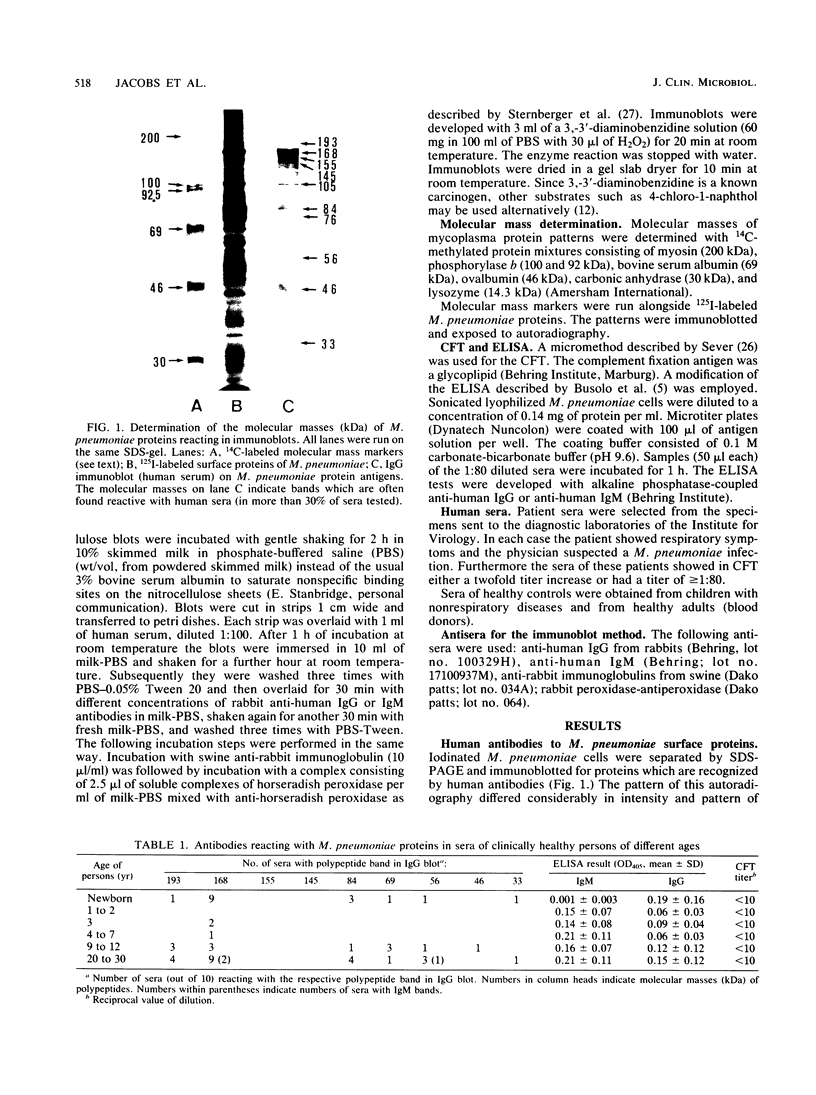
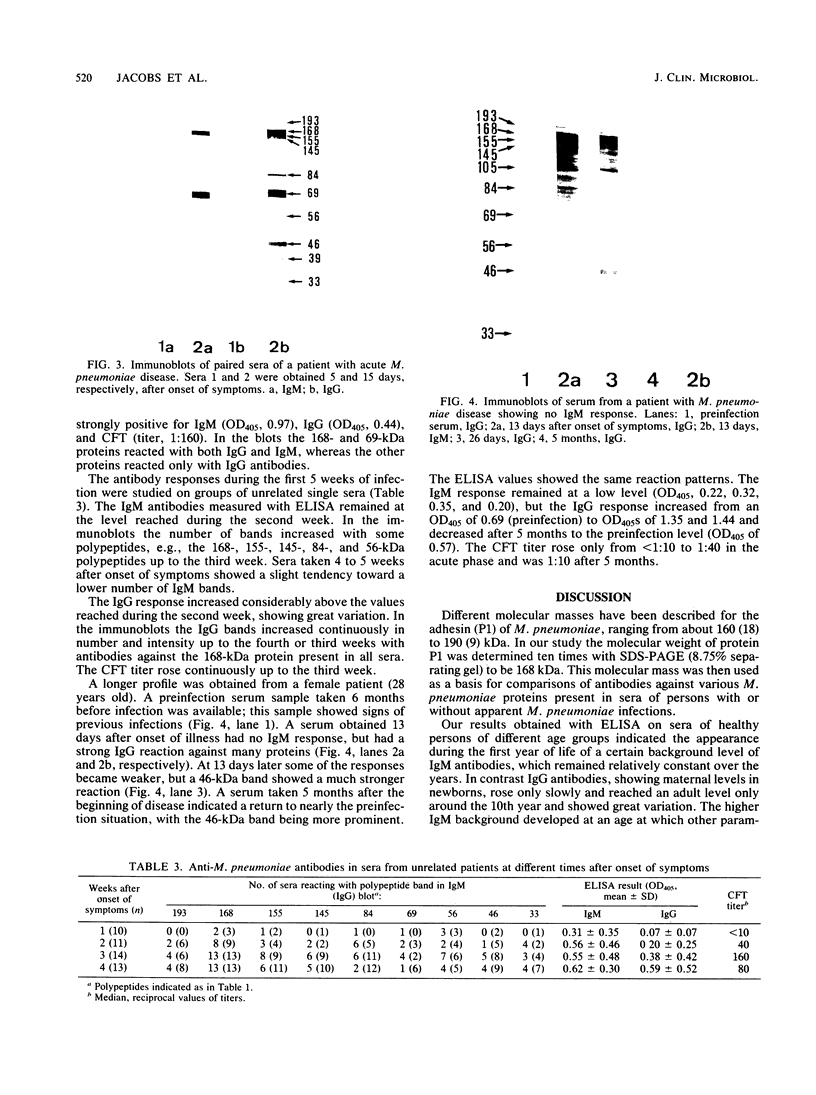
Abstract
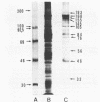
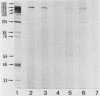
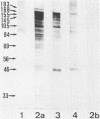
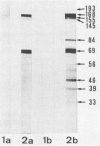
In serological diagnosis of Mycoplasma pneumoniae disease the frequently used complement fixation test is based on a cross-reacting glycolipid. Recently enzyme-linked immunoassays have been developed to overcome this lack of specificity. To study the involvement of the various proteins and the influence of age on the level of antiprotein antibodies present, we investigated by enzyme-linked immunoassays (immunoglobulin M [IgM] and IgG) and immunoblotting the sera of healthy persons of different age groups as well as sera of patients (including paired sera) with M. pneumoniae infection. In sera of children with nonrespiratory diseases and in healthy blood donors the IgM antibodies rose during the first 2 years of life to a relatively constant background level (optical density at 405 nm of 0.15 to 0.21). In contrast IgG remained low up to the seventh year and then increased to moderate levels (optical density of 0.15). The blotting patterns showed few IgM bands in the age group of 20 to 30 years. IgG blots revealed, up to 7 years, only very few reactions with a 168-kilodalton protein, but in higher age groups a considerable number of reactions with proteins of 193, 168, 84, 69, and 56 kilodaltons were detected. In the sera of patients, positive IgM blots were most numerous in the third week, whereas the number of IgG blots increased up to the fourth and the fifth week. At this time all sera contained antibodies against the 168-kilodalton protein, which is identical with the adhesin of M. pneumoniae. In a patient with acute infection, who had a high preinfection IgG level, no IgM response developed. The data indicate a relatively high background of antibodies against M. pneumoniae proteins in older age groups, suggesting a requirement for paired sera. Furthermore, reinfections of adults may occur without a concomitant IgM response.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baseman J. B., Drouillard D. L., Leith D. K., Tully J. G. Absence of Mycoplasma pneumoniae cytadsorption protein P1 in Mycoplasma genitalium and Mycoplasma gallisepticum. Infect Immun. 1984 Mar;43(3):1103–1105. doi: 10.1128/iai.43.3.1103-1105.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biberfeld G. Antibodies to brain and other tissues in cases of Mycoplasma pneumoniae infection. Clin Exp Immunol. 1971 Feb;8(2):319–333. [PMC free article] [PubMed] [Google Scholar]
- Brunner H. Protective efficacy of Mycoplasma pneumoniae polysaccharides. Isr J Med Sci. 1981 Jul;17(7):678–681. [PubMed] [Google Scholar]
- Feldner J., Bredt W., Razin S. Role of energy metabolism in Mycoplasma pneumoniae attachment to glass surfaces. Infect Immun. 1981 Jan;31(1):107–113. doi: 10.1128/iai.31.1.107-113.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner J., Göbel U., Bredt W. Mycoplasma pneumoniae adhesin localized to tip structure by monoclonal antibody. Nature. 1982 Aug 19;298(5876):765–767. doi: 10.1038/298765a0. [DOI] [PubMed] [Google Scholar]
- Fernald G. W., Clyde W. A. Protective Effect of Vaccines in Experimental Mycoplasma pneumoniae Disease. Infect Immun. 1970 Jun;1(6):559–565. doi: 10.1128/iai.1.6.559-565.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Cole R. M., Huang Y. S., Graham J. A., Gardner D. E., Collier A. M., Clyde W. A., Jr Mycoplasma pneumoniae infection: role of a surface protein in the attachment organelle. Science. 1982 Apr 16;216(4543):313–315. doi: 10.1126/science.6801766. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Huang C. H., Collier A. M., Clyde W. A., Jr Demonstration of antibodies to Mycoplasma pneumoniae attachment protein in human sera and respiratory secretions. Infect Immun. 1983 Jul;41(1):437–439. doi: 10.1128/iai.41.1.437-439.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E., Schöpperle K., Bredt W. Adherence inhibition assay: a specific serological test for detection of antibodies to Mycoplasma pneumoniae. Eur J Clin Microbiol. 1985 Apr;4(2):113–118. doi: 10.1007/BF02013574. [DOI] [PubMed] [Google Scholar]
- Kenny G. E., Cartwright F. D. Immunoblotting for determination of the antigenic specificities of antibodies to the Mycoplasmatales. Isr J Med Sci. 1984 Oct;20(10):908–911. [PubMed] [Google Scholar]
- Kleemola M., Käyhty H. Increase in titers of antibodies to Mycoplasma pneumoniae in patients with purulent meningitis. J Infect Dis. 1982 Aug;146(2):284–288. doi: 10.1093/infdis/146.2.284. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lederman M. M., Ellner J. J. Presence of antibodies to Mycoplasma pneumoniae in patients with bacterial meningitis. J Infect Dis. 1983 Aug;148(2):363–365. doi: 10.1093/infdis/148.2.363. [DOI] [PubMed] [Google Scholar]
- Leinikki P. O., Panzar P., Tykkä H. Immunoglobulin M antibody response against Mycoplasma pneumoniae lipid antigen in patients with acute pancreatitis. J Clin Microbiol. 1978 Aug;8(2):113–118. doi: 10.1128/jcm.8.2.113-118.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leith D. K., Trevino L. B., Tully J. G., Senterfit L. B., Baseman J. B. Host discrimination of Mycoplasma pneumoniae proteinaceous immunogens. J Exp Med. 1983 Feb 1;157(2):502–514. doi: 10.1084/jem.157.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind K. Immunological relationships between Mycoplasma pneumoniae and Streptococcus MG. Acta Pathol Microbiol Scand. 1968;73(2):237–244. doi: 10.1111/j.1699-0463.1968.tb00497.x. [DOI] [PubMed] [Google Scholar]
- Lind K., Lindhardt B. O., Schütten H. J., Blom J., Christiansen C. Serological cross-reactions between Mycoplasma genitalium and Mycoplasma pneumoniae. J Clin Microbiol. 1984 Dec;20(6):1036–1043. doi: 10.1128/jcm.20.6.1036-1043.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. P., Wenzel R. P., Senterfit L. B., Beam W. E., Jr Relationship of pre-existing antibody to subsequent infection by Mycoplasma pneumoniae in adults. Infect Immun. 1974 Jan;9(1):53–59. doi: 10.1128/iai.9.1.53-59.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pönkä A., Pönkä T., Sarna S., Penttinen K. Questionable specificity of lipid antigen in the Mycoplasma pneumoniae complement fixation test in patients with extrapulmonary manifestations. J Infect. 1981 Dec;3(4):332–338. doi: 10.1016/s0163-4453(81)91901-0. [DOI] [PubMed] [Google Scholar]
- Räisänen S., Suni J. I., Vaheri A. Mycoplasma pneumoniae protein involved in the antibody response in human infection. J Clin Pathol. 1984 Oct;37(10):1129–1133. doi: 10.1136/jcp.37.10.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Senterfit L. B. Laboratory diagnosis of mycoplasma infections. Isr J Med Sci. 1984 Oct;20(10):905–907. [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Griethuysen A. J., de Graaf R., van Druten J. A., Heessen F. W., van der Logt J. T., van Loon A. M. Use of the enzyme-linked immunosorbent assay for the early diagnosis of Mycoplasma pneumoniae infection. Eur J Clin Microbiol. 1984 Apr;3(2):116–121. doi: 10.1007/BF02014328. [DOI] [PubMed] [Google Scholar]