Abstract
Paraproteinemia potentially causes peripheral neuropathy via an unknown underlying pathogenetic mechanism. We report a case of pathologically proven amyloid neuropathy with AL amyloidosis with an IgA kappa light chain, which was initially diagnosed as neuropathy associated with monoclonal gammopathy of undetermined significance. This case indicates that in cases of neuropathy with paraproteinemia, the other potential causes should be excluded by appropriate means, especially pathological evaluations.
Keywords: Amyloidosis, Paraproteinemia, Polyneuropathy
Monoclonal gammopathy is a condition in which aberrant monoclonal immunoglobulin is produced from clonally expanded B lymphocyte and is present in excessive amounts in the blood, and occurs in about 1% of the general population. Identifying monoclonal gammopathy requires the physician to work-up the possible underlying lymphoproliferative disorders, such as multiple myeloma, osteosclerotic myeloma, and Waldenstrom's macroglobulinemia.1 It has also been claimed to be a cause of otherwise idiopathic polyneuropathy, which is present in 10% of neuropathic patients without any systemic disorder known to cause neuropathy,2 such as diabetes mellitus, alcoholism, and connective-tissue disorders. Anti-myelin-associated glycoprotein (MAG) antibody, which is identified in about a half of neuropathic patients with IgM paraproteinemia,3 has been considered to have a major pathogenetic role in neuropathies. However, in most instances there are no nerve-specific antibodies identifiable in neuropathy with monoclonal gammopathy, and hence the causative role of paraproteinemia is not clear, and its nosological entity, so-called paraproteinemic neuropathy, is dubious. Furthermore, although neuropathy is associated exceedingly more frequently with IgM than with IgG or IgA monoclonal gammopathy, IgG/IgA paraproteinemia-associated neuropathies also have been reported infrequently, and their underlying cause has not been determined.4,5 Here we present a case of neuropathy associated with IgA monoclonal gammopathy, and discuss some diagnostic considerations related to identifying the underlying cause.
CASE REPORT
A 69-year-old man visited a hospital with complaints of orthostatic intolerance, burning ache, and weakness in distal extremities that had been present for more than 5 months. He had previously been evaluated at another hospital for the same complaints, where he had been diagnosed as having 'sensorimotor polyneuropathy associated with IgA kappa (κ) light-chain monoclonal gammopathy of undetermined significance' (MGUS) based on an electrophysiological investigation, protein electrophoresis (PEP), immunoelectrophoresis (IEP), and the normal appearance of a bone marrow biopsy specimen, but the pathology of his peripheral nerves had not been investigated.
He had taken oral prednisolone at 1 mg/kg/day for more than 3 months, but his symptoms had gradually worsened. On admission, he was alert and cooperative. A physical examination revealed symmetric distal muscle atrophy and weakness, but tendon reflexes were exceptionally well preserved even at the ankle. In the same distal areas of limbs he complained of numbness, paresthesia, allodynia, and anhidrosis. His supine blood pressure of 130/80 mmHg dropped to 106/80 mmHg at 3 minutes after standing, during which he complained of lightheadedness. Nerve conduction and electromyography investigations revealed diffuse axonal sensorimotor polyneuropathy without apparent electrophysiologic indication of demyelination or myopathy. The sympathetic skin response and the Valsalva ratio were compatible with dysfunction of the autonomic nervous system. PEP and IEP reaffirmed the presence of IgA κ light-chain monoclonal gammopathy with a serum IgA concentration of 509 mg/dl as determined by nephelometry (normal range: 70~400 mg/dl). Radiological investigations revealed neither lytic nor sclerotic bone lesions, and blood data including complete blood count, serum creatinine, and blood urea nitrogen were normal.
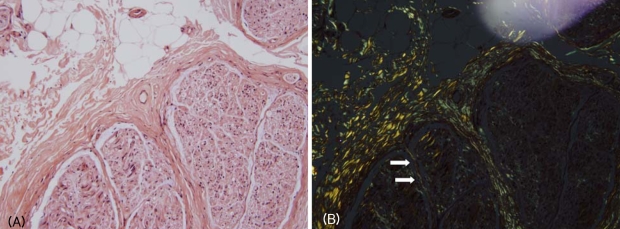
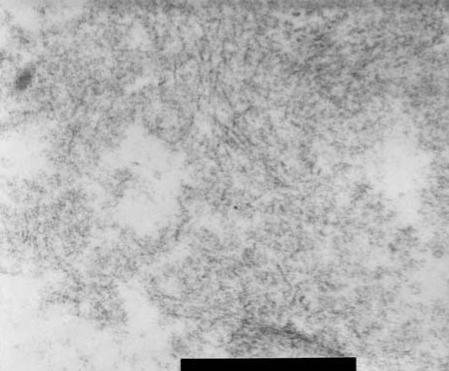
Considering the clinical picture of autonomic and sensory dysfunction and the presence of a rare association of neuropathy with IgA MGUS, we biopsied the right sural nerve due to suspected other causes of neuropathy including amyloidosis. The deposition of amorphous materials in the endoneurium was indicated in sections stained with H&E (Fig. 1-A) that exhibited birefringence under polarized-light microscopy with Congo red staining (Fig. 1-B). Transverse cryostat sections were examined by direct immunofluorescence microscopy using rabbit antisera monospecific to human IgG, IgM, and IgA κ and λ light chains, C3, fibrinogen, and C1d, which revealed no immunofluorescence reactions. Ultrastructural examination disclosed moderately decreased myelinated and unmyelinated fibers with haphazardly arranged nonbranching amyloid fibrils in the endoneurium (Fig. 2). Thus the patient was diagnosed as having amyloid neuropathy complicating primary immunoglobulin-light-chain-related (AL) amyloidosis, rather than neuropathy associated with IgA MGUS. A comprehensive work-up revealed that no organ outside the peripheral nervous system was involved in the amyloidosis.
Figure 1.
Deposition of amorphous materials in the endoneurium was indicated in sections stained with H&E (A) that exhibited birefringence under polarized-light microscopy with Congo red staining (B, arrows).
Figure 2.
Ultrastructural examination showed that both myelinated and unmyelinated fibers had nonbranching amyloid fibrils in a haphazard arrangement in the endoneurium.
Administration of corticosteroid and melphalan for more than 6 months produced no objective clinical or electrophysiological improvement. His neuropathic pain was only partially relieved by a transdermal fentanyl patch, and no evidence of other organ involvement was detected.
DISCUSSION
About 60% of cases with monoclonal gammopathy are classified as MGUS after excluding various lymphoproliferative disorders. MGUS is defined by the presence of serum monoclonal protein at less than 30 g/L, fewer than 10% of plasma cells in bone marrow, the absence of lytic bone lesions, and no related anemia, hypercalcemia, or renal insufficiency.1 In our patient, evaluation of paraproteinemia via the recommended modalities produced a diagnosis of MGUS. However, when monoclonal gammopathy is associated with sensorimotor polyneuropathy (particularly if autonomic involvement is present), nephritic syndrome, congestive heart failure, or idiopathic malabsorption syndrome, the possibility of AL amyloidosis must be considered.
Nerve tissue can be damaged by paraproteins directly through deposition of the antibodies specific for a myelin or axonal component, as evident in neuropathic cases with anti-MAG antibodies. Moreover, amyloid deposition or local ischemia due to serum hyperviscosity in cryoglobulinemia can also result in nerve damage.6 The anti-MAG antibody is identified in more than 50% of cases with IgM monoclonal gammopathy, whereas it is rare for a specific neural autoantibody to be present in IgG or IgA monoclonal gammopathy. Various clinical features of peripheral neuropathy associated with IgG/IgA paraproteinemia contrast with the relatively homogenous clinical features of peripheral neuropathy with IgM neuropathy, which is described under the various terms 'IgM-MGUS neuropathy', 'anti-MAG neuropathy', and 'distal acquired demyelinating symmetric neuropathy'. These observations suggest nonimmunological pathomechanisms of neuropathy, which is supported by reports of only a few cases with histologically proven immunoglobulin deposition in the setting of IgG or IgA paraproteinemia.7-10
The presence of paraproteinemia increases the probability of neuropathy tenfold, with it being present in about 10% of patients with paraproteinemia. This means that about 90% of paraproteinemic patients do not exhibit peripheral neuropathy. Therefore, the mere presence of paraproteinemia itself serves as a strong risk factor for peripheral neuropathy, but cannot be considered as indicative of peripheral neuropathy, especially in the case of IgG or IgA paraproteinemia, for which the risk for neuropathy is lower than that of IgM paraproteinemia. Some previous investigations of neuropathy associated with IgG/IgA monoclonal gammopathy did not perform the histological evaluations required for a definitive diagnosis.4,5
Bone marrow biopsy, skeletal investigation, peripheral blood smear, and other blood and urine assays are sufficient for diagnosing MGUS, but paraproteinemia associated with neuropathy is more problematic. Even after the exclusion of lymphoproliferative disorder, excluding the rare disorder amyloid neuropathy by pathological evaluation in the setting of paraproteinemic neuropathy is preferable not only for diagnosis accuracy but also for selecting the appropriate management. Our patient provided no clinical or laboratory evidence of other organ involvement, and was a good example for illustrating the importance of histological evaluation.
References
- 1.Kyle RA, Rajkumar SV, Lust JA. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. In: Greer JP, Foerster J, Lukens JN, Rodgers GM, Paraskevas F, Glader B, editors. Wintrobe's Clinical Hematology. 11th edn. Vol. 2. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 2565–2581. [Google Scholar]
- 2.Kelly JJ, Kyle RA, O'Brien PC, Dyck PJ. Prevalence of monoclonal protein in peripheral neuropathy. Neurology. 1981;31:1480–1483. doi: 10.1212/wnl.31.11.1480. [DOI] [PubMed] [Google Scholar]
- 3.Braun PE, Frail DE, Latov N. Myelin-associated glycoprotein is the antigen for a monoclonal IgM in polyneuropathy. J Neurochem. 1982;39:1261–1265. doi: 10.1111/j.1471-4159.1982.tb12563.x. [DOI] [PubMed] [Google Scholar]
- 4.Nobile-Orazio E, Barbieri S, Baldini L, Marmiroli P, Carpo M, Premoselli S, et al. Peripheral neuropathy in monoclonal gammopathy of undetermined significance: prevalence and immunopathogenetic studies. Acta Neurol Scand. 1992;85:383–390. doi: 10.1111/j.1600-0404.1992.tb06033.x. [DOI] [PubMed] [Google Scholar]
- 5.Magy L, Chassande B, Maisonobe T, Bouche P, Vallat JM, Leger JM. Polyneuropathy associated with IgG/IgA monoclonal gammopathy: a clinical and electrophysiological study of 15 cases. Eur J Neurol. 2003;10:677–685. doi: 10.1046/j.1468-1331.2003.00687.x. [DOI] [PubMed] [Google Scholar]
- 6.Pletz MW, Duda PW, Kappos L, Steck AJ. Immunemediated neuropathies: etiology and pathogenic relationship to aging processes. J Neuroimmunol. 2003;137:1–11. doi: 10.1016/s0165-5728(03)00010-9. [DOI] [PubMed] [Google Scholar]
- 7.Sewell HF, Matthews JB, Gooch E, Millac P, Willox A, Stern MA, et al. Autoantibody to nerve tissue in a patient with a peripheral neuropathy and an IgG paraprotein. J Clin Pathol. 1981;34:1163–1166. doi: 10.1136/jcp.34.10.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhib-Jalbut S, Liwnicz BH. Binding of serum IgA of multiple myeloma to normal peripheral nerve. Acta Neurol Scand. 1986;73:381–387. doi: 10.1111/j.1600-0404.1986.tb03293.x. [DOI] [PubMed] [Google Scholar]
- 9.Bleasel AF, Hawke SH, Pollard JD, McLeod JG. IgG monoclonal paraproteinaemia and peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1993;56:52–57. doi: 10.1136/jnnp.56.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehndiratta MM, Sen K, Tatke M, Bajaj BK. IgA monoclonal gammopathy of undetermined significance with peripheral neuropathy. J Neurol Sci. 2004;221:99–104. doi: 10.1016/j.jns.2004.02.020. [DOI] [PubMed] [Google Scholar]