Abstract
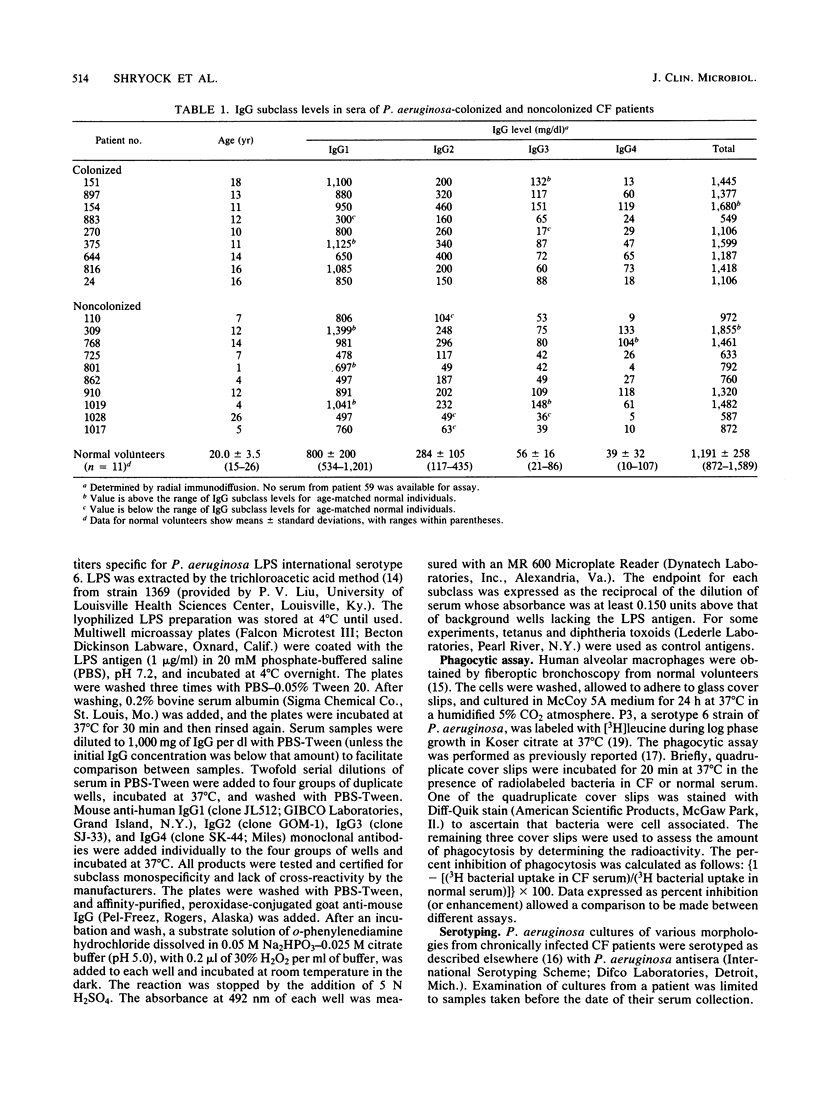
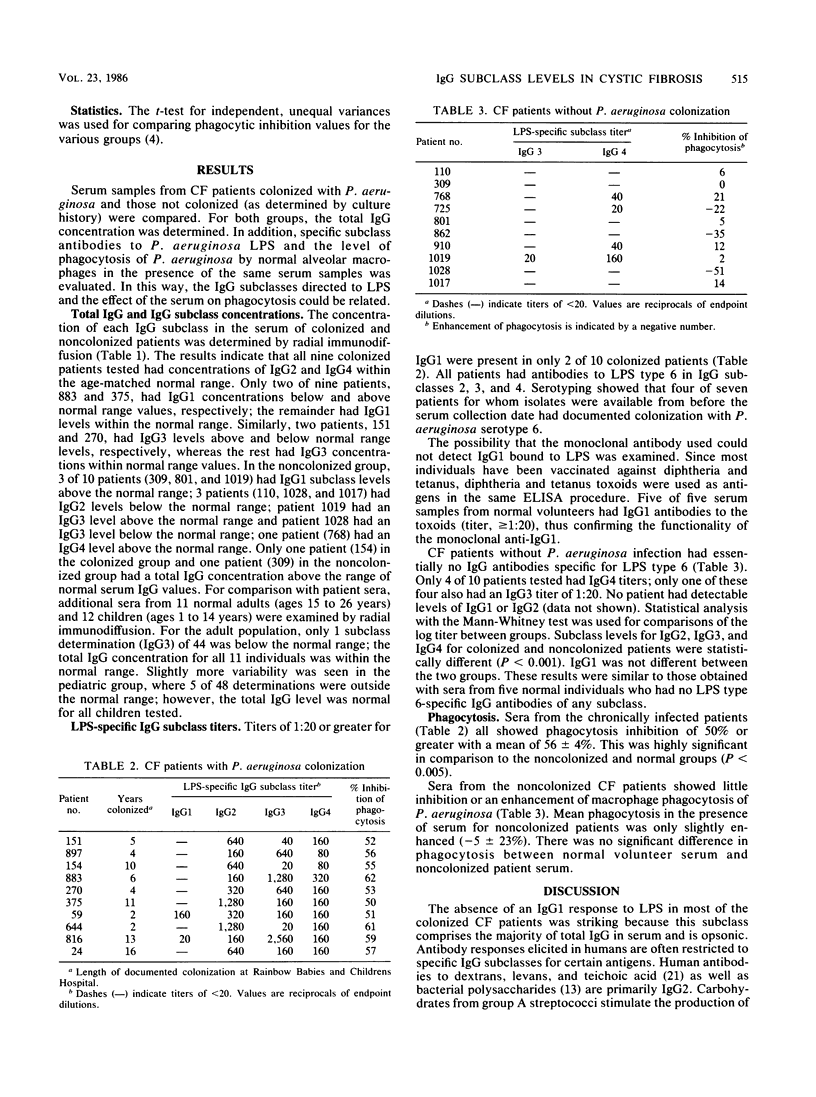
Serum from cystic fibrosis patients colonized with Pseudomonas aeruginosa specifically inhibits phagocytosis of P. aeruginosa by alveolar macrophages. Serum was examined for P. aeruginosa lipopolysaccharide-specific immunoglobulin G (IgG) subclass levels (by enzyme-linked immunosorbent assay) and for the effect on macrophage phagocytosis (by radiolabeled P. aeruginosa uptake). Sera from cystic fibrosis patients with no known P. aeruginosa colonization history had negligible amounts of lipopolysaccharide-specific IgG and a mean phagocytic enhancement of 5%. The sera of normal volunteers also had negligible amounts of lipopolysaccharide-specific IgG. Serum from cystic fibrosis patients with P. aeruginosa respiratory tract infections had substantial titers (range, 1:20 to 1:1,280) of lipopolysaccharide-specific IgG2, IgG3, and IgG4 and a mean phagocytic inhibition of 56%. However, these patients had low or absent titers of lipopolysaccharide-specific IgG1. No consistent variation in the level of individual IgG subclasses in the sera of colonized patients was observed, as determined by radial immunodiffusion. The results suggest that during P. aeruginosa infection phagocytosis-inhibitory activity develops coincident with production of lipopolysaccharide-specific IgG subclasses.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. D., Andrews J. A., Leslie R. G., Wood N. J. The binding of human and guinea-pig IgG subclasses to homologous macrophage and monocyte Fc receptors. Immunology. 1978 Jul;35(1):115–123. [PMC free article] [PubMed] [Google Scholar]
- Fick R. B., Jr, Naegel G. P., Matthay R. A., Reynolds H. Y. Cystic fibrosis pseudomonas opsonins. Inhibitory nature in an in vitro phagocytic assay. J Clin Invest. 1981 Oct;68(4):899–914. doi: 10.1172/JCI110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay F. C., Torrigiani G., Roitt I. M. The binding of human IgG subclasses to human monocytes. Eur J Immunol. 1972 Jun;2(3):257–261. doi: 10.1002/eji.1830020312. [DOI] [PubMed] [Google Scholar]
- Hsu Y. P., Moss R. B. Microtiter immunoenzymatic assay (micro-ELISA) for antigen-specific IgG4 subclass antibody. Clin Rev Allergy. 1983 Jun;1(2):231–236. doi: 10.1007/BF02991158. [DOI] [PubMed] [Google Scholar]
- Oxelius V. A. IgG subclass levels in infancy and childhood. Acta Paediatr Scand. 1979 Jan;68(1):23–27. doi: 10.1111/j.1651-2227.1979.tb04424.x. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Atkinson J. P., Newball H. H., Frank M. M. Receptors for immunoglobulin and complement on human alveolar macrophages. J Immunol. 1975 Jun;114(6):1813–1819. [PubMed] [Google Scholar]
- Riesen W. F., Skvaril F., Braun D. G. Natural infection of man with group A streptococci. Levels; restriction in class, subclass, and type; and clonal appearance of polysaccharide-group-specific antibodies. Scand J Immunol. 1976;5(4):383–390. doi: 10.1111/j.1365-3083.1976.tb00292.x. [DOI] [PubMed] [Google Scholar]
- Schur P. H., Rosen F., Norman M. E. Immunoglobulin subclasses in normal children. Pediatr Res. 1979 Mar;13(3):181–183. doi: 10.1203/00006450-197903000-00010. [DOI] [PubMed] [Google Scholar]
- Shakib F., Stanworth D. R., Drew R., Catty D. A quantitative study of the distribution of IgG sub-classes in a group of normal human sera. J Immunol Methods. 1975;8(1-2):17–28. doi: 10.1016/0022-1759(75)90077-0. [DOI] [PubMed] [Google Scholar]
- Shakib F., Stanworth D. R., Smalley C. A., Brown G. A. Elevated serum IgG4 levels in cystic fibrosis patients. Clin Allergy. 1976 May;6(3):237–240. doi: 10.1111/j.1365-2222.1976.tb01902.x. [DOI] [PubMed] [Google Scholar]
- Siber G. R., Schur P. H., Aisenberg A. C., Weitzman S. A., Schiffman G. Correlation between serum IgG-2 concentrations and the antibody response to bacterial polysaccharide antigens. N Engl J Med. 1980 Jul 24;303(4):178–182. doi: 10.1056/NEJM198007243030402. [DOI] [PubMed] [Google Scholar]
- Thomassen M. J., Boxerbaum B., Demko C. A., Kuchenbrod P. J., Dearborn D. G., Wood R. E. Inhibitory effect of cystic fibrosis serum on pseudomonas phagocytosis by rabbit and human alveolar macrophages. Pediatr Res. 1979 Sep;13(9):1085–1088. doi: 10.1203/00006450-197909000-00030. [DOI] [PubMed] [Google Scholar]
- Thomassen M. J., Demko C. A., Winnie G. B., Sherman J. M. Function of human alveolar macrophages from normal volunteers and cigarette smokers: effect of cystic fibrosis serum. J Leukoc Biol. 1984 Apr;35(4):345–355. doi: 10.1002/jlb.35.4.345. [DOI] [PubMed] [Google Scholar]
- Thomassen M. J., Demko C. A., Wood R. E., Tandler B., Dearborn D. G., Boxerbaum B., Kuchenbrod P. J. Ultrastructure and function of alveolar macrophages from cystic fibrosis patients. Pediatr Res. 1980 May;14(5):715–721. doi: 10.1203/00006450-198005000-00003. [DOI] [PubMed] [Google Scholar]
- Winnie G. B., Klinger J. D., Sherman J. M., Thomassen M. J. Induction of phagocytic inhibitory activity in cats with chronic Pseudomonas aeruginosa pulmonary infection. Infect Immun. 1982 Dec;38(3):1088–1093. doi: 10.1128/iai.38.3.1088-1093.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R. E., Boat T. F., Doershuk C. F. Cystic fibrosis. Am Rev Respir Dis. 1976 Jun;113(6):833–878. doi: 10.1164/arrd.1976.113.6.833. [DOI] [PubMed] [Google Scholar]
- Yount W. J., Dorner M. M., Kunkel H. G., Kabat E. A. Studies on human antibodies. VI. Selective variations in subgroup composition and genetic markers. J Exp Med. 1968 Mar 1;127(3):633–646. doi: 10.1084/jem.127.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]


