Abstract
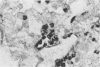
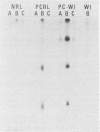
It has been difficult to obtain pure Pneumocystis carinii antigen either from cultures or from infected lungs for use in producing a specific antibody against P. carinii. This report describes an approach toward producing a monoclonal antibody that bypasses the antigen purification steps. P. carinii infection was developed in Sprague-Dawley rats by the method of immunosuppression with cortisone. The infected lungs were homogenized, and the homogenate was used to immunize Sprague-Dawley rats. Rat spleen cells were then fused with SP2/0 mouse myeloma cells. Hybridoma clones were screened for antibody production against P. carinii by immunoperoxidase staining techniques and by enzyme-linked immunosorbent assay, using as antigens homogenates of normal rat lung, homogenates of P. carinii-infected rat lung, and harvests of P. carinii grown with WI-38 cells. Out of six hybridoma clones obtained that produced antibodies against P. carinii, one was able to produce ascitic fluid. This monoclonal antibody reacted with two P. carinii antigens with masses of about 35,000 and 65,000 daltons in P. carinii-infected lungs and three proteins with masses of about 35,000, 65,000, and 110,000 daltons in P. carinii that was harvested from a WI-38 cell culture.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett M. S., Verbanac P. A., Smith J. W. Cultivation of Pneumocystis carinii with WI-38 cells. J Clin Microbiol. 1979 Dec;10(6):796–799. doi: 10.1128/jcm.10.6.796-799.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel J. K., Good J. T., Shultz J. A. Latent Pneumocystis infection of rats, relapse, and chemotherapy. Lab Invest. 1966 Oct;15(10):1559–1577. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Meuwissen J. H., Tauber I., Leeuwenberg A. D., Beckers P. J., Sieben M. Parasitologic and serologic observations of infection with Pneumocystis in humans. J Infect Dis. 1977 Jul;136(1):43–49. doi: 10.1093/infdis/136.1.43. [DOI] [PubMed] [Google Scholar]
- Pifer L. L., Niell H. B., Morrison B. J., Counce J. D., Jr, Freeman J. M., Woods D. R., Neely C. L. Pneumocystis carinii antigenemia in adults with malignancy, infection, or pulmonary disease. J Clin Microbiol. 1984 Nov;20(5):887–890. doi: 10.1128/jcm.20.5.887-890.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer P. D., Rutledge M. E., Yoneda K., Stahr B. J. Pneumocystis carinii: new separation method from lung tissue. Exp Parasitol. 1979 Jun;47(3):356–368. doi: 10.1016/0014-4894(79)90088-2. [DOI] [PubMed] [Google Scholar]