Abstract
Descriptions of symptomatic focal dystonia caused by focal lesions of the central nervous system (CNS) are rare in the literature. We report a 9-year-old child who experienced sudden-onset left-hand dystonia for 6 months. Brain magnetic resonance imaging showed a mass lesion involving the putamen, globus pallidus, head of caudate, and the anterior limb of the internal capsule. Histopathological and immunocytochemical examinations of the mass revealed features characteristic of malignant germinoma. CNS germinoma in the basal ganglia is very rare. Combining previous reports in the literature with the anatomical and clinical presentation of our case suggests that this phenomenon results from disruption of the pathways within and adjacent to the basal ganglia.
Keywords: Basal ganglia, Germinoma, Focal dystonia
Descriptions of symptomatic (secondary) dystonia caused by isolated lesions in the brain are rare.1 Whilst central nervous system (CNS) germinoma is a malignant tumor that is mostly found in the pineal region and hypothalamic area,2 basal ganglia germinoma can occur very rarely. This is the first report of a CNS germinoma presenting with focal hand dystonia.
CASE REPORT
A 9-year-old boy presented with a 6-month history of left-hand dystonia. Six months previously the patient had experienced the sudden onset of involuntary twitching movement on the left hand. Since then the patient developed abnormal cramping whenever he played the piano or a computer game. During the subsequent months, this involuntary movement progressed to occur in all other activities during wakefulness, but disappeared during sleep. His medical and developmental histories were unremarkable. He had never been taken any neuroleptic or dopaminergic drugs, and there was no family history of neurological diseases.
A neurological examination was normal except for the dystonia of the left hand, which consisted of abduction and extension of the fingers and flexion of the wrist (Fig. 1). The dystonia appeared both at rest and during voluntary movements, but was aggravated by actions such as spreading out his fingers or playing the piano. It disappeared during sleep.
Figure 1.
Photograph showing dystonia of the left hand during arm extension (A) and playing the piano (B, C).
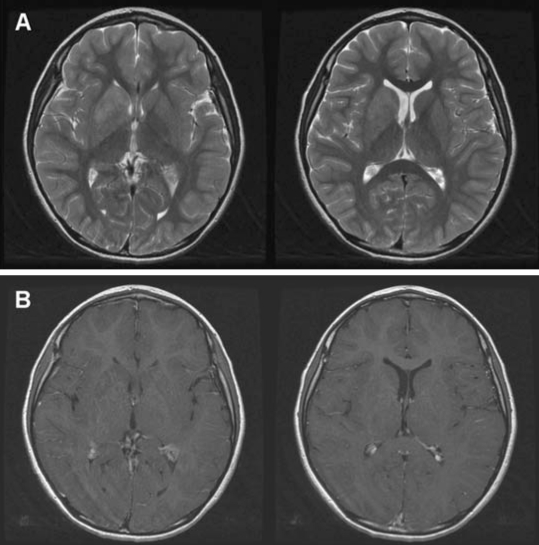
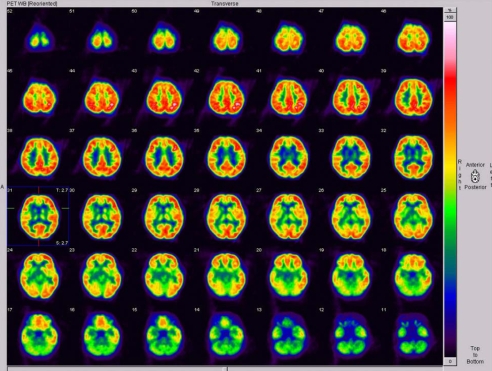
The results of our routine investigations, including hematological and biochemical screening, and measurements of the serum ceruloplasmin and copper levels, were all normal. Magnetic resonance imaging scans of the brain showed an ill-defined mass lesion in the right basal ganglia, especially involving the putamen, globus pallidus, head of caudate, and the anterior limb of the internal capsule (Fig. 2). Positron-emission tomography using 18F-deoxyglucose demonstrated diffuse hypometabolism in the right basal ganglia, right thalamus, and adjacent insular and temporal cortices (Fig. 3).
Figure 2.
Magnetic resonance imaging showing an ill-defined mass lesion involving the right basal ganglia. The lesion was located in the putamen, globus pallidus, head of caudate, and the anterior limb of the internal capsule. (A) Axial T2- weighted images, and (B) axial T1-weighted contrast enhancement images.
Figure 3.
Positron-emission tomography using 18F-deoxyglucose demonstrated diffuse hypometabolism in the right basal ganglia, right thalamus, and right insular and temporal cortices.
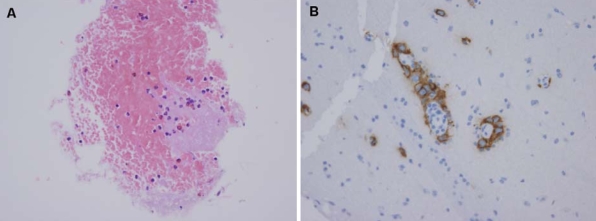
A stereotaxic biopsy was performed at another university hospital. The histopathological examination showed characteristic features of pure germinoma, and immunohistochemical staining using PLAP (placental alkaline phosphatase) was positive (Fig. 4).
Figure 4.
Histopathological and immunohistochemical findings revealed (A) large tumorous cells (germ cells) with a clear nucleus and a light-colored and vacuolated cytoplasm (hematoxylin-eosin stain, ×400) and (B) a placental alkaline phosphatase (PLAP) profile with large tumorous cells labeled for PLAP in the cell membrane and cytoplasm (×400).
DISCUSSION
CNS germinoma is a malignant germ cell tumor that is mostly found in the pineal region and hypothalamic area. Other uncommon cerebral locations of germinoma include the basal ganglia, thalamus, midbrain, posterior fossa, fourth ventricle, cerebellopontine angle, medulla oblongata, and cerebral hemispheres.3-8 This tumor mostly arises in children and young adults.
We present a unique case of a 9-year-old boy with a right basal ganglia germinoma presenting with focal hand dystonia. A radiological examination prompted various possible diagnoses, such as low-grade astrocytoma, lymphoma, primitive neuroectodermal tumor, or germ cell tumor.
Clinical observations of symptomatic dystonia have led to suggestions that focal hand dystonia is related to lesions of the posterolateral thalamic nuclei and, more rarely, of the caudate nucleus, putamen, and parietal cortex,9,10 whereas hemidystonia has been related to large caudatocapsulolenticular lesions and small lesions in the putamen.9-12 Because of the variable location of lesions causing focal dystonia, Marsden et al. and Pettigrew and Jankovic suggested that symptomatic dystonia is produced by abnormal thalamicmediated input to the premotor cortex and ultimately the motor cortex from the basal ganglia.9,12 This inappropriate information delivered to the premotor area can be produced by a lesion anywhere in the striatopallidothalamic connections to the premotor cortex. Dystonia therefore may be viewed as a consequence of the release of premotor cortical activity from thalamic control.9-12
Our patient experienced focal hand dystonia associated with a germinoma involving the putamen, globus pallidus, head of caudate, and the anterior limb of internal capsule. The dystonia was most probably produced by a mass effect of the germinoma. However, the pathophysiological mechanism underlying why dystonia was largely restricted to one limb in our patient remains unclear. A possible explanation is the presence of a somatotopic representation of the foot, hand, and face within the basal ganglia. Recent functional neuroimaging and clinicoradiological studies suggest the presence of a somatotopic organization of the putamen, but precise characterization of this has not been possible.13-15
In conclusion, CNS germinoma in the basal ganglia is very rare, and combining its anatomical and clinical presentation in our patient with data in the literature suggests that the focal hand dystonia can result from disruption of the pathways within and adjacent to the basal ganglia.
Footnotes
This study was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (no. A060093).
References
- 1.Kostic VS, Stojanovic-Svetel M, Kacar A. Symptomatic dystonias associated with structural brain lesions: report of 16 cases. Can J Neurol Sci. 1996;23:53–56. doi: 10.1017/s0317167100039184. [DOI] [PubMed] [Google Scholar]
- 2.Matsutani M, Sano K, Takakura K, Fujimaki T, Nakamura O, Funata N, et al. Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg. 1997;86:446–455. doi: 10.3171/jns.1997.86.3.0446. [DOI] [PubMed] [Google Scholar]
- 3.Evanson EJ, Lewis PD, Colquhoun IR. Primary germinoma of the posterior cranial fossa: a case report. Neuroradiology. 1997;39:716–718. doi: 10.1007/s002340050493. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Santos JM, Torres del Rio S, Sanchez A, Martinez-Lage JF. Basal ganglia and thalamic tumours: an imaging approximation. Childs Nerv Syst. 2002;18:412–425. doi: 10.1007/s00381-002-0606-z. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi T, Yoshida J, Kida Y. Bilateral germ cell tumors involving the basal ganglia and thalamus. Neurosurgery. 1989;24:579–583. doi: 10.1227/00006123-198904000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto K, Tabuchi A, Tamesa N, Nakashima H, Ohmoto T. Primary intracranial germinoma involving the midbrain. Clin Neurol Neurosurg. 1998;100:292–295. doi: 10.1016/s0303-8467(98)00050-x. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima H, Iwai Y, Yamanaka K, Yasui T, Kishi H. Primary intracranial germinoma in the medulla oblongata. Surg Neurol. 2000;53:448–451. doi: 10.1016/s0090-3019(00)00224-x. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida K, Nakao Y, Yamamoto T, Mori K, Maeda M. Germinoma in the fourth ventricle. Acta Neurochir (Wien) 2003;145:789–792. doi: 10.1007/s00701-003-0043-0. [DOI] [PubMed] [Google Scholar]
- 9.Marsden CD, Obeso JA, Zarranz JJ, Lang AE. The anatomical basis of symptomatic hemidystonia. Brain. 1995;108:463–483. doi: 10.1093/brain/108.2.463. [DOI] [PubMed] [Google Scholar]
- 10.Obeso JA, Gimenez-Roldan S. Clinicopathological correlation in symptomatic dystonia. In: Fahn S, editor. Dystonia 2, Advances in Neurology. New York: Raven Press; 1988. pp. 113–122. [PubMed] [Google Scholar]
- 11.Narbona J, Obeso JA, Tunon T, Martinez-Lage JM, Marsden CD. Hemi-dystonia secondary to localised basal ganglia tumour. J Neurol Neurosurg Psychiatry. 1984;47:704–709. doi: 10.1136/jnnp.47.7.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pettigrew LC, Jankovic J. Hemidystonia: A report of 22 patients and a review of the literature. J Neurol Neurosurg Psychiatry. 1985;48:650–657. doi: 10.1136/jnnp.48.7.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krystkowiak P, Martinat P, Defebvre L, Pruvo JP, Leys D, Destee A. Dystonia after striatopallidal and thalamic stroke: clinicoradiological correlations and pathophysiological mechanisms. J Neurol Neurosurg Psychiatry. 1998;65:703–708. doi: 10.1136/jnnp.65.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maillard L, Ishii K, Bushara K, Waldvogel D, Schulman AE, Hallett M. Mapping the basal ganglia: fMRI evidence for somatotopic representation of face, hand, and foot. Neurology. 2000;55:377–383. doi: 10.1212/wnl.55.3.377. [DOI] [PubMed] [Google Scholar]
- 15.Gerardin E, Lehericy S, Pochon JB, Tezenas du Montcel S, Mangin JF, Poupon F, et al. Foot, hand, face and eye representation in the human striatum. Cereb Cortex. 2003;13:162–169. doi: 10.1093/cercor/13.2.162. [DOI] [PubMed] [Google Scholar]