Abstract
The P2X7 receptor is widely referred to as the paradigmatic cytotoxic nucleotide receptor, and is often taken as an epitome of cytotoxic receptors as a whole. However, cytotoxicity is the result of sustained pharmacological stimulation, which is likely to occur in vivo only under severe pathological conditions. Over the years, we have gathered robust experimental proof that led us to adopt an entirely different view, pointing to P2X7 as a survival/growth-promoting rather than death-inducing receptor. Evidence in favour of this role is manifold: (1) extracellular ATP and benzoyl ATP support cell proliferation in peripheral T lymphocytes via a P2X7-like receptor; (2) P2X7 transfection into several cell lines confers growth advantage; (3) HEK293 cells transfected with P2X7 show enhanced mitochondrial metabolic activity and growth; (4) lipopolysaccharide (LPS)-dependent growth arrest of microglia is mediated via P2X7 down-modulation; (5) several malignant tumours express high P2X7 levels and (6) the ATP concentration in tumour interstitium is several-fold higher than in healthy tissues, to a level in principle sufficient to activate the P2X7 receptor. The molecular basis of P2X7-mediated growth-promoting activity is poorly known, but mitochondria appear to play a central role. A deeper understanding of the role played by P2X7 in cell proliferation might provide an insight into the mechanism of normal and malignant cell growth and suggest novel anti-tumour therapies.
Keywords: Extracellular ATP-P2 receptors-cancer
Introduction
Cell life and death are crucially dependent on a tight control of intracellular ion homeostasis. Agents that increase ion conductance across the plasma membrane or the membrane of intracellular organelles often cause cell death. Examples of such a lethal activity are the complement membrane attack complex, bacterial toxins or mitochondrial uncoupling agents. Thus, we were not surprised when we observed about at the same time as Sitkovsky and co-workers that stimulation of lymphocytes or tumour cells by high extracellular ATP doses caused a potent cytotoxic effect in many (but by no means all!) cell lines expressing the P2X7 receptor (at the time known as P2Z) [1, 2]. Accordingly, it was not surprising that blockade of P2X7 significantly reduced spontaneous cell death in cultures of selected cell lines [3]. Problems started when we found that proliferation of ex vivo cultures of human T lymphocytes far from being inhibited was in fact enhanced by pharmacological stimulation of a nucleotide receptor/channel with properties very similar to P2X7 [4]. Shaken by this unexpected finding, we started and in depth investigation of the underlying mechanism.
Initially, we explored the effect of heterologous P2X7 expression in human erythroleukemic and lymphoblastoid cell lines. In agreement with our previous findings in T lymphocytes, the mere P2X7 transfection, in the absence of stimulation with exogenous ATP, conferred a strong growth advantage but only in the absence of serum [5]. We then extended these observations to human chronic lymphocytic leukemia (CLL) cells. Peripheral lymphocytes from these patients turned out to have a several-fold higher P2X7 expression level compared to lymphocytes isolated from healthy subjects and larger increases in cytoplasmic Ca2+ in response to P2X7 agonists [6]. These concordant findings instigated a thorough examination of the biochemical intracellular changes involved.
We knew from earlier unpublished observations performed in our laboratory that cells transfected with P2X7 displayed morphological alterations in the mitochondrial network. The mitochondria appeared “thicker” and more ramified in P2X7- than in mock-transfected or wild-type (wt) cells (Adinolfi and Di Virgilio, unpublished observations). Investigation of the biochemical changes associated with these structural alterations unveiled that mitochondrial potential, intramitochondrial Ca2+ and ATP synthetic activity were enhanced in P2X7-transfected cells [7]. Further support to a role of P2X7 in cell growth came from the elucidation of the mechanism of action of bacterial lipopolysaccharide (LPS) in microglia. It is known that microglia, under the effect of LPS, stop proliferating and differentiate into an activated phenotype. Verderio and co-workers showed that inhibition of proliferation of mouse microglia by LPS is mediated by down-modulation of the P2X7 receptor [8]. In the same year, Raffaghello et al. [9] reported that P2X7 receptor expression sustained growth of human neuroblastoma cells.
P2X7 is an unusual receptor in that its activation causes opening of a cation-selective ion channel as well as a non-selective pore (this issue, Pelegrin and Surprenant). The molecular basis of this bifunctional behaviour is far from clear. It has been proposed that the large pore might be intrinsic to the receptor that would thus undergo dilatation and, therefore, a “channel-to-pore” transition, upon sustained activation, or that the “channel” and the “pore” are separate molecular entities [10, 11, 12, 13]. A few candidates to the “pore” role have been put forward, among which pannexin-1 seems to enjoy wider support [13, 14]. However, whichever the molecular identity, there is no doubt that pore opening is a potentially catastrophic event for the cell, unless it is tightly controlled. If controlled, pore opening might allow generation of a tonic intracellular signal (Ca2+?) that would then support growth. On the other hand, in the case of unchecked pore opening, the large Ca2+ influx would also be a trigger of death. This opens an intricate but fascinating scenario in which the level of P2X7 activation sets a delicate balance between growth and demise.
Tonic P2X7 stimulation supports cell growth
Human T lymphocytes express an ATP receptor/channel with properties akin to P2X7 in that it is activated by BzATP, is inhibited by oxidized ATP, triggers Ca2+ influx and plasma membrane depolarization, but not large pore opening [4]. Normal human B lymphocytes lack a receptor with these same properties. In T lymphocytes, ATP as such had little if any growth-promoting effect, moreover, high concentrations (0.5–1 mM) were needed to trigger Ca2+ influx or plasma membrane depolarization. This observation made us rather uncomfortable initially, but was readily explained later by the finding that T lymphocytes continuously leak ATP, causing the desensitization of the P2X7-like receptor. A brief treatment with apyrase to hydrolyze endogenously secreted ATP was sufficient to restore a near-normal sensitivity to ATP [4]. Thus, lymphocytes, like all other cell types, are continuously exposed to autocrine/paracrine stimulation by secreted ATP which likely reaches a much higher concentration in the pericellular space than in the bulk solution.
Normal T lymphocyte cultures do not proliferate unless mitogenically stimulated. Extracellular ATP has been reported to have diverse modulatory effects on mouse T lymphocytes, depending on the dose and incubation time, among which are stimulation of DNA synthesis and blastogenesis [15, 16, 17, 18]. ATP, as such, has little or no effect at all on human T lymphocyte proliferation but behaves as a strong co-stimulus when added together with known mitogens such as phytohemoagglutinin (PHA) or anti-CD3 Abs [4]. Conversely, mitogenic stimulation is strongly blocked by pre-incubation in the presence of oxidized ATP (oATP). Curiously, oxidized UTP is almost as potent an inhibitor as oATP, clearly indicating that different compounds able to form Schiff bases with plasma membrane receptors might be valuable as immunomodulators [19].
However, despite scattered evidence that human-resting non-neoplastic T lymphocytes express the P2X7 receptor but little P2Ys [4], the identity of the receptor mediating the growth-promoting effects of extracellular nucleotides in these cells has remained largely unknown. To explore the possible contribution of the P2X7 receptor to growth stimulation we investigated the effect of P2X7 transfection in K562 and LG14 cells, two cell lines lacking endogenous P2X7. Under normal culture conditions, proliferation rate of P2X7-transfected cells did not differ from that of mock-transfected cells; however, while proliferation of mock-transfected cells was arrested by removal of serum, proliferation of P2X7-transfected cells progressed unabated [5]. Interestingly, P2X7 but not mock-transfectants were sensitive to the growth-inhibition by apyrase, further supporting the role of an ATP-based autocrine–paracrine loop. Indirect evidence for the role of P2X7 in proliferation came from the finding that peripheral B lymphocytes from patients affected by the aggressive variant of chronic lymphocytic leukemia express P2X7 to a level higher than patients with the indolent variant or healthy controls [6]. In B-leukemic lymphocytes, P2X7 stimulation causes a large increase in intracellular Ca2+, while its blockade stops proliferation. We extended these findings to HEK293 cells, a cell line lacking endogenous P2X7. Also, in this cell type, transfection of P2X7 conferred a growth advantage as in other model systems [7].
In 2006, Verderio and co-workers provided an important and independent support to the growth-promoting activity of P2X7 by showing that proliferation of microglia cell clones and primary microglia was modulated by this receptor [8]. Microglia are resident cells of the brain that participate in reaction to traumas and in defence against foreign microorganisms. When exposed to bacterial products, microglia stop proliferating and differentiate into effector cells. During this process, microglia undergo a profound change of purinergic signalling involving a striking down-modulation of P2X7. This is not a mere correlation but rather a causal relationship because all treatments that selectively decrease P2X7 expression or function invariably decrease microglia proliferation. Furthermore, microglia cell clones lacking P2X7 are completely insensitive to the proliferation block caused by bacterial products (e.g. lipopolysaccharide). Thus, these experiments convincingly show that growth-promoting activity is a feature of the native as well as recombinant P2X7 receptor.
The biochemical basis of P2X7-mediated growth stimulation
The P2X7 receptor is a ligand-gated cation channel that, depending on the level of activation, may cause opening of a cation-selective channel or drive formation of a large conductance plasma membrane pore. The process leading to “large pore formation” is poorly understood, but there is sound preliminary evidence that it might be due to recruitment and activation of ubiquitous plasma membrane hemi-channels such as pannexin-1 [13, 14]. Thus, the most profound change observed in intracellular physiology upon P2X7 stimulation is a dramatic upset of intracellular ion homeostasis.
Although P2X7 has been reported to cause caspase, MAP kinase or Rho/ROCK protein activation [20, 21, 22], ion fluxes are still thought to be the most important signal transduction device associated to P2X7. Uncontrolled Na+ and Ca2+ entry is the main trigger of the well-known P2X7-dependent cytotoxicity, while K+ efflux is thought to be the main mechanism by which P2X7 drives pro-IL-1β processing. However, such dramatic upset in intracellular ion homeostasis is only observed upon P2X7 stimulation with high doses of exogenous ATP, or with pharmacological agonists such as benzoyl ATP, i.e. under conditions that might be considered pathological rather than physiological. Thus, we started an investigation on the effect on cell physiology of basal P2X7 activity, i.e. in the absence of exogenous stimulants. The first intriguing finding was that P2X7 transfection into lymphoblastoid or leukemic cell clones, in the absence of any additional stimulation, caused a small but significant increase in resting cytosolic Ca2+ ([Ca2+]i) levels [5]. This finding, together with the observation that P2X7-transfected cells had a thicker mitochondrial network (Adinolfi and Di Virgilio, unpublished observations), focused our attention on the mitochondria, an organelle central to intracellular ion homeostasis and energy metabolism.
Mitochondria have a key role in cell death, whether by necrosis or apoptosis [23], thus it is not surprising that cytotoxicity due to P2X7 stimulation is preceded by mitochondrial swelling or even disruption of the mitochondrial network [24, 7]. However, it is likely that this disruptive effect on mitochondrial physiology and morphology occurs only under extreme pathological conditions, while under more physiological conditions (i.e. no pharmacological stimulation by exogenous ATP), the P2X7–mitochondria liaison might be less catastrophic. Experiments performed with the mitochondrial potential probe TMRM showed that basal P2X7 activation may be not only harmless but even beneficial to mitochondria, as mitochondria of P2X7-transfected cells displayed a membrane potential 20–30 mV more negative than mock-transfected or wt control cells. The hyperpolarized mitochondrial potential was entirely dependent of P2X7 function as it returned to control values following addition of oATP or apyrase, or chelation of extracellular Ca2+ [7]. Thus, as already hypothesized in our previous study [5], the messenger linking P2X7 to mitochondria might be Ca2+.
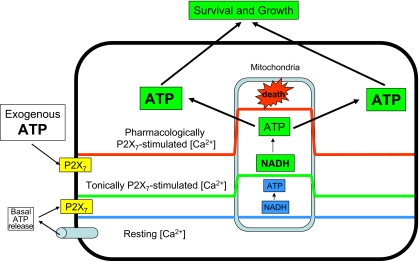
There is a delicate relationship between Ca2+ and mitochondria in that on the one hand a higher mitochondrial potential enhances the electrochemical gradient for Ca2+ import into the mitochondrial matrix, and on the other, small increases in the mitochondrial Ca2+ concentration cause hyperpolarization [25]. Mitochondrial Ca2+ in P2X7-transfected cells turns out to be at least twice as high as in mock-transfected or wt cells, and also intramitochondrial Ca2+ transients triggered by activation of plasma membrane receptors are several-fold higher than in control cells. Higher intramitochondrial Ca2+ is fully dependent on influx across the plasma membrane as it reverts to normal level upon chelation of extracellular Ca2+ or addition of apyrase. Intramitochondrial Ca2+ level is tightly linked to energy metabolism. It was originally showed by Denton and McCormack that three key rate-limiting enzymes in NADH synthesis and oxidative metabolism, pyruvate dehydrogenase, NAD+-isocitrate dehydrogenase and 2-oxoglutarate dehydrogenase are modulated by Ca2+ [26], and that NADH production is accordingly increased by raising mitochondrial Ca2+. Altogether, these two findings, hyperpolarized mitochondrial potential and higher NADH mitochondrial content, lead us to put forward the obvious prediction that P2X7-transfected cells should have a more efficient oxidative phosphorylation and higher intracellular ATP content. Thus, we were not surprised to find that P2X7-transfected cells have a total cellular ATP content four- to sixfold higher than wt or mock-transfected cells, as well as faster growth rate (Fig. 1).
Fig. 1.
Schematic rendition of the differential effects of P2X7-stimulated intracellular Ca2+ increases. ATP is continuously released into the extracellular environment via as yet poorly characterized pathways. This causes tonic stimulation of the P2X7 receptor leading to moderately increased cytoplasmic Ca2+, which translates into an elevation of intramitochondrial Ca2+ from 0.1–0.2 μM (blue line) to about 1.5–2.0 μM (green line). Such a Ca2+ increase stimulates NADH synthesis and ATP production via oxidative phosphorylation. Increased cellular ATP content then facilitates cell survival and growth. However, in the presence of massive amounts of extracellular ATP, the P2X7 receptor is overstimulated, this leads to uncontrolled Ca2+ influx and unchecked increase in the cytoplasm as well as the mitochondria (red line), with catastrophic consequences on the cell
P2X7 and cancer
The link between oxidative phosphorylation and cancer has always been ambiguous since the early observations of Otto Warburg [27]. Neoplastic phenotype has often been associated to alterations in mitochondria physiology and morphology, to the point that a defect in oxidative phosphorylation was considered a hallmark of cancer [28, 29]. Although there is no real indication that defective phosphorylation is an absolute feature of cancer cells, there is no doubt that many cancers display aerobic glycolysis. Although the glycolytic pathway is much less efficient than respiration as a source of ATP, cancer cells may utilize glucose in such an efficient fashion to maintain more than adequate intracellular ATP levels [28]. In any case, whether generated via respiration or glycolysis, ATP remains the key metabolic factor supporting cell growth. If, as shown by our findings, the P2X7 receptor increases cellular ATP stores and confers growth advantage, it might be expected that malignant tumours overexpress this receptor.
So far, no extensive screening of P2X7 expression in malignant tumours has been performed; however, different laboratories have reported increased expression of this receptor in prostate [30], breast and skin cancers [31, 32], neuroblastoma [9], leukemia [6] and thyroid papillary carcinoma [33]. In most cases, these studies simply report histological evidence of receptor overexpression in tissue slices, with no analysis of functional activity. In a few studies, P2X7 function has been investigated in cell lines established from the primary tumours [9, 33]. The interesting finding stemming from these more-detailed studies is that the P2X7 receptor in cancer may also support growth by indirect means, for example, by supporting secretion of growth factors.
In human thyroid papillary carcinoma, P2X7 stimulation triggers release of IL-6, a growth factor for thyreocytes [33], while in human neuroblastoma, this receptor supports release of substance P, a growth factor for these cancer cells [9]. Neuroblastoma cells display an additional intriguing feature: although P2X7 is fully functional and induces formation of the typical “large conductance pore”, ATP has little if any cytotoxic effect, as if neuroblastoma cells had learned to uncouple pore formation from the death-inducing pathway. This “death escape” mechanism has not been investigated, but it might be related to a faulty coupling to caspases, as neuroblastoma cells, in contrast to many other non-tumour cell types, show no caspase-3 activation in response to P2X7 stimulation [9].
P2X7-mediated growth-promoting activity might provide a biological rationale for overexpression of this receptor in cancer as it would be odd, to say the least, if tumours overexpressed a potentially lethal receptor without any advantage for growth or survival. We hypothesize several potential advantages for cancer cells expressing P2X7: (1) more efficient mitochondrial metabolism and ATP synthesis; (2) more active MAP kinase pathway; (3) increased ability to release growth- or survival-promoting factors and (4) increased ability to release ATP, which may act as a growth factor in its own, or stimulate release of additional factors. However, whether cancers really benefit of these potential advantages is, so far, purely speculative as the role of P2X7 in tumour growth and progression has never been investigated. Furthermore, while there is vast literature correlating the drop in mitochondrial potential with apoptosis or with an overall “reduced cellular fitness” under various experimental conditions, very few investigators have explored the link between hyperpolarized mitochondria and tumour growth. Early works reported the intriguing observation that mitochondria of cancer cells have an unusually high membrane potential [34, 35]. These observations were recently confirmed and extended in colonic cancer cells, one of the fastest-growing tissues in the body. A strong correlation was shown to exist between increased mitochondrial potential and (1) resistance to hypoxia, (2) anchorage-independent growth, and (3) invasion of the basement membrane [36]. The biochemical mechanism by which an increased mitochondrial potential allows better survival and growth is unknown, but our finding that cells with hyperpolarized mitochondria accumulate higher cellular ATP stores may provide a reasonable physiological explanation as adequate energy stores are a pre-requisite for any cell function.
Finally, a crucial question is whether enough ATP accumulates in tumour interstitium to activate the low-affinity P2X7 receptor. It is well known that in vitro P2X7 stimulation requires hundreds of micromolar, if not millimolar, ATP concentration, levels that many believe almost impossible to achieve in vivo. A recent study of ours shows, on the contrary, and rather surprisingly, that such high ATP levels, although unattainable within healthy tissues, may be reached in the tumour interstitium [37].
Conclusion
P2X7 is generally known as a “death-inducing” receptor. While there is no doubt that this is indeed the case when it is overstimulated, our findings show that tonic, low-level stimulation by endogenously released ATP generates a trophic stimulus that enhances the efficiency of mitochondrial metabolism and overall fitness of P2X7-expressing cells. Thus, high P2X7 expression by nearly all cancers so far investigated might not be coincidental, but on the contrary, causally related to tumour growth and progression.
Acknowledgements
This research was supported by grants from the Italian Association for Cancer Research, Telethon of Italy (n. GGP06070), the Italian Space Agency (ASI-OSMA), the Italian Ministry of University and Scientific Research (PRIN), the Commission of European Communities (7th Framework Program HEALTH-F2-2007-202231), the Regione Emilia-Romagna, and institutional funds from the University of Ferrara.
References
- 1.Di Virgilio F, Bronte V, Collavo D, Zanovello P (1989) Responses of mouse lymphocytes to extracellular adenosine 5′-triphosphate (ATP). Lymphocytes with cytotoxic activity are resistant to the permeabilizing effects of ATP. J Immunol 143:1955–1960 [PubMed]
- 2.Filippini A, Taffs RE, Agui T, Sitkovsky MV (1990) Ecto-ATPase activity in cytolytic T-lymphocytes. Protection from the cytolytic effects of extracellular ATP. J Biol Chem 265:334–340 [PubMed]
- 3.Chiozzi P, Murgia M, Falzoni S, Ferrari D, Di Virgilio F (1996) Role of the purinergic P2Z receptor in spontaneous cell death in J774 macrophage cultures. Biochem Biophys Res Commun 218:176–181 [DOI] [PubMed]
- 4.Baricordi OR, Ferrari D, Melchiorri L, Chiozzi P, Hanau S, Chiari E et al (1996) An ATP-activated channel is involved in mitogenic stimulation of human T lymphocytes. Blood 87:682–690 [PubMed]
- 5.Baricordi OR, Melchiorri L, Adinolfi E, Falzoni S, Chiozzi P, Buell G et al (1999) Increased proliferation rate of lymphoid cells transfected with the P2X(7) ATP receptor. J Biol Chem 274:33206–33208 [DOI] [PubMed]
- 6.Adinolfi E, Melchiorri L, Falzoni S, Chiozzi P, Morelli A, Tieghi A et al (2002) P2X7 receptor expression in evolutive and indolent forms of chronic B lymphocytic leukemia. Blood 99:706–708 [DOI] [PubMed]
- 7.Adinolfi E, Callegari MG, Ferrari D, Bolognesi C, Minelli M, Wieckowski MR et al (2005) Basal activation of the P2X7 ATP receptor elevates mitochondrial calcium and potential, increases cellular ATP levels, and promotes serum-independent growth. Mol Biol Cell 16:3260–3272 [DOI] [PMC free article] [PubMed]
- 8.Bianco F, Ceruti S, Colombo A, Fumagalli M, Ferrari D, Pizzirani C et al (2006) A role for P2X7 in microglial proliferation. J Neurochem 99:745–758 [DOI] [PubMed]
- 9.Raffaghello L, Chiozzi P, Falzoni S, Di Virgilio F, Pistoia V (2006) The P2X7 receptor sustains the growth of human neuroblastoma cells through a substance P-dependent mechanism. Cancer Res 66:907–914 [DOI] [PubMed]
- 10.Di Virgilio F (1995) The P2Z purinoceptor: an intriguing role in immunity, inflammation and cell death. Immunol Today 16:524–528 [DOI] [PubMed]
- 11.North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067 [DOI] [PubMed]
- 12.Coutinho-Silva R, Persechini PM (1997) P2Z purinoceptor-associated pores induced by extracellular ATP in macrophages and J774 cells. Am J Physiol 273:C1793–C1800 [DOI] [PubMed]
- 13.Pelegrin P, Surprenant A (2006) Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 25:5071–5082 [DOI] [PMC free article] [PubMed]
- 14.Pelegrin P, Surprenant A (2007) Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem 282:2386–2394 [DOI] [PubMed]
- 15.el-Moatassim C, Dornand J, Mani JC (1987) Extracellular ATP increases cytosolic free calcium in thymocytes and initiates the blastogenesis of the phorbol 12-myristate 13-acetate-treated medullary population. Biochim Biophys Acta 927:437–444 [DOI] [PubMed]
- 16.DosReis GA, Nobrega AF, de Carvalho RP (1986) Purinergic modulation of T-lymphocyte activation: differential susceptibility of distinct activation steps and correlation with intracellular 3’,5’-cyclic adenosine monophosphate accumulation. Cell Immunol 101:213–231 [DOI] [PubMed]
- 17.Lin J, Krishnaraj R, Kemp RG (1985) Exogenous ATP enhances calcium influx in intact thymocytes. J Immunol 135:3403–3410 [PubMed]
- 18.Chused TM, Apasov S, Sitkovsky M Murine T (1996) Lymphocytes modulate activity of an ATP-activated P2Z-type purinoceptor during differentiation. J Immunol 157:1371–1380 [PubMed]
- 19.Rhodes J (1996) Covalent chemical events in immune induction: fundamental and therapeutic aspects. Immunol Today 17:436–441 [DOI] [PubMed]
- 20.Ferrari D, Los M, Bauer MK, Vandenabeele P, Wesselborg S, Schulze-Osthoff K (1999) P2Z purinoreceptor ligation induces activation of caspases with distinct roles in apoptotic and necrotic alterations of cell death. FEBS Lett 447:71–75 [DOI] [PubMed]
- 21.Pfeiffer ZA, Aga M, Prabhu U, Watters JJ, Hall DJ, Bertics PJ (2004) The nucleotide receptor P2X7 mediates actin reorganization and membrane blebbing in RAW 264.7 macrophages via p38 MAP kinase and Rho. J Leukoc Biol 75:1173–1182 [DOI] [PubMed]
- 22.Morelli A, Chiozzi P, Chiesa A, Ferrari D, Sanz JM, Falzoni S et al (2003) Extracellular ATP causes ROCK I-dependent bleb formation in P2X7-transfected HEK293 cells. Mol Biol Cell 14:2655–2664 [DOI] [PMC free article] [PubMed]
- 23.Giorgi C, Romagnoli A, Pinton P, Rizzuto R (2008) Ca2+ signaling, mitochondria and cell death. Curr Mol Med 8:119–130 [DOI] [PubMed]
- 24.MacKenzie A, Adinolfi E, Grainge A, Surprenant A (2005) Pseudoapoptosis induced by brief activation of ATP-gated P2X7 receptors. J Biol Chem 280:33968–33976 [DOI] [PubMed]
- 25.Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R (1999) Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci U S A 96:13807–13812 [DOI] [PMC free article] [PubMed]
- 26.Denton RM, McCormack JG (1980) On the role of the calcium transport cycle in heart and other mammalian mitochondria. FEBS Lett 119:1–8 [DOI] [PubMed]
- 27.Warburg O (1956) On respiratory impairment in cancer cells. Science 124:269–270 [PubMed]
- 28.Kim Jw, Dang CV (2006) Cancer’s molecular sweet tooth and the Warburg Effect. Cancer Res 66:8927–8930 [DOI] [PubMed]
- 29.Schulz TJ, Thierbach R, Voigt A, Drewes G, Mietzner B, Steinberg P et al (2006) Induction of oxidative metabolism by mitochondrial frataxin inhibits cancer growth: Otto Warburg revisited. J Biol Chem 281:977–981 [DOI] [PubMed]
- 30.Slater M, Danieletto S, Gidley-Baird A, Teh LC, Barden JA (2004) Early prostate cancer detected using expression of non-functional cytolytic P2X7 receptors. Histopathology 44:206–215 [DOI] [PubMed]
- 31.Slater M, Danieletto S, Pooley M, Cheng TL, Gidley-Baird A, Barden JA (2004) Differentiation between cancerous and normal hyperplastic lobules in breast lesions. Breast Cancer Res Treat 83:1–10 [DOI] [PubMed]
- 32.Greig AV, Linge C, Healy V, Lim P, Clayton E, Rustin MH et al (2003) Expression of purinergic receptors in non-melanoma skin cancers and their functional roles in A431 cells. J Invest Dermatol 121:315–327 [DOI] [PubMed]
- 33.Solini A, Cuccato S, Ferrari D, Santini E, Gulinelli S, Callegari MG et al (2008) Increased P2X7 receptor expression and function in thyroid papillary cancer: a new potential marker of the disease? Endocrinology 149:389–396 [DOI] [PubMed]
- 34.Damdimopoulos AE, Miranda-Vizuete A, Pelto-Huikko M, Gustafsson JA, Spyrou G (2002) Human mitochondrial thioredoxin. Involvement in mitochondrial membrane potential and cell death. J Biol Chem 277:33249–33257 [DOI] [PubMed]
- 35.Heerdt BG, Houston MA, Wilson AJ, Augenlicht LH (2003) The intrinsic mitochondrial membrane potential (Deltapsim) is associated with steady-state mitochondrial activity and the extent to which colonic epithelial cells undergo butyrate-mediated growth arrest and apoptosis. Cancer Res 63:6311–6319 [PubMed]
- 36.Heerdt BG, Houston MA, Augenlicht LH (2006) Growth properties of colonic tumor cells are a function of the intrinsic mitochondrial membrane potential. Cancer Res 66:1591–1596 [DOI] [PubMed]
- 37.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F (2008) Increased level of extracellular atp at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS ONE 3:e2599 [DOI] [PMC free article] [PubMed]