Abstract
Antiepileptic drugs (AEDs) can adversely affect cognitive function by suppressing neuronal excitability or enhancing inhibitory neurotransmission. The main cognitive effects of AEDs are impaired attention, vigilance, and psychomotor speed, but secondary effects can manifest on other cognitive functions. Although the long-term use of AEDs can obviously elicit cognitive dysfunction in epilepsy patients, their cognitive effects over short periods of up to a year are inconclusive due to methodological problems. In general, the effects on cognition are worse for older AEDs (e.g., phenobarbital) than for placebo, nondrug condition, and newer AEDs. However, topiramate is the newer AED that has the greatest risk cognitive impairment irrespective of the comparator group. Since the cognitive impact of AEDs can be serious, clinicians should be alert to adverse events by evaluating cognitive function using screening tests. Adverse cognitive events of AEDs can be avoided by slow titration to the lowest effective dosage and by avoiding polytherapy.
Keywords: Cognition, Antiepileptic drug, Adverse event
Introduction
Patients with epilepsy often experience cognitive dysfunction. Multiple factors can adversely affect cognition in epilepsy, including the etiology of the seizures, cerebral lesions acquired before the onset of seizures, seizure type, age at onset of epilepsy, seizure frequency, duration, and severity, intraictal and interictal physiologic dysfunction, structural cerebral damage caused by repetitive or prolonged seizures, hereditary factors, psychosocial factors, and sequelae of treatment for epilepsy, including antiepileptic drugs (AEDs) and epilepsy surgery.1-6 All these interrelated factors make complex contributions to cognitive deficits.5 AEDs affect cognition by suppressing neuronal excitability or enhancing inhibitory neurotransmission. Patients, and even some clinicians, tend to blame cognitive problems on AEDs because they are more identifiable than other factors. However, AED effects should not be overrated. Psychosocial problems, which are common, can be overlooked as a source of cognitive impairment. The stigma of epilepsy and the fear of having seizures in public can lead to low self-esteem, social isolation, and depression, all of which can negative affect cognitive function. Similarly, subclinical epileptiform activity is another important contributor to cognitive dysfunction that can go unrecognized, especially in patients with infrequent seizures.
Whilst AEDs are rarely the sole source of cognitive deficits, they do have the potential to affect cognition significantly in certain patients. AEDs exert dosage-dependent effects on cognitive functioning, which can be exacerbated by AED polytherapy. The main cognitive effects of AEDs involve attention/vigilance, psychomotor speed, and secondary involvement of other cognitive functions (e.g., memory).4 The effects of AEDs on cognition are especially significant since AEDs are often be selected based on both traditional measures of treatment effectiveness such as efficacy and tolerability, and their negative neuropsychological side effects. The presence of AED-induced cognitive side effects is an important concern of epilepsy patients taking medications.7 Consequently, the neuropsychologist should attempt to determine the potential effects of AEDs on cognitive performance, as well as the patient's subjective perception of performance, which can also be mediated by the mood and affective state.6
The magnitude of AED-related cognitive dysfunction is generally modest in monotherapy and when the AED is present at therapeutic serum concentrations. However, there are circumstances in which decreased cognitive function assumes greater importance, such as learning in school children,5,8 when driving or operating machinery, and when cognitive skills might be especially vulnerable, such as in the elderly.4 It is especially important to identify and minimize the cognitive effects of AEDs in children, whose developing nervous systems can be more vulnerable to the long-term consequences of AED-induced cognitive impairment.9 Individuals older than 65 years are more susceptible to the cognitive effects of AEDs due to both pharmacodynamic and pharmacokinetic factors.10 Nevertheless, very few studies have specifically examined the cognitive effects of AEDs in this population.
Attempts to identify and quantify the cognitive effects of AEDs have had limited success. Although hundreds of studies have been performed, their methodological flaws, differences in study designs, and contradictory results have resulted in no clear picture emerging. There are three primary factors to consider when assessing the cognitive risk of AEDs, which is a difficult area in which to perform studies in a largely clinical setting.6 The first results from failure to randomize the treatment regimen, since patients with epilepsy that is more difficult to control are more likely to try newer drugs, and hence have cognitive skills prior to treatment that are not representative of the population. The second factor is the difficulty of establishing comparable drug levels, since patients are treated clinically across a wide range of effective dosages, which might have varying effects on cognitive function. Third, the absence of blinding is a concern in many studies. Other methodological issues might also be present. Some studies have employed healthy volunteers rather than patients in order to control for the confounding effects of seizures and pre-existing brain abnormalities. The duration of treatment used in some crossover studies might be inadequate to determine the effects of long-term therapy on neuropsychological status, a concern that is especially relevant to pediatric AED studies since the cognitive effects occur against the backdrop of normal cognitive development. Here we review the current literature on the cognitive effects of AEDs, drawing general conclusions whenever possible whilst taking into account the many underlying methodological problems.
Older AEDs
AEDs are often categorized into "older"(developed before the 1990s) and "newer" (introduced during in the 1990s or later) agents. Since numerous reviews and clinical data are available for older AEDs, here only a brief overview of their cognitive effects is provided. Phenytoin (PHT), carbamazepine (CBZ), valproate (VPA), phenobarbital (PB), and benzodiazepines have been used in epilepsy treatment for many years, and are categorized as older AEDs. The large Veterans Administration (VA) Cooperative Study comparing the cognitive effects of CBZ, PB, PHT, and primidone in a parallel study of patients with new-onset epilepsy found no consistent pattern across AEDs and little change in cognition after AED treatment.11 However, the study design did not control adequately for test-retest effects. The second VA Cooperative Study found no cognitive differences between CBZ and VPA.12 Another study comparing CBZ and PHT in patients with epilepsy also found modest negative effects on cognition for both drugs, and few differential effects.13 Randomized, double-blind crossover studies in healthy volunteers directly comparing AEDs have demonstrated that CBZ, PHT, and VPA have similar cognitive side effects.14,15 In contrast, PB scored significantly worse than PHT or VPA on 32% of the variables. All of these older AEDs affected cognition relative to the nondrug conditions, with approximately half of the variables being significantly worse for CBZ, PHT, and VPA. It was recently reported that total Intelligence Quotient (IQ) (especially performance items) improved in children who discontinued PB compared to those who continued to take PB.16 Thus, among the older AEDs, CBZ, PHT, and VPA exert similar effects on cognitive function, with PB producing greater cognitive impairment.
Newer AEDs
Many newer AEDs have been introduced since the early 1990s, including felbamate (FBM), gabapentin (GBP), lamotrigine (LTG), oxcarbazepine (OXC), topiramate (TPM), tiagabine (TGB), vigabatrin (VGB), zonisamide (ZNS), pregabalin (PGB), and levetiracetam (LEV). Because of serious safety issues, including aplastic anemia and hepatotoxicity, FBM therapy is recommended only for epilepsy patients who are refractory to other AEDs, and its cognitive effects have not been systematically studied.
Despite the large number of newer agents, most neuropsychological studies have compared newer drugs to older AEDs with a higher risk of cognitive impairment, or against newer drugs at dosages that do not reflect current prescribing patterns, making the comparative effects of newer agents unclear.6 Since these methodologies might be unduly influenced by the pharmaceutical companies that financially support cognitive studies, direct head-to-head comparative studies of newer drugs will probably require government support.
Gabapentin (Neurontin®)
GBP has a novel mode of action, which is thought to involve potentiation of GABA-mediated inhibition and possibly inactivation of sodium channels. GBP has few side effects on the central nervous system (CNS), even at relatively high dosages or with rapid dosage escalation,17 and exerts few adverse cognitive effects. A study of patients with partial epilepsy added double-blind placebo or GBP to a stable baseline of one or two other AEDs.18 Patients were treated for 3 months at increasing dosages (up to 2,400 mg/day) and were then crossed over to the alternative therapy. None of the cognitive measures differed significantly between GBP and placebo. A longer term healthy volunteer study comparing GBP with CBZ with a randomized double-blind crossover design found that performance was better with GBP than with CBZ in 8 of the 31 neuropsychological measures assessed.19 Another study found no differences between CBZ and GBP in brief neuropsychological tests or in quantitative electroencephalogram (EEG) in healthy volunteers.20 The use of GBP as an add-on treatment in patients on established AED treatments improved the performance in 1 of 10 cognitive measures.21 Thus, multiple studies involving patients and healthy volunteers suggest that the cognitive effects of GBP are modest, and generally less than those associated with CBZ.
Lamotrigine (Lamictal®)
LTG appears to act by stabilizing sodium channels and reducing glutamate. One study found that the neuropsychological outcome was better for LTG than for CBZ in more than half of the neuropsychological measures assessed in healthy volunteers.22 The superiority of LTG over older AEDs has been demonstrated in studies involving healthy volunteers.23-25 Similar neuropsychological findings have been obtained in patient studies, with add-on treatments producing no incremental impairment noted compared to placebo.26,27 The results of a computerized battery of cognitive tests in children did not differ significantly between LTG and placebo.28 Moreover, LTG and VPA produced the same results in adult epilepsy patients.29 Both of these AEDs improve the performance of list learning and in the Trail-Making Test. The results were also the same for LTG and OXC in adult epilepsy patients.30 The available data appear to indicate that the neuropsychological performance in both adults and children with epilepsy improves after LTG treatment.
Oxcarbazepine (Trileptal®)
OXC is a novel AED that is chemically related to CBZ and is approved as an initial or add-on treatment for partial seizures. Three studies have evaluated the cognitive effects of OXC in adult epilepsy patients.31-33 In the first study, newly diagnosed patients received OXC or other AEDs as a monotherapy for 4 months.31 Compared with baseline, OXC-treated patients improved in 1 of 20 cognitive tasks and worsened in none, and the results were similar in patients receiving CBZ, VPA, PB, or PHT monotherapy. The second study had a double-blind design, and found no differences between OXC and PHT monotherapy in newly diagnosed patients in any of the seven cognitive variables measured at any time point.32 The third study used neuropsychological tests and event-related potentials, and found that 1 year of OXC monotherapy had no negative effects on cognition.33 However, OXC induced mild cognitive impairment accompanied by slight EEG slowing in healthy volunteers, although the magnitude of the effect of OXC was smaller than that observed with PHT.34 In children with newly diagnosed partial epilepsy, no difference between OXC and CBZ, VPA, or combined CBZ/VPA polytherapy in various standard neuropsychological measures and specialized computerized tasks was found during a 6-month follow-up.35,36 In summary, OXC does not appear to afford significant cognitive benefit in adults and children with epilepsy compared to traditional AEDs.
Topiramate (Topamax®)
TPM is a broad-spectrum AED that has multiple mechanisms of action, including blockade of voltage-dependent sodium channels, potentiation of GABA-mediated effects, carbonic anhydrase inhibition, and glutamate antagonism. TPM is the newer AED that generates the greatest concern over its potential negative neuropsychological effects, which include decreased function in language and frontal execution.37-39 TPM can induce somnolence, mental slowing, memory deficit, and language problems in clinical trials. Head-to-head comparative studies have found that neuropsychological impairments are greater for TPM (at 300-400 mg/day) than for LTG, VPA, GBP, and TGB in both epilepsy patients and healthy volunteers.40-44 Importantly, some individuals are disproportionately sensitive to TPM, although the ability to predict this does not presently exist.41 In a monotherapy comparative trial in adult epilepsy patients, the effect on cognition was worse even for TPM at 87 mg/day than for OXC at 825 mg/day.45
The cognitive risk of TPM increases with the dosage. Across various cognitive measures there appears to be a strong relationship between the risk of cognitive impairment and the total daily dose. A prospective study in adult epilepsy patients found that 1 year of TPM monotherapy at 89 mg/day induced cognitive impairment.46 The cognitive effects of TPM were dosage-dependent, and prominent for dosages higher than 75 mg/day. Very similar results were obtained in a short-term prospective study of TPM prophylaxis for migraine.47 Dosage-related cognitive deficits of TPM monotherapy have also been demonstrated in children with epilepsy. Although the cognitive effects were more harmful for TPM than for CBZ in children with benign rolandic epilepsy, the outcome with the minimum target dosage did not differ significantly between the treatment groups.48 In conclusion, although most patients will tolerate TPM, certain situations are associated with clinically significant adverse cognitive events. The factors affecting these events include titration rate, maintenance time, dosage, use of polytherapy, and individual susceptibility.
Tiagabine (Gabatril®)
TGB is a GABA-reuptake inhibitor that is used to treat partial epilepsy. Despite being introduced into Europe in 1995, it is not yet available in Korea. A parallel-group, add-on, randomized, double-blind dosage-response study in epilepsy patients did not reveal any significant cognitive or behavioral effects of TGB.49 In a monotherapy study in newly treated epilepsy patients treated for 52 weeks, the effect of TGB was comparable to that of CBZ, except for CBZ being more harmful to verbal fluency.50 Thus, the cognitive effects of TGB appear modest, and generally comparable to those of CBZ.
Vigabatrin (Sabril®)
VGB is a structural analogue of GABA that irreversibly inhibits the degradative enzyme GABA-transaminase, which increases brain GABA levels. Its use in epilepsy treatment is limited due to evidence of visual field constriction as a side effect. Compared to placebo, VGB produced few adverse effects on either cognitive or quality-of-life measures in patients with epilepsy in a double-blind, randomized, add-on study.51 VGB also produced fewer adverse events than CBZ in a small, open-label, randomized, parallel-group patient study.52 Therefore, VGB does not appear to exert negative effects on cognition.
Zonisamide (Exegran®)
ZNS was introduced into the South Korean market in 1992, and approved as a mono- and adjunctive therapy in treating partial and generalized epilepsy. It works by blocking the presynaptic voltage-sensitive sodium and calcium channels in neurons, or increasing cortical GABA concentrations, and also mildly inhibiting carbonic anhydrase. Two preliminary studies found that ZNS appeared to affect cognitive functions such as attention, memory, and language function when it was used for 12 to 24 weeks.53,54 The worse cognitive performance at 12 weeks of medication tended to improve at 24 weeks of medication.53
However, a significant proportion of epilepsy patients who took ZNS as a monotherapy complained of memory loss (35%) and attention deficit (27%) even after 6 months of therapy,55 which might indicate the intolerable cognitive dysfunction is induced by long-term ZNS treatment. A prospective, randomized, open-label study recently clarified the long-term cognitive effects of ZNS monotherapy in epilepsy patients.56 Despite 1 year of ZNS treatment decreasing the seizure frequency and EEG abnormalities, several cognitive tests also revealed negative effects. The poor performance might have been related to the dosage, especially those higher than 300 mg/day. In summary, the few related studies suggest that ZNS exerts harmful effects on cognition.
Pregabalin (Lyrica®)
PGB was introduced into the South Korean market in 2006, and approved as an add-on therapy for partial onset seizure. PGB binds to the alpha2-delta (A2D) receptors of voltage-gated calcium channels in CNS tissues, and thereby inhibits calcium influx and the release of glutamate, norepinephrine, substance P, and other neurotransmitters. A randomized doubleblind three-period crossover study administering 450 mg/day PGB to healthy volunteers found no significant effects on the objective measures of reaction time, vigilance, and short-term memory, although it was associated with subjective sedation, critical flicker fusion, and divided attention.57 In a short-term comparative add-on trial in adult patients with refractory partial epilepsy, add-on PGB induced partly significant impairments in the episodic memory of verbal and visual information, whereas add-on LEV improved visual short-term memory performance.58 The negative neuropsychological effects of PGB are thought to be temporary under titration. In summary, the few related studies suggest that the cognitive effects of PGB are modest, and greater than those associated with LEV
Levetiracetam (Keppra®)
LEV was introduced into the South Korean market in 2007, and approved as a monotherapy and add-on therapy for partial onset seizure. Recently, it began to be approved as addon therapy for generalized seizure. The mechanism of action of LEV is unique, putatively involving modulation of the functions of the synaptic vesicle protein SV2A, which is its binding site. A healthy-volunteer study found that cognitive deficits were less for LEV than for CBZ.59 Significant differences were present for 42% (23 of 55) of the investigated measures, all favoring LEV. Compared to the nondrug average, the effects of CBZ were worse in 65% (36 of 55) of the investigated measures, and those of LEV were worse in 12% (4 of 33). An observational study found that there was no change in cognitive functioning between patients being treated with LEV and TPM after AED titration.60 Thus, data from both healthy volunteers and patients suggest that LEV exerts few adverse effects on cognition.
How to Minimize the Adverse Cognitive Events
The ideal AED would reduce neuronal irritability without affecting neuronal excitability and cognitive function. The following techniques are used to reduce the cognitive side effects of AEDs: treat underlying disease processes, titrate slowly when initiating AEDs, use the lowest AED dosage possible, use AED monotherapy if possible, avoid AEDs with greater adverse events (e.g., PB), avoid adverse pharmacokinetic interactions, balance all factors with best seizure control, and confirm the seizure diagnosis if the patient is refractory to AEDs. Some patients are best controlled (with the least side effects) on low-dosage, addictive polytherapy.
Conclusions
Patients with epilepsy often experience cognitive dysfunction. An AED treatment can affect cognition either positively or negatively. Since greater seizure frequency, duration, and severity increase the likelihood of impaired cognition, AED intake can reduce cognitive dysfunction by controlling seizures. However, even though the cognitive effects of AEDs are usually modest when these agents are used as a monotherapy at blood concentrations within the standard therapeutic ranges, significant effects such as decreased quality of life or neuropsychological impairment can occur. Furthermore, intolerance of long-term AED-induced cognitive dysfunction will have longer lasting harmful effects on the quality of life.
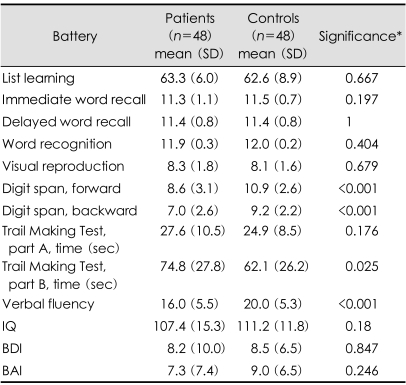
Our epilepsy clinic investigated cognition using neuropsychological tests in patients with well-controlled epilepsy treated with AEDs for at least 1 year to determine whether cognitive deficits were induced by the long-term use of AEDs. We selected 48 patients who had been seizure-free for more than 1 year, normal EEG in neuropsychological tests, and normal MRI in order to exclude cognitive effects of interictal epileptiform discharges or structural brain lesions. Our patients were exposed to AEDs for a mean of 5.8 years (range: 1-30 years) and had a mean seizure-free period of 3 years (range: 1-9 years). Ninety percent of them received monotherapy at the usual dosages, being treated with older or newer AEDs as follows: VPA (n=16), LTG (n=12), OXC (n=7), TPM (n=6), CBZ (n=4), ZNS (n=4), PHT (n=1), Clonazopam (n=1), and PGN (n=1). We compared cognitive measures in these patients with those of 48 age- and educationmatched healthy controls. As indicated in Table 1, the IQ and mood status were normal in both of the epilepsy patients and controls, but the working memory, executive function, and verbal fluency were worse in the patients. The performances of learning and memory tests did not differ between the two groups. These cognitive deficits were not correlated with patient characteristics, epilepsy variables, or type of AEDs. Interestingly, the verbal-fluency performance was well correlated with the AED treatment duration (r=-0.304, p=0.035). Executive dysfunction is a common cognitive side effect of AEDs, and we concluded that any AED can elicit cognitive impairment when it is used for a sufficiently long time.
Table 1.
Neuropsychological test scores of well-controlled epilepsy patients and controls
Higher scores indicate better performance for all cognitive tests except the Trail Making Test. *Independent t test comparing patients and controls. IQ: intelligence quotient, BDI: Beck Depression Inventory, BAI: Beck Anxiety Inventory
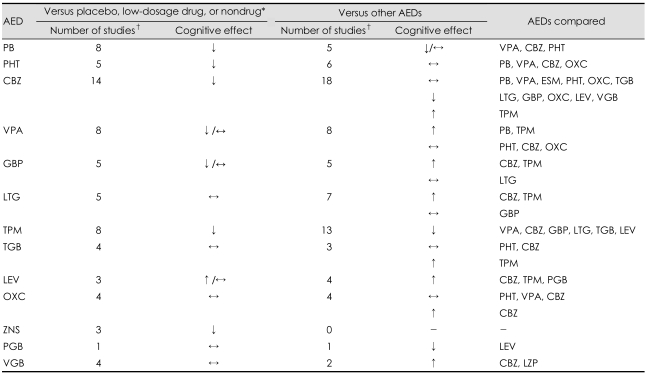
It is clear that the long-term use of AEDs can elicit cognitive dysfunction in epilepsy patients, but the short-term cognitive effects of AEDs (for periods of up to a year) are not conclusive from recent studies. We attempted to summarize the current literature and draw general conclusions despite previous cognitive studies exhibiting many methodological problems. Table 2 summarizes the cognitive effects of AEDs obtained by searching MEDLINE up to January 2008. In general, comparisons of mean data across groups revealed that cognitive performances are worse for older AEDs than for placebo or a nondrug condition. However, comparisons of older AEDs reveal few differences, with only PB showing some consistency in a trend for inferior performance compared to other AEDs. Data availability is worse for newer AEDs than for older AEDs. In general, it appears that newer AEDs performed more favorably compared with placebo or nondrug than have the older AEDs and also performed as well or better than the older AEDs in comparative studies. Especially, there appear to be fewer adverse cognitive effects for GBP, LTG, and LEV than for CBZ. TPM is the newer AED that is associated with the greatest risk of cognitive impairment irrespective of the comparator group, although this risk decreases with the use of slow titration and low target dosages. Although ZNS and PGB are tentatively associated with some cognitive risks, the current data are insufficient for drawing definitive conclusions.
Table 2.
Summary of cognitive effects of antiepileptic drugs (AEDs) obtained by searching MEDLINE up to January 2008
Subject type varied between studies, and included adult and pediatric subjects, normal volunteers, and epilepsy patients. Some studies were counted twice because they included comparisons with placebo or nondrug as well as comparisons with other AEDs. *Depending on the study, AED was compared with placebo, a lower dosage of the same AED, a nondrug baseline, or a non-treated control group, †Includes both double-blind and open-label studies lasting more than 1 week. PB: phenobarbital, VPA: valproic acid, CBZ: carbamazepine, PHT: phenytoin, OXC: oxcarbazepine, ESM: ethosuximide, TGB: tiagabine, LTG: lamotrigine, GBP: gabapentin, LEV: levetiracetam, TPM: topiramate, PGB: pregabalin, ZNS: zonisamide, VGB: vigabatrin, LZP: lorazepam. ↓: generally negative effects on cognition versus comparison group, ↑: generally positive effects on cognition versus comparison group, ↔: generally equal effects on cognition versus comparison group
Various factors can affect cognition in patients with epilepsy, and clinicians require considerable skill to elucidate these factors and mitigate those that can be influenced. The cognitive impact of AEDs can potentially be alleviated, and is therefore important to address. This should be seriously considered in those who are at an age vulnerable to cognitive function, e.g., children and the elderly, and who require maximal cognitive efficiency for their job, school, or daily activities. We therefore recommend that clinicians consider evaluating cognitive function prior to adding or switching an AED, with this evaluation including neuropsychological screening. Since neuropsychological tests can objectively discriminate subtle changes in cognitive function, they might assist when deciding the appropriate AED for improving cognition.
Since findings from studies usually only provide information on the "average" patient, individualization of AED therapy is essential. If a certain AED impairs cognition, trying another AED with a differing pharmacologic profile might be successful. Most of all, it is critical to guard against overmedication. Slow titration to the lowest effective dosage and avoiding polytherapy when possible are keys to success. Refractoriness to AEDs should be determined as rapidly as possible. In refractory patients, clinicians should consider video EEG monitoring to confirm the seizure diagnosis and determine if the patient is a candidate for epilepsy surgery.
Acknowledgment
The authors thank Geum-Ye Bae (a neuropsychologist) for conducting the neuropsychological tests of cognition.
References
- 1.Motamedi G, Meador K. Epilepsy and cognition. Epilepsy Behav. 2003;4(Suppl 2):S25–S38. doi: 10.1016/j.yebeh.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Aldenkamp AP, De Krom M, Reijs R. Newer antiepileptic drugs and cognitive issues. Epilepsia. 2003;44(Suppl 4):S21–S29. doi: 10.1046/j.1528-1157.44.s4.3.x. [DOI] [PubMed] [Google Scholar]
- 3.Ortinski P, Meador KJ. Cognitive side effects of antiepileptic drugs. Epilepsy Behav. 2004;5(Suppl 1):S60–S65. doi: 10.1016/j.yebeh.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Meador KJ. Cognitive outcomes and predictive factors in epilepsy. Neurology. 2002;58:S21–S26. doi: 10.1212/wnl.58.8_suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- 5.Bourgeois BF. Determining the effects of antiepileptic drugs on cognitive function in pediatric patients with epilepsy. J Child Neurol. 2004;19(Suppl 1):S15–S24. doi: 10.1177/088307380401900103. [DOI] [PubMed] [Google Scholar]
- 6.Loring DW, Marino S, Meador KJ. Neuropsychological and behavioral effects of antiepilepsy drugs. Neuropsychol Rev. 2007;17:413–425. doi: 10.1007/s11065-007-9043-9. [DOI] [PubMed] [Google Scholar]
- 7.Carpay JA, Aldenkamp AP, van Donselaar CA. Complaints associated with the use of antiepileptic drugs: results from a community-based study. Seizure. 2005;14:198–206. doi: 10.1016/j.seizure.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Loring DW, Meador KJ. Cognitive side effects of antiepileptic drugs in children. Neurology. 2004;62:872–877. doi: 10.1212/01.wnl.0000115653.82763.07. [DOI] [PubMed] [Google Scholar]
- 9.Holmes GL. Epilepsy in the developing brain: lessons from the laboratory and clinic. Epilepsia. 1997;38:12–30. doi: 10.1111/j.1528-1157.1997.tb01074.x. [DOI] [PubMed] [Google Scholar]
- 10.Garrard J, Harms S, Hardie N, Eberly LE, Nitz N, Bland P, et al. Antiepileptic drug use in nursing home admissions. Ann Neurol. 2003;54:75–85. doi: 10.1002/ana.10593. [DOI] [PubMed] [Google Scholar]
- 11.Smith DB, Mattson RH, Cramer JA, Collins JF, Novelly RA, Craft B. Results of a nation-wide Veterans Administration Cooperative Study comparing the efficacy and toxicity of carbamazepine, phenobarbital, phenytoin, and primidone. Epilepsia. 1987;28(Suppl 3):S50–S58. doi: 10.1111/j.1528-1157.1987.tb05778.x. [DOI] [PubMed] [Google Scholar]
- 12.Prevey ML, Delaney RC, Cramer JA, Cattanach L, Collins JF, Mattson RH The Department of Veterans Affairs Epilepsy Cooperative Study 264 Group. Effect of valproate on cognitive functioning. Comparison with carbamazepine. Arch Neurol. 1996;53:1008–1016. doi: 10.1001/archneur.1996.00550100086018. [DOI] [PubMed] [Google Scholar]
- 13.Pulliainen V, Jokelainen M. Effects of phenytoin and carbamazepine on cognitive functions in newly diagnosed epileptic patients. Acta Neurol Scand. 1994;89:81–86. doi: 10.1111/j.1600-0404.1994.tb01640.x. [DOI] [PubMed] [Google Scholar]
- 14.Meador KJ, Loring DW, Allen ME, Zamrini EY, Moore EE, Abney OL, et al. Comparative cognitive effects of carbamazepine and phenytoin in healthy adults. Neurology. 1991;41:1537–1540. doi: 10.1212/wnl.41.10.1537. [DOI] [PubMed] [Google Scholar]
- 15.Meador KJ, Loring DW, Moore EE, Thompson WO, Nichols ME, Oberzan RE, et al. Comparative cognitive effects of phenobarbital, phenytoin, and valproate in adults subjects. Neurology. 1995;45:1494–1499. doi: 10.1212/wnl.45.8.1494. [DOI] [PubMed] [Google Scholar]
- 16.Tonekaboni SH, Beyraghi N, Tahbaz HS, Bahreynian SA, Aghamohammadpoor M. Neurocognitive effects of phenobarbital discontinuation in epileptic children. Epilepsy Behav. 2006;8:145–148. doi: 10.1016/j.yebeh.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Chadwick DW, Anhut H, Greiner MJ, Alexander J, Murray GH, Garofalo EA, et al. International Gabapentin Monotherapy Study Group 945-77. A double-blind trial of gabapentin monotherapy for newly diagnosed partial seizures. Neurology. 1998;51:1282–1288. doi: 10.1212/wnl.51.5.1282. [DOI] [PubMed] [Google Scholar]
- 18.Leach JP, Girvan J, Paul A, Brodie MJ. Gabapentin and cognition: a double blind, dose ranging, placebo controlled study in refractory epilepsy. J Neurol Neurosurg Psychiatry. 1997;62:372–376. doi: 10.1136/jnnp.62.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meador KJ, Loring DW, Ray PG, Murro AM, King DW, Nichols ME, et al. Differential cognitive effects of carbamazepine and gabapentin. Epilepsia. 1999;40:1279–1285. doi: 10.1111/j.1528-1157.1999.tb00858.x. [DOI] [PubMed] [Google Scholar]
- 20.Salinsky MC, Binder LM, Oken BS, Storzbach D, Aron CR, Dodrill CB. Effects of gabapentin and carbamazepine on the EEG and cognition in healthy volunteers. Epilepsia. 2002;43:482–490. doi: 10.1046/j.1528-1157.2002.22501.x. [DOI] [PubMed] [Google Scholar]
- 21.Mortimore C, Trimble M, Emmers E. Effects of gabapentin on cognition and quality of life in patients with epilepsy. Seizure. 1998;7:359–364. doi: 10.1016/s1059-1311(05)80003-2. [DOI] [PubMed] [Google Scholar]
- 22.Meador KJ, Loring DW, Ray PG, Murro AM, King DW, Perrine KR, et al. Differential cognitive and behavioral effects of carbamazepine and lamotrigine. Neurology. 2001;56:1177–1182. doi: 10.1212/wnl.56.9.1177. [DOI] [PubMed] [Google Scholar]
- 23.Aldenkamp AP, Arends J, Bootsma HP, Diepman L, Hulsman J, Lambrechts D, et al. Randomized double-blind parallel-group study comparing cognitive effects of a low-dose lamotrigine with valproate and placebo in healthy volunteersa. Epilepsia. 2002;43:19–26. doi: 10.1046/j.1528-1157.2002.29201.x. [DOI] [PubMed] [Google Scholar]
- 24.Cohen AF, Ashby L, Crowley D, Land G, Peck AW, Miller AA. Lamotrigine (BW430C), a potential anticonvulsant: Effects on the central nervous system in comparison with phenytoin and diazepam. Br J Clin Pharmacol. 1985;20:619–629. doi: 10.1111/j.1365-2125.1985.tb05120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamilton MJ, Cohen AF, Yuen AW, Harkin N, Land G, Weatherley BC, et al. Carbamazepine and lamotrigine in healthy volunteers: relevance to early tolerance and clinical trial dosage. Epilepsia. 1993;34:166–173. doi: 10.1111/j.1528-1157.1993.tb02393.x. [DOI] [PubMed] [Google Scholar]
- 26.Placidi F, Marciani MG, Diomedi M, Scalise A, Pauri F, Giacomini P, et al. Effects of lamotrigine on nocturnal sleep, daytime somnolence and cognitive functions in focal epilepsy. Acta Neurol Scand. 2000;102:81–86. doi: 10.1034/j.1600-0404.2000.102002081.x. [DOI] [PubMed] [Google Scholar]
- 27.Smith D, Baker G, Davies G, Dewey M, Chadwick DW. Outcomes of add-on treatment with lamotrigine in partial epilepsy. Epilepsia. 1993;34:312–322. doi: 10.1111/j.1528-1157.1993.tb02417.x. [DOI] [PubMed] [Google Scholar]
- 28.Pressler RM, Binnie CD, Coleshill SG, Chorley GA, Robinson RO. Effect of lamotrigine on cognition in children with epilepsy. Neurology. 2006;66:1495–1499. doi: 10.1212/01.wnl.0000216273.94142.84. [DOI] [PubMed] [Google Scholar]
- 29.Kang KH, Lee JM, Lee HW, Jung DK, Suh CK, Kwon SH, et al. Cognitive profiles of lamotrigine in epilepsy patients: a comparative study with valproate. J Korean Epilepsy Soc. 2006;10:146–152. [Google Scholar]
- 30.Seo JG, Lee DI, Hwang YH, Lee HW, Jung DK, Suh CK, et al. Comparison of cognitive effects of lamotrigine and oxcarbazepine in epilepsy patients. J Clin Neurol. 2007;3:31–37. doi: 10.3988/jcn.2007.3.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabers A, Møller A, Dam M, Smed A, Arlien-Søborg P, Buchman J, et al. Cognitive function and anticonvulsant therapy: effect of monotherapy in epilepsy. Acta Neurol Scand. 1995;92:19–27. doi: 10.1111/j.1600-0404.1995.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 32.Aikiä M, Kälviäinen R, Sivenius J, Halonen T, Riekkinen PJ. Cognitive effects of oxcarbazepine and phenytoin monotherapy in newly diagnosed epilepsy: one year follow-up. Epilepsy Res. 1992;11:199–203. doi: 10.1016/0920-1211(92)90099-f. [DOI] [PubMed] [Google Scholar]
- 33.Park SP, Hwang YH, Kim JI, Kim JY, Kwon SH, Jung BW, et al. Cognitive function in epileptic patients treated with oxcarbazepine: neuropsychologic test and event-related potential. J Korean Neurol Assoc. 2002;20:27–33. [Google Scholar]
- 34.Salinsky MC, Spencer DC, Oken BS, Storzbach D. Effects of oxcarbazepine and phenytoin on the EEG and cognition in healthy volunteers. Epilepsy Behav. 2004;5:894–902. doi: 10.1016/j.yebeh.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Donati F, Gobbi G, Campistol J, Rapatz G, Daehler M, Sturm Y, et al. Effects of oxcarbazepine on cognitive function in children and adolescents with partial seizures. Neurology. 2006;67:679–682. doi: 10.1212/01.wnl.0000230138.46508.5b. [DOI] [PubMed] [Google Scholar]
- 36.Donati F, Gobbi G, Campistol J, Rapatz G, Daehler M, Sturm Y, et al. The cognitive effects of oxcarbazepine versus carbamazepine or valproate in newly diagnosed children with partial seizures. Seizures. 2007;16:670–679. doi: 10.1016/j.seizure.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Mula M, Trimble MR, Thompson P, Sander JW. Topiramate and word-finding difficulties in patients with epilepsy. Neurology. 2003;60:1104–1107. doi: 10.1212/01.wnl.0000056637.37509.c6. [DOI] [PubMed] [Google Scholar]
- 38.Lee S, Sziklas V, Andermann F, Farnham S, Risse G, Gustafson M, et al. The effects of adjunctive topiramate on cognitive function in patients with epilepsy. Epilepsia. 2003;44:339–347. doi: 10.1046/j.1528-1157.2003.27402.x. [DOI] [PubMed] [Google Scholar]
- 39.Kockelmann E, Elger CE, Helmstaedter C. Significant improvement in frontal lobe associated neuropsychological functions after withdrawal of topiramate in epilepsy patients. Epilepsy Res. 2003;54:171–178. doi: 10.1016/s0920-1211(03)00078-0. [DOI] [PubMed] [Google Scholar]
- 40.Fritz N, Glogau S, Hoffmann J, Rademacher M, Elger CE, Helmstaedter C. Efficacy and cognitive side effects of tiagabine and topiramate in patients with epilepsy. Epilepsy Behav. 2005;6:373–381. doi: 10.1016/j.yebeh.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Meador KJ, Loring DW, Hulihan JF, Kamin M, Karim R CAPSS-027 Study Group. Differential cognitive and behavioral effects of topiramate and valproate. Neurology. 2003;60:1483–1488. doi: 10.1212/01.wnl.0000063308.22506.19. [DOI] [PubMed] [Google Scholar]
- 42.Meador KJ, Loring DW, Vahle VJ, Ray PG, Werz MA, Fessler AJ, et al. Cognitive and behavioral effects of lamotrigine and topiramate in healthy volunteers. Neurology. 2005;64:2108–2114. doi: 10.1212/01.WNL.0000165994.46777.BE. [DOI] [PubMed] [Google Scholar]
- 43.Salinsky MC, Storzbach D, Spencer DC, Oken BS, Landry T, Dodrill CB. Effects of topiramate and gabapentin on cognitive abilities in healthy volunteers. Neurology. 2005;64:792–798. doi: 10.1212/01.WNL.0000152877.08088.87. [DOI] [PubMed] [Google Scholar]
- 44.Blum D, Meador K, Biton V, Fakhoury T, Shneker B, Chung S, et al. Cognitive effects of lamotrigine compared with topiramate in patients with epilepsy. Neurology. 2006;67:400–406. doi: 10.1212/01.wnl.0000232737.72555.06. [DOI] [PubMed] [Google Scholar]
- 45.Kim SY, Lee HW, Jung DK, Suh CK, Park SP. Cognitive effects of low-dose topiramate compared with oxcarbazepine in epilepsy patients. J Clin Neurol. 2006;2:126–133. doi: 10.3988/jcn.2006.2.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee HW, Jung DK, Suh CK, Kwon SH, Park SP. Cognitive effects of low-dose topiramate monotherapy in epilepsy patients: A 1-year follow-up. Epilepsy Behav. 2006;8:736–741. doi: 10.1016/j.yebeh.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 47.Park SP, Jung DK. Topiramate related cognitive dysfunction during migraine prevention. J Korean Neurol Assoc. 2005;23:55–61. [Google Scholar]
- 48.Kang HC, Eun BL, Lee C Wu, Moon H Ku, Kim JS, D Wook Kim, et al. The effects on cognitive function and behavioral problems of topiramate compared to carbamazepine as monotherapy for children with benign rolandic epilepsy. Epilepsia. 2007;48:1716–1723. doi: 10.1111/j.1528-1167.2007.01160.x. [DOI] [PubMed] [Google Scholar]
- 49.Dodrill CB, Arnett JL, Sommerville KW, Shu V. Cognitive and quality of life effects of differing dosages of tiagabine in epilepsy. Neurology. 1997;48:1025–1031. doi: 10.1212/wnl.48.4.1025. [DOI] [PubMed] [Google Scholar]
- 50.Aikiä M, Jutila L, Salmenperä T, Mervaala E, Kälviäinen R. Comparison of the cognitive effects of tiagabine and carbamazepine as monotherapy in newly diagnosed adult patients with partial epilepsy: pooled analysis of two long-term, randomized, follow-up studies. Epilepsia. 2006;47:1121–1127. doi: 10.1111/j.1528-1167.2006.00545.x. [DOI] [PubMed] [Google Scholar]
- 51.Dodrill CB, Arnett JL, Sommerville KW, Sussman NM. Evaluation of the effects of vigabatrin on cognitive abilities and quality of life in epilepsy. Neurology. 1993;43:2501–2507. doi: 10.1212/wnl.43.12.2501. [DOI] [PubMed] [Google Scholar]
- 52.Kälviäinen R, Aikiä M, Saukkonen AM, Mervaala E, Riekkinen PJ., Sr Vigabatrin vs carbamazepine monotherapy in patients with newly diagnosed epilepsy. A randomized, controlled study. Arch Neurol. 1995;52:989–996. doi: 10.1001/archneur.1995.00540340081016. [DOI] [PubMed] [Google Scholar]
- 53.Berent S, Sackellares JC, Giordani B, Wagner JG, Donofrio PD, Abou-Khalil B. Zonisamide (CI-912) and cognition: results from preliminary study. Epilepsia. 1987;28:61–67. doi: 10.1111/j.1528-1157.1987.tb03624.x. [DOI] [PubMed] [Google Scholar]
- 54.Wilensky AJ, Friel PN, Ojemann LM, Dodrill CB, McCormick KB, Levy RH. Zonisamide in epilepsy: a pilot study. Epilepsia. 1985;26:212–220. doi: 10.1111/j.1528-1157.1985.tb05408.x. [DOI] [PubMed] [Google Scholar]
- 55.Park SP, Kim SY, Hwang YH, Lee HW, Suh CK, Kwon SH. Long-term efficacy and safety of zonisamide monotherapy in epilepsy patients. J Clin Neurol. 2007;3:175–180. doi: 10.3988/jcn.2007.3.4.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park SP, Hwang YH, Lee HW, Suh CK, Kwon SH, Lee BI. Long-term cognitive and mood effects of zonisamide monotherapy in epilepsy patients. Epilepsy Behav. 2008;12:102–108. doi: 10.1016/j.yebeh.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 57.Hindmarch I, Trick L, Ridout F. A double-blind, placebo- and positive-internal-controlled (alprazolam) investigation of the cognitive and psychomotor profile of pregabalin in healthy volunteers. Psychopharmacology (Berl) 2005;183:133–143. doi: 10.1007/s00213-005-0172-7. [DOI] [PubMed] [Google Scholar]
- 58.Ciesielski AS, Samson S, Steinhoff BJ. Neuropsychological and psychiatric impact of add-on titration of pregabalin versus levetiracetam: a comparative short-term study. Epilepsy Behav. 2006;9:424–431. doi: 10.1016/j.yebeh.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 59.Meador KJ, Gevins A, Loring DW, McEvoy LK, Ray PG, Smith ME, et al. Neuropsychological and neurophysiologic effects of carbamazepine and levetiracetam. Neurology. 2007;69:2076–2084. doi: 10.1212/01.wnl.0000281104.55418.60. [DOI] [PubMed] [Google Scholar]
- 60.Gomer B, Wagner K, Frings L, Saar J, Carius A, Härle M, et al. The influence of antiepileptic drugs on cognition: a comparison of levetiracetam with topiramate. Epilepsy Behav. 2007;10:486–494. doi: 10.1016/j.yebeh.2007.02.007. [DOI] [PubMed] [Google Scholar]