kindly provided by Dr. G. Kreibich (New York University, New York, NY, USA). Mouse Abs against GFAP, Abs against P2X7 (C-term and N-term), and IL-1β were from Alomone Labs (Israel). Rabbit Abs against pannexin-1 was from Chemicon (UK).
Statistical analysis The data are presented as means + SE. Statistical significance was evaluated using either Student’s t test or one-way ANOVA. Differences were considered to be significant if p < 0.05 and are indicated by an asterisk; those at p < 0.01 are indicated by double asterisks.
Results and discussion
Expression of P2X7 in cultured astrocytes from cortex and hippocampus
The analysis by immunoblotting of P2X7 receptor with an antibody directed against the C-terminal region (C-ter Abs) revealed the presence of a protein band of the predicted molecular weight (66 kDa) in both hippocampal and cortical astrocytes (Fig. 1a). An additional band of about 27 kDa was detected in cortical cultures, whose specificity was indicated by the use of competitive peptide. The presence of a band corresponding to the full-length receptor in both astrocyte cultures was confirmed by an antibody directed against the N-terminal region (N-ter Abs).This antibody recognized in the hippocampal but not in cortical cultures a major band of about 27 kDa (Fig. 1b), likely corresponding to a cytoplasmic tail-deleted variant of the receptor, given it was not detected by the C-ter antibody (Fig. 1a). All together these results suggest the possible presence of different isoforms of the P2X7 receptor in astrocytes cultured from distinct brain region.
Fig. 1.
P2X7 receptor expression in cortical and hippocampal astrocytes in cultures. Lysates of cortical or hippocampal astrocytes were immunoblotted with two different anti-P2X7 receptor antibodies, directed against the C-terminal (a) and the N-terminal (b) region. While the C-ter antibody reveals the presence of a main protein band of about 60 kDa in both hippocampal and cortical astrocytes, the N-ter antibody reveals in the hippocampal astrocytes a prominent band of lower molecular weight (27 kDa), besides the 60 kDa. Band specificity is indicated by the use of competitive peptide. Staining with antibodies against the ER marker ribophorin was used as loading control
Functional P2X7 receptors in hippocampal and cortical astrocytes
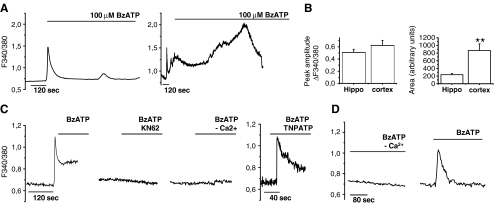
The presence of functional P2X7 receptors in cultured astrocytes was evaluated analyzing [Ca++]i responses induced by the rather selective agonist BzATP (100 μM). Cells were loaded with the fluorescent calcium dye Fura-2 and imaged by single cell digital system. When stimulated by prolonged agonist application, P2X7 receptor can induce the opening of large membrane pores that are permeable to molecules of up to about 1,000 Da in size [5, 28]. Formation of these pores allows the efflux of cytoplasmic water-soluble molecules such as Fura-2-free acid [10, 12, 28] and eventually results in cell lysis. As shown in Fig. 2a, prolonged BzATP application induced a transient increase of [Ca2+]i in hippocampal astrocytes (Fig. 2a, left) while a [Ca2+]i peak followed by a sustained calcium response in cortical glial cells (Fig. 2a, right). The secondary [Ca2+]i response lasted several minutes and eventually resulted in loss of Fura-2, as indicated by drop of the Fura-2 fluorescence signal, thus suggesting the opening of large membrane pores. Quantification of area under the response curve revealed significant smaller BzATP-induced calcium rises in hippocampal versus cortical astrocytes (Fig. 2b, right histogram). However, mean amplitude of peak [Ca2+]i (Fig. 2b, ΔF340/380 = 0.51 ± 0.05, hippocampal astrocytes; 0.62 ± 0.08, cortical astrocytes; n = 10; p = 0.28, Student’s t test) and percentages of BzATP-responsive cells (55.56 ± 9.85%, hippocampal astrocytes; 62.28 ± 5.02 cortical astrocytes; p = 0.3, n = 10, Student’s t test) were similar in cortical and hippocampal cultures, thus excluding a different level of receptor expression between the two glial cell types (Fig. 2b, left histogram). BzATP-induced [Ca2+]i responses were strongly inhibited in cells pre-incubated with the P2X7 antagonists adenosine 5′-triphosphate-2′,3′-dialdehyde (oATP; 100 μM, not shown) or KN-62 (Fig. 2c) in both glial cells types. The P2X antagonist TNP-ATP, which mainly acts on P2X1,3 and P2X4 receptors, did not affect the amplitude of peak [Ca2+]i rises, thus excluding a major contribution of other P2X receptors in BzATP-induced [Ca2+]i response (Fig. 2c). Furthermore, in astrocytes from both brain regions, BzATP-induced calcium responses were abolished when the agonist was applied in the absence of extracellular calcium ions, thus excluding a possible activation of metabotropic receptors by BzATP (Fig. 2c, d).
Fig. 2.
Activation of P2X7 receptor in cortical and hippocampal astrocytes. a Kinetics of [Ca2+]i changes in hippocampal (left) and cortical (right) cultures upon prolonged exposure to 100 μM BzATP. Note the long-lasting [Ca2+]i increase induced by BzATP in cortical but not hippocampal astrocytes. b Quantitative analysis of peak and area under curve of BzATP-induced [Ca2+]i rises in astrocytes from rat hippocampus or cortex. c BzATP-induced [Ca2+]i responses in the presence or in the absence of P2X antagonists or calcium ions in cortical cultures. d Representative traces of [Ca2+]i changes evoked by BzATP in the absence or in the presence of extracellular calcium ions in hippocampal astrocytes
P2X7 receptor-induced pore opening in cortical but not hippocampal astrocytes
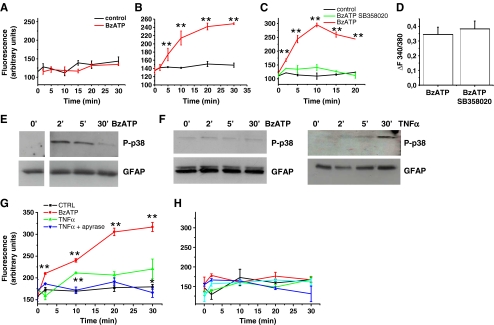
To confirm the capability of P2X7 receptor to induce pore opening in cortical but not in hippocampal astrocytes, the uptake of the fluorescent dye Yo-Pro-1, a reliable test for P2X7-dependent pore formation [29], was analyzed in both astrocyte types. A time-dependent Yo-Pro-1 uptake was evident in cortical (Fig. 3b) but not hippocampal (Fig. 3a) astrocytes exposed to BzATP. Dye internalization was evaluated in the absence of extracellular calcium ions, a condition which is known to favor pore formation [27].
Fig. 3.
P2X7 receptor activation induces pore opening in cortical but not hippocampal astrocytes. a, b Time course analysis of Yo-Pro-1 uptake in hippocampal (a) and cortical (b), astrocytes exposed to BzATP in the absence of extracellular calcium ions. c Time course analysis of BzATP-induced Yo-Pro-1 uptake in cortical astrocytes in the presence or in the absence of the p38 MAPK inhibitor SB358020. d Quantitative analysis of peak [Ca2+]i responses evoked by BzATP in cortical astrocytes exposed or not to the p38 MAPK inhibitor SB358020. Note that P2X7 channel activity is not altered by the p38 MAPK inhibitor. e, f Western blot analysis of p38 MAPK phosphorylation (P-p38) in cortical (e) or hippocampal (f) astrocyte cultures exposed to BzATP or TNF-α (10 μM) for different time points. GFAP staining was used as loading control. g Time course analysis of Yo-Pro-1 uptake in cortical astrocytes exposed to TNF-α to activate p38 in the presence or in the absence of apyrase to prevent P2X7 receptor activation by endogenous ATP. Note that dye uptake in cells exposed 30 min to TNF-α in the absence of apyrase is significantly higher than in control. h Time course analysis of Yo-Pro-1 uptake in hippocampal astrocytes exposed to TNF-α and BzATP either alone or in association to activate p38 MAPK and the P2X7 receptor. No significant Yo-Pro-1 uptake is detectable upon co-stimulation of P2X7 receptor and p38 MAPK
P2X7 receptor-induced p38 MAPK phosphorylation
The P2X7 receptor is coupled to several downstream effectors, including P38 MAPK [9] and previous evidence suggested that phosphorylation of this kinase is required for the opening of membrane pores upon the receptor activation [17]. In line with these findings, incubation of cortical astrocytes with the p38 inhibitor SB203580 (400 nM) strongly prevented BzATP-induced membrane permeabilization, as assayed by Yo-Pro-1 uptake (Fig. 3c). No significant alteration of BzATP-induced peak calcium rises was observed in SB203580-treated cortical cells (Fig. 3d), thus excluding an inhibitory effect of the drug on P2X7 channel activity. To evaluate whether the lack of pore formation upon P2X7 receptor stimulation in hippocampal cultures could be due to a defective activation of p38 MAPK, phosphorylation of the MAPK was evaluated by western blot in glial cultures exposed to BzATP. We observed that a brief agonist exposure induced a transient p38 MAPK phosphorylation in cortical astrocytes (Fig. 3e) but failed to activate the MAPK in hippocampal cultures (Fig. 3f, left). These data support the possibility that the lack of pore formation in hippocampal astrocytes is due to a defective coupling of the P2X7 receptor to p38 MAPK. On the other hand, treatment of the cultures with TNF-α, which is known to activate p38 MAPK cascade in mammalian cells, induced p38 phosphorylation in both hippocampal (Fig. 3f, right) and cortical cultures (not shown), 30 min after drug addition. In line with the requirement of p38 MAPK activation in P2X7-induced pore opening, exposure of cortical astrocytes to TNF-α induced Yo-Pro-1 uptake in the absence but not in the presence of the ATP-degrading enzyme apyrase (Fig. 3g). These data suggest that P2X7 activation by endogenous ATP is not sufficient to induce pore formation per se, but becomes able to induce pore formation when p38 MAPK is phosphorylated by TNF-α in cortical cells. Differently, treatment of hippocampal astrocytes with TNF-α, which phosphorylates p38 MAPK (Fig. 3f), failed to induce Yo-Pro-1 uptake (Fig. 3h), even when administered in the presence of BzATP. These data suggest that the lack of pore formation in hippocampal astrocytes could not be simply attributed to a defective coupling of the receptor to p38 MAPK signaling.
Pannexin-1 expression in astrocytes from hippocampus or cortex
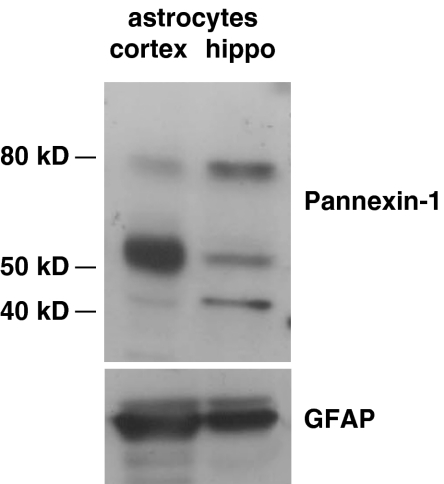
Recently, it has been suggested that pore formation does not depend on recruitment and clustering of P2X7 receptor subunits [1–3], but it rather involves the opening of a distinct membrane protein, pannexin-1, which can form hemichannels. We thus evaluated by western blot pannexin-1 expression in cortical and hippocampal astrocytes.
Whereas previous studies were suggestive of either low or very restricted expression of pannexin-1 in certain glial types [30] and endogenous expression of pannexin-1 gene, it has been recently described in rat primary astrocytes [31]. In the brain and in the retina, pannexin-1 is detectable in the plasma membrane and much more abundantly in the cytoplasm, with a prominent accumulation in organelles such as the Golgi apparatus and ER [32]. Different pannexin-1 isoforms have been described. Of these, the monomeric form, which matches the predicted size of pannexin-1 (43–48 kDa), has been shown in mouse-cultured astrocytes and is mainly detectable in organelle-enriched fractions [33], while the 58-kDa isoform is ubiquitously expressed and is associated with cell membranes [32]. Western blot analysis of cultured hippocampal or cortical astrocytes for pannexin-1 revealed three expected molecular weight bands of about of 43, 55–58, and 80 kDa (Fig. 4). Interestingly, the membrane associated 55–58-kDa isoform is the dominant band in cortical astrocytes while the 43- and the 80-kDa isoforms are more abundant in hippocampal cells. Overall, these results suggest that lack of pore opening in hippocampal astrocytes may be due to a low expression of pannexin-1 hemichannels associated to the plasma membrane.
Fig. 4.
Pannexin-1 expression in cortical and hippocampal astrocytes. Western blot analysis of pannexin-1 in cultured astrocytes from cortex or hippocampus. Note that the 58-kDa membrane isoform of pannexin-1 is expressed at a higher level in cortical than hippocampal astrocytes
P2X7-dependent release of IL-1β from cortical but not hippocampal astrocytes
We have previously shown that hippocampal astrocytes, primed with LPS, express the immature form of IL-1β (37 kDa) but they are not able to process and release the cytokine upon P2X7 receptor activation [34]. To investigate whether the different properties of P2X7 receptor in cortical and hippocampal astrocytes could influence IL-1β processing and release, either cortical or hippocampal cultures were stimulated for 30 min with 1 mM ATP after 6 h of LPS priming, to activate transcription of IL-1β gene and accumulation of the immature form of the cytokine. Although the pro-cytokine was clearly detectable in homogenates of both astrocyte types, the mature form of IL-1β (17 kDa) was detected, by Western blot analysis, only in the supernatant of cortical glial cells (Fig. 5a). The ELISA analysis of IL-1β levels in the cell supernatants further confirmed that ATP selectively induces IL-1β release from cortical astrocytes (Fig. 5b). Maturation of pro-IL-1β is catalyzed by caspase-1, which, in resting cells, is present in an inactive form. Caspase-1 becomes activated by the protein complex inflammosoma [35], whose assembly is driven by depletion of cytoplasmic K+. Due to the fact that P2X7 receptor stimulation induces K+ efflux, it has been hypothesized that massive K+ efflux upon P2X7-dependent pore formation leads to inflammosoma and caspase-1 activation. We asked therefore whether the lack of IL-1β release from the hippocampal astrocytes upon P2X7 stimulation could be due to detective caspase-1 activation. Quantitative analysis of active caspase-1 by fluorochrome inhibitor of caspases (FLICA) showed an increase in the number of active caspase-1 enzymes upon ATP stimulation in cortical but not hippocampal cultures (Fig. 5c). In line with this finding, a processed band of caspase-1 appeared in cortical but not hippocampal homogenates (not shown). These data indicate that the lack of IL-1β release from hippocampal cultures is most likely due to unprocessing of pro-caspase-1 and that functional properties and signaling coupling of P2X7 receptor can strongly influence the immune response of glial cells.
Fig. 5.
P2X7 receptor activation induces IL-1β release from cortical but not from hippocampal astrocytes. a Western blot analysis for IL-1β on cell lysates and supernatants of cortical (AC) and hippocampal cells (AH), primed 6 h with 100 ng/ml LPS and incubated with 100 μM BzATP for 30 min. Note the presence of the cytokine only in the supernatant of cortical astrocytes. b IL-1β detection by ELISA in the supernatants collected from hippocampal and cortical astrocytes primed 6 h with 100 ng/ml LPS and exposed to BzATP for 30 min. Values are presented as mean ± SE picograms per milliliter and are normalized to protein concentration of cell extracts. c Quantitative analysis of active caspase-1 by FLICA assay. Note the increase in the number of active caspase-1 upon BzATP exposure in cortical but not hippocampal astrocytes. d ELISA evaluation of IL-1β levels in the supernatants of cortical astrocytes maintained in static condition or mechanically stimulated with or without apyrase (one-way ANOVA, post hoc Dunn’s method, p = 0.003, n = 3) or the P2X7 antagonist oATP, showing that astrocyte-derived ATP induces IL-1β release from cortical cultures
In a previous study, we reported that ATP released by astrocytes during calcium wave propagation can activate P2X7 receptors on adjacent microglial cells [10]. Therefore, in principle, astrocyte-released ATP could be relevant for the control of cytokine secretion from astrocytes themselves, which express functional P2X7 receptors. To evaluate whether ATP release, induced by mechanical stimulation, can trigger IL-1β secretion, purified culture of cortical astrocytes was mechanically stimulated on an orbital shaker, as previously described [36]. ELISA analysis of collected media indicated a strong increase of IL-1β concentration in the supernatant of mechanically stimulated astrocytes (29.44 ± 5.5 pg/ml), as compared with supernatants from control cultures maintained under static conditions (0.78 ± 0.52 pg/ml; Fig. 5d). Interestingly, IL-1β decreased even below control levels (0.5 ± 0.05 pg/ml) when mechanical stimulation was performed in the presence of the ATP-degrading enzyme apyrase (30 U/ml), or the P2X7 antagonist oATP. Taken together, these results indicate that, besides microglial cells, astrocytes at least from cortex but not hippocampus can serve as a major source of the pro-inflammatory cytokine in the CNS upon injury or inflammation.
Conclusions
In this study, we provide evidence for a different capability of the P2X7 receptor to induce large pore opening in astrocytes cultured from cortex and hippocampus. Although functionally expressed in both brain regions, the receptor induces large membrane pore opening only in astrocytes cultured from the cortex. The capability of P2X7 receptor of opening large pore influences the cellular responses triggered in astrocytes by the receptor activation. Indeed, P2X7 receptor activation induces the release of the pro-inflammatory cytokine IL-1β from reactive cortical astrocytes, primed with LPS, but not hippocampal astrocytes. Interestingly, IL-1β release from cortical astrocytes can be induced also by P2X7 receptor activation by endogenous ATP, released from the cultures upon mechanical stimulation [36]. Association of IL-1β release to propagating calcium wave among reactive astrocytes supports the possibility that the process is more relevant in pathological than in physiological conditions [37]. Given that the cortex is the brain region more subject to traumatic injury, distinct properties of P2X7 receptor in hippocampal versus cortical astrocytes could be linked to the different needs of the two brain regions to cope with traumatic insults.
Acknowledgments
We thank Roberto Mele and Marta Fumagalli for their help in some experiments. This work was supported by FISM (2007/R35) to CV and CARIPLO 20060948 to MM.
References
- 1.Pelegrin P, Surprenant A (2006) Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 25:5071–5082 [DOI] [PMC free article] [PubMed]
- 2.Pelegrin P, Surprenant A (2007) Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem 282:2386–2394 [DOI] [PubMed]
- 3.Locovei S, Scemes E, Qiu F, Spray DC, Dahl G (2007) Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett 581:483–488 [DOI] [PMC free article] [PubMed]
- 4.Chen L, Brosnan CF (2006) Regulation of immune response by P2X7 receptor. Crit Rev Immunol 26:499–513 [DOI] [PubMed]
- 5.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G (1996) The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272:735–738 [DOI] [PubMed]
- 6.North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067 [DOI] [PubMed]
- 7.Smart ML et al (2003) P2X7 receptor cell surface expression and cytolytic pore formation are regulated by a distal C-terminal region. J Biol Chem 278:8853–8860 [DOI] [PubMed]
- 8.Cheewatrakoolpong B, Gilchrest H, Anthes JC, Greenfeder S (2005) Identification and characterization of splice variants of the human P2X7 ATP channel. Biochem Biophys Res Commun 332:17–27 [DOI] [PubMed]
- 9.Duan S, Neary JT (2006) P2X(7) receptors: properties and relevance to CNS function. Glia 54:738–746 [DOI] [PubMed]
- 10.Verderio C, Matteoli M (2001) ATP mediates calcium signaling between astrocytes and microglial cells: modulation by IFN-gamma. J Immunol 166:6383–6391 [DOI] [PubMed]
- 11.Neary JT, Kang Y, You-Fang S (2005) Cell cycle regulation of astrocytes by extracellular nucleotides and fibroblast growth factor-2. Purinergic Signal 1(4):329–336 doi:10.1007/s11302-005-8075-y [DOI] [PMC free article] [PubMed]
- 12.Fumagalli M et al (2003) Nucleotide-mediated calcium signaling in rat cortical astrocytes: role of P2X and P2Y receptors. Glia 43:218–230 [DOI] [PubMed]
- 13.Ferrari D et al (1997) ATP-mediated cytotoxicity in microglial cells. Neuropharmacology 36:1295–1301 [DOI] [PubMed]
- 14.Budagian V et al (2003) Signaling through P2X7 receptor in human T cells involves p56lck, MAP kinases, and transcription factors AP-1 and NF-kappa B. J Biol Chem 278:1549–1560 [DOI] [PubMed]
- 15.Panenka W et al (2001) P2X7-like receptor activation in astrocytes increases chemokine monocyte chemoattractant protein-1 expression via mitogen-activated protein kinase. J Neurosci 21:7135–7142 [DOI] [PMC free article] [PubMed]
- 16.Gendron FP et al (2003) Mechanisms of P2X7 receptor-mediated ERK1/2 phosphorylation in human astrocytoma cells. Am J Physiol Cell Physiol 284:C571–C581 [DOI] [PubMed]
- 17.Suzuki T et al (2004) Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci 24:1–7 [DOI] [PMC free article] [PubMed]
- 18.Ballerini P et al (1996) Rat astroglial P2Z (P2X7) receptors regulate intracellular calcium and purine release. Neuroreport 7:2533–2537 [DOI] [PubMed]
- 19.Sperlagh B, Vizi ES, Wirkner K, Illes P (2006) P2X7 receptors in the nervous system. Prog Neurobiol 78:327–346 [DOI] [PubMed]
- 20.Fellin T, Pozzan T, Carmignoto G (2006) Purinergic receptors mediate two distinct glutamate release pathways in hippocampal astrocytes. J Biol Chem 281:4274–4284 [DOI] [PubMed]
- 21.Jabs R et al (2007) Lack of P2X receptor mediated currents in astrocytes and GluR type glial cells of the hippocampal CA1 region. Glia 55:1648–1655 [DOI] [PubMed]
- 22.Nolte C et al (2001) GFAP promoter-controlled EGFP-expressing transgenic mice: a tool to visualize astrocytes and astrogliosis in living brain tissue. Glia 33:72–86 [DOI] [PubMed]
- 23.Matthias K et al (2003) Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci 23:1750–1758 [DOI] [PMC free article] [PubMed]
- 24.Steinhauser C, Berger T, Frotscher M, Kettenmann H (1992) Heterogeneity in the membrane current pattern of identified glial cells in the hippocampal slice. Eur J Neurosci 4:472–484 [DOI] [PubMed]
- 25.Zhou M, Kimelberg HK (2001) Freshly isolated hippocampal CA1 astrocytes comprise two populations differing in glutamate transporter and AMPA receptor expression. J Neurosci 21:7901–7908 [DOI] [PMC free article] [PubMed]
- 26.Calegari F et al (1999) A regulated secretory pathway in cultured hippocampal astrocytes. J Biol Chem 274:22539–22547 [DOI] [PubMed]
- 27.Virginio C, Church D, North RA, Surprenant A (1997) Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology 36:1285–1294 [DOI] [PubMed]
- 28.Falzoni S et al (1995) The purinergic P2Z receptor of human macrophage cells. Characterization and possible physiological role. J Clin Invest 95:1207–1216 [DOI] [PMC free article] [PubMed]
- 29.Virginio C, MacKenzie A, North RA, Surprenant A (1999) Kinetics of cell lysis, dye uptake and permeability changes in cells expressing the rat P2X7 receptor. J Physiol 519(Pt 2):335–346 [DOI] [PMC free article] [PubMed]
- 30.Shestopalov VI, Panchin Y (2008) Pannexins and gap junction protein diversity. Cell Mol Life Sci 65:376–394 [DOI] [PMC free article] [PubMed]
- 31.Lai CP et al (2007) Tumor-suppressive effects of pannexin-1 in C6 glioma cells. Cancer Res 67:1545–1554 [DOI] [PubMed]
- 32.Dvoriantchikova G, Ivanov D, Panchin Y, Shestopalov VI (2006) Expression of pannexin family of proteins in the retina. FEBS Lett 580:2178–2182 [DOI] [PubMed]
- 33.Huang Y, Grinspan JB, Abrams CK, Scherer SS (2007) Pannexin1 is expressed by neurons and glia but does not form functional gap junctions. Glia 55:46–56 [DOI] [PubMed]
- 34.Bianco F et al (2005) Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol 174:7268–7277 [DOI] [PubMed]
- 35.Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10:417–426 [DOI] [PubMed]
- 36.Coco S et al (2003) Storage and release of ATP from astrocytes in culture. J Biol Chem 278:1354–1362 [DOI] [PubMed]
- 37.Nedergaard M, Ransom B, Goldman SA (2003) New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci 26:523–530 [DOI] [PubMed]