Abstract
Background and purpose
Progression of motor deficits in the acute period is frequently observed in patients with subcortical striatocapsular infarctions. Therefore, we sought to determine the factors associated with early motor progression in patients with infarcts confined to the striatocapsular region.
Methods
We studied 80 consecutive patients with striatocapsular-region infarction, as defined by clinical and MRI criteria, within 24 hours after stroke onset. Motor progression was defined as an increase of >2 points in the motor items of the National Institutes of Health Stroke Scale (NIHSS) within 7 days of stroke onset. The study population was divided into patients with and without motor progression, and risk factors, clinical features, and brain MRI/magnetic resonance angiograpy (MRA) findings were compared between these groups.
Results
Motor progression was observed in 40% of the 80 patients. The independent variables associated with motor progression were a history of hypertension (OR=7.8, 95% CI=1.5-39.8, p=0.013) and an initial infarct extent of ≥15 mm (OR=9.2, 95% CI=1.8-45.7, p=0.006). However, there were no differences in the initial NIHSS score, other stroke risk factors, vascular stenosis pattern, or cardioembolic source.
Conclusions
Early motor progression in patients with striatocapsular-region infarction is associated with the initial extent of the lesion. However, the stroke mechanism and vascular stenosis did not differ between the motor progression and stable groups.
Keywords: Striatocapsular infarction, Magnetic resonance imaging, Acute stroke, Stroke progression, Motor deficit, Subcortical infarct
Introduction
Stroke patients with unilateral motor weakness often have deep subcortical infarctions. Several studies have focused on the worsening of motor deficits in patients with lacunar strokes.1-3 However, the worsening of motor deficits in patients with subcortical striatocapsular infarctions has not been widely investigated. Because the classification of deep subcortical infarctions (e.g., striatocapsular and lacunar infarct) is not firmly established and little is known about the mechanism of progression in striatocapsular infarction, we sought to investigate the clinical and radiologic features of progressive motor deficits in patients with strict striatocapsular-region infarctions, regardless of the infarct size.
Methods
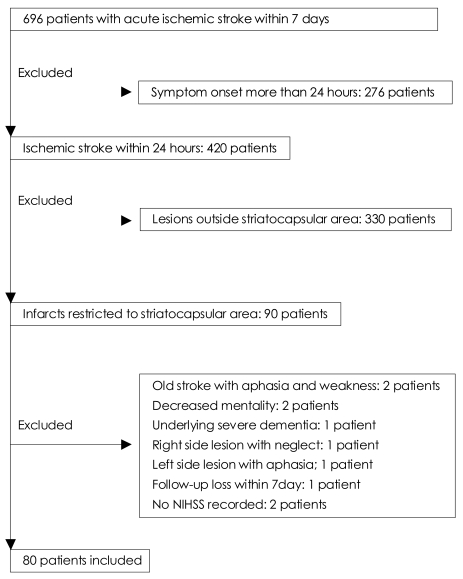
From January 2005 to September 2007, we retrospectively studied consecutive patients with acute ischemic stroke from a prospectively collected acute stroke registry. The inclusion criteria were that the patients had suffered motor deficits with symptom onset within the preceding 24 hours and had no cortical symptoms such as aphasia, neglect, visual field defect, or mental status change. Of 696 patients with acute ischemic stroke within 7 days of symptom onset who were admitted during the study period, 80 were included in this study (Fig. 1). All patients had acute infarction confined to the striatocapsular region as detected by diffusion-weighted imaging (DWI). The infarct size was not an exclusion criterion as long as the infarct was in an appropriate location. All patients underwent DWI within 24 hours of admission, and other sequences of brain MRI and magnetic resonance angiography (MRA) covering the cerebral and carotid arteries within 3 days of admission. MRI was performed using a 1.5-tesla scanner (Intera, Philips Medical Systems, Best, The Netherlands). Axial T2-weighted fast spin-echo imaging (TR: 4,000 ms, TE: 100 ms) and DWI (b=0 and 1,000 s/mm2, TR: 6,000 ms, TE: 74 ms) were included in the routine protocol. The diagnosis of striatocapsular-region infarct was made using previously published templates.4 The area of the infarction had to be confined to the striatocapsular region, regardless of lesion size, in order to be diagnosed as striatocapsular-region infarction (Fig. 2). Striatocapsular infarcts extending into the corona radiata were included. However, isolated corona radiata infarcts were excluded. The extent of the infarct was quantified as the longest diameter of the lesion on axial DWI. Involvement of the posterolateral striatum and lesion extension to the corona radiata were analyzed. Middle cerebral artery (MCA) stenosis was categorized into mild (signal reduction <50%), severe (signal reduction >50%), and occlusion. Carotid artery stenosis was considered severe when the degree of stenosis measured by the North American Symptomatic Carotid Endarterectomy Trial (NASCET) method was >50%. Severe stenosis or occlusion in the MCA or extracranial or intracranial carotid artery on the same hemisphere as the infarct was considered to be a relevant arterial stenosis. We reviewed the medical history, general physical and neurological examination findings, and laboratory test results of each patient. The following risk factors were considered: (1) hypertension, defined as receiving antihypertensive medication or blood pressure >140/90 mmHg on repeated measurement at least 1 week after stroke onset; (2) diabetes mellitus, defined as taking diabetes medication, or fasting blood glucose >126 mg/dL, or 2 hour plasma glucose >200mg/dL; (3) current cigarette smoking; (4) hypercholesterolemia, defined as taking a cholesterol-reducing agent or fasting cholesterol level >220 mg/dL; and (5) cardioembolism was considered if patients had high- or medium-risk sources of cardioembolism according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification.5 All patients underwent electrocardiography and transthoracic echocardiography. Patients with systolic blood pressure >220 mmHg or diastolic blood pressure >120 mmHg received antihypertensive medication during the first 7 days. Otherwise, hypertension was not controlled during the acute stage of stroke.
Fig. 1.
Selection of patients. NIHSS: National Institutes of Health Stroke Scale.
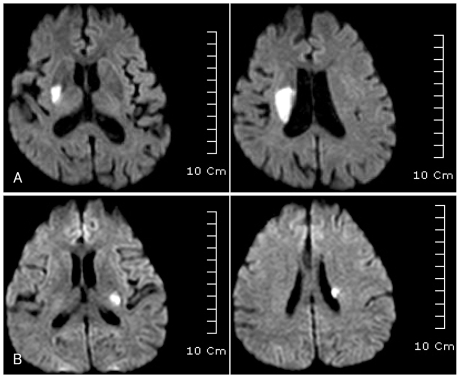
Fig. 2.
MRI findings of striatocapsular-region infarction. A: Initial DWI image of an 81-year-old female with an initial score of 6 on the NIHSS who suffered motor progression (NIHSS score of 10) during admission, which shows right striatocapsular infarct (lesion diameter of 32 mm) extending to the corona radiata. B: Initial DWI image of a 51-year-old man without motor progression (NIHSS score of 3), which shows small striatocapsular infarct (lesion diameter of 14 mm) extending to the corona radiata. DWI: diffusion-weighted imaging, NIHSS: National Institutes of Health Stroke Scale.
The patients' National Institutes of Health Stroke Scale (NIHSS) scores6 were checked at admission, on days 1, 2, 3, 4, 5, and 7, and at discharge. Any changes in the NIHSS score between recording days were also noted. Early motor progression was defined as an increase of at least 2 points in the motor item of the NIHSS lasting for at least 24 hours and within 7 days of stroke onset. Stroke scale was scored by neurologists certified in NIHSS and each patient was examined by the same investigator during the follow-up.
This study was approved by the institutional review board of Kangbuk Samsung Hospital as an anonymous retrospective study.
Statistical analysis was performed using the chi-square test or Fisher's exact test for dichotomized variables and Student's t-test for continuous variables. Potential predictors of motor progression were analyzed by the multiple logistic regression test with the factors of age, gender, hypertension, hyperlipidemia, diabetes mellitus, parent-artery lesion, size of lesion, involvement of the posterolateral striatum, lesion extension to the corona radiata, and presence of a cardioembolic source. Results were expressed as adjusted odds ratios and corresponding 95% confidence intervals. A probability value of p<0.05 was considered statistically significant. Statistical analysis was performed with SPSS software (version 10.0, SPSS, Chicago, IL).
Results
The 80 patients who met the inclusion criteria comprised 43 (54%) men and 37 women aged 29 to 91 years (mean, 61 years). Thirty-two patients (40%) had motor progression, and 48 were classified into the stable motor deficit group.
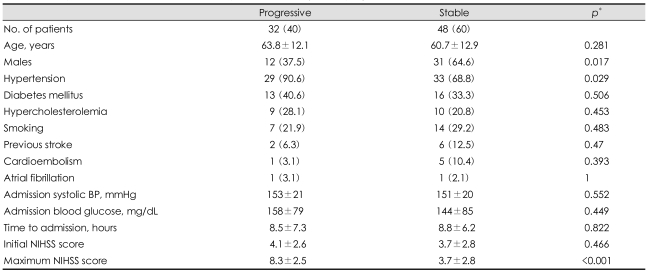
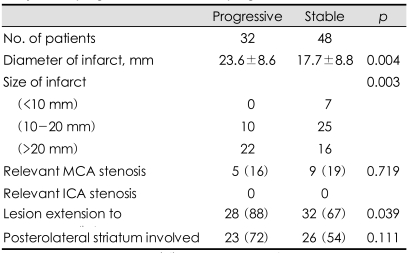
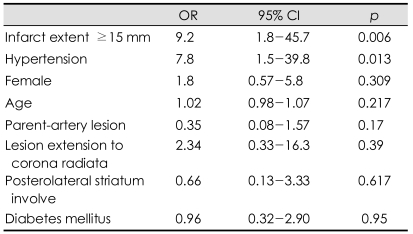
Univariate analysis showed that motor progression was more frequent in female subjects and hypertensive subjects. However, the age, risk factors for stroke except hypertension, admission systolic blood pressure, admission delay, and initial NIHSS scores did not differ between the motor progression and stable patient groups (Table 1). The stroke etiology also did not differ between these two groups. Cardioembolic sources were rarely detected in either group. One patient in the motor progression group and five patients in the stable motor group had cardioembolic sources (p=0.393 by Fisher's exact test). Relevant MCA stenosis was found in nine patients (19%) in the stable motor group and in five patients (16%) in the motor progression group (p=0.719). There were no patients with relevant stenosis of the internal carotid artery. The infarct diameter was 17.7±8.8 mm (mean+SD) in the stable motor group and 23.6±8.6 mm in the motor progression group (p=0.004). No patients with a lesion size of <10 mm showed motor progression (0/7), while 10 patients (29%) with a lesion size of 10-20 mm and 22 patients (58%) with a lesion size of >20 mm showed motor progression (p=0.003). Patients with motor progression had a higher incidence of lesions extending to the corona radiata in univariate analysis (Table 2). Multiple logistic regression analyses showed that an initial infarct extent ≥15 mm and history of hypertension were independent predictors of early motor progression (Table 3).
Table 1.
Comparison of clinical features between patients with early motor progression and without progression
Data are mean±SD or n (%) values. *Chi-square test or Fisher's exact test for categorical variables, difference between means by Student's t-test. BP: blood pressure, NIHSS: National Institutes of Health Stroke scale
Table 2.
Comparison of radiologic features between patients with early motor progression and without progression
Data are mean±SD or n (%) values. MCA: middle cerebral artery, ICA: internal carotid artery
Table 3.
Factors independently associated with early motor progression in multiple logistic regression analysis
OR: odds ratio, CI: confidence interval
Discussion
The present study shows that early motor progression is primarily associated with the initial infarct size in patients with striatocapsular-region infarction. The presumed stroke mechanism and pattern of vascular stenosis did not differ between the motor progression and stable motor deficit patients.
Striatocapsular infarction has been described as a distinct form of subcortical infarction in the MCA territory.7 However, the introduction of DWI and sophisticated methods for vascular imaging has challenged the concept of a distinction between striatocapsular and lacunar infarctions. Several studies have found that the pathogenesis of small lacunar-like infarctions is variable, with the 15 mm size criterion for a lacunar infarction appearing to have no rationale.8-10 Moreover, a recent DWI study suggested that cortical symptoms due to striatocapsular infarction are attributable to cortical infarct demonstrated by DWI, even though cortical infarcts might not be visible on conventional MRI.11 Therefore, the arbitrary separation of striatocapsular infarction and lacunar infarction based on size remains controversial, and hence our study included all patients with striatocapsular-region infarction as long as their motor symptoms were compatible with the inclusion criteria. Motor progression was more frequent in patients with a larger infarct than in those with a smaller infarct, but the stroke etiology and parent-artery lesion did not differ between the two groups. In contradiction to our results, a previous study of deep subcortical infarcts found that the infarct diameter differed with the underlying pathologic mechanism.12 Therefore, we speculated that anatomic variations in the branching pattern of the lenticulostriate artery as well as the stroke mechanism determine the size of the striatocapsular-region infarct. It is well known that the lenticulostriate arteries exhibit widely varying branching patterns and ramification areas.13,14 Intraoperative observations of the variations in the origin of the lenticulostriate arteries demonstrated that multiple lenticulostriate arteries arise from one large trunk in 52% of cases, from two large trunks in 35% of cases, and arise separately in only 13% of cases.15 Therefore, the size of the ischemic zone depends on the caliber of the affected artery, proximity of the lesion and the extent of the ramification.
Progression of motor deficits is highly prevalent in patients with small deep infarcts.3 However, comparison of the neurologic progression between large subcortical infarction and lacunar infarction has not been reported. A study evaluating patients with supratentorial lacunar size infarction in the internal capsule or the corona radiata found that 27% of patients had progressive hemiparesis and that the size of the infarct was slightly larger in patients with progressive infarction.1 Another study also found that the infarct volume was larger in patients with progressive lacunar stroke than in those with stable infarct.16 Both studies showed that larger infarct size was associated with the progression of neurologic deficits; however, both studies included only patients with small lacunar infarct, and brain imaging was only performed after progression had occurred in most patients.
Significant stenosis of the relevant MCA was found in 14 of our patients (18%), and did not differ between the progression and stable groups (Table 2). The association between vascular stenosis and progression of motor deficits has been rarely investigated. Stenotic lesions of the MCA were associated with progressive clinical courses in patients with striatocapsular small, deep infarction.17 Also, in contrast to our study, Adachi et al. reported that small subcortical infarction resulting from large-vessel disease showed more frequent progression than lacunar infarction.8 However, the progression of neurological deficits was not clearly defined in that study.
Our results suggest that the infarct mechanism is not the sole determinant of neurological progression and lesion size in striatocapsular infarction. We postulate that anatomic variations in the lenticulostriate artery also affect the extent of a striatocapsular-region infarction, and that a larger ischemic area with a longer perforating artery requires more time for an infarct to complete, which clinically recognized as progression. This hypothesis was also proposed in a previous report on supratentorial lacunar infarction.1
It is also presumed that occlusion of the distal part of the perforating artery without sufficient length of thrombus propagation would manifest as stable motor deficits as compared with the occlusion of the proximal portion of the lenticulostriate artery. Proximity of an ischemic lesion to the pyramidal tract might also be related to stroke progression, as suggested by MRI tractography.18
There are several noteworthy limitations of our study. First, the sample size was relatively small and especially the small number of patients with MCA stenosis might have lessened the significance of MCA lesions. Second, our study included patients admitted within 24 hours of stroke onset, and therefore progression before admission could not be detected. Third, the infarct volume has been shown to increase during the acute phase,19 and the different timing of the MRI scanning might have influenced the determination of lesion size in our study. However, DWI scanning was performed within 24 hours of admission in all patients, and the significant difference in lesion size between our study groups could not be neglected. Fourth, mild atherosclerotic changes that might cause the striatocapsular infarct might have been underdiagnosed due to the technical limitations of MRA.20
Finally, although hypertension was significantly associated with stroke progression in our study, we did not investigate the relation between temporal changes in blood pressure and stroke progression. Future studies should investigate the relation between acute blood pressure change and stroke progression in detail.
In conclusion, this study suggests that the initial extent of an infarct is predictive of early motor progression in patients with striatocapsular-region infarction. However, the stroke mechanism and parent-artery stenosis are not associated with early motor progression.
References
- 1.Nakamura K, Saku Y, Ibayashi S, Fujishima M. Progressive motor deficits in lacunar infarction. Neurology. 1999;52:29–33. doi: 10.1212/wnl.52.1.29. [DOI] [PubMed] [Google Scholar]
- 2.Serena J, Leira R, Castillo J, Pumar JM, Castellanos M, Dávalos A. Neurological deterioration in acute lacunar infarctions: the role of excitatory and inhibitory neurotransmitters. Stroke. 2001;32:1154–1161. doi: 10.1161/01.str.32.5.1154. [DOI] [PubMed] [Google Scholar]
- 3.Steinke W, Ley SC. Lacunar stroke is the major cause of progressive motor deficits. Stroke. 2002;33:1510–1516. doi: 10.1161/01.str.0000016326.78014.fe. [DOI] [PubMed] [Google Scholar]
- 4.Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of the human brain: cerebral hemispheres. Neurology. 1998;50:1699–1708. doi: 10.1212/wnl.50.6.1699. [DOI] [PubMed] [Google Scholar]
- 5.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 6.Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S, et al. NINDS tPA Stroke Study Group. Improved reliability of the NIH Stroke Scale using video training. Stroke. 1994;25:2220–2226. doi: 10.1161/01.str.25.11.2220. [DOI] [PubMed] [Google Scholar]
- 7.Bladin PF, Berkovic SF. Striatocapsular infarction: large infarcts in the lenticulostriate arterial territory. Neurology. 1984;34:1423–1430. doi: 10.1212/wnl.34.11.1423. [DOI] [PubMed] [Google Scholar]
- 8.Adachi T, Kobayashi S, Yamaguchi S, Okada K. MRI findings of small subcortical "lacunar-like" infarction resulting from large vessel disease. J Neurol. 2000;247:280–285. doi: 10.1007/s004150050584. [DOI] [PubMed] [Google Scholar]
- 9.Waterston JA, Brown MM, Butler P, Swash M. Small deep cerebral infarcts associated with occlusive internal carotid artery disease. A hemodynamic phenomenon? Arch Neurol. 1990;47:953–957. doi: 10.1001/archneur.1990.00530090023007. [DOI] [PubMed] [Google Scholar]
- 10.Cho AH, Kang DW, Kwon SU, Kim JS. Is 15 mm size criterion for lacunar infarction still valid? A study on strictly subcortical middle cerebral artery territory infarction using diffusion-weighted MRI. Cerebrovasc Dis. 2007;23:14–19. doi: 10.1159/000095753. [DOI] [PubMed] [Google Scholar]
- 11.Han MK, Kang DW, Jeong SW, Roh JK. Aphasia following striatocapsular infarction may be explained by concomitant small cortical infarct on diffusion-weighted imaging. Cerebrovasc Dis. 2005;19:220–224. doi: 10.1159/000083886. [DOI] [PubMed] [Google Scholar]
- 12.Bang OY, Yeo SH, Yoon JH, Seok JI, Sheen SS, Yoon SR, et al. Clinical MRI cutoff point for predicting lacunar stroke may not exist: need for a grading rather than a dichotomizing system. Cerebrovasc Dis. 2007;24:520–529. doi: 10.1159/000110422. [DOI] [PubMed] [Google Scholar]
- 13.Marinkovic SV, Milisavljevic MM, Kovacevic MS, Stevic ZD. Perforating branches of the middle cerebral artery. Microanatomy and clinical significance of their intracerebral segments. Stroke. 1985;16:1022–1029. doi: 10.1161/01.str.16.6.1022. [DOI] [PubMed] [Google Scholar]
- 14.Umansky F, Gomes FB, Dujovny M, Diaz FG, Ausman JI, Mirchandani HG, et al. The perforating branches of the middle cerebral artery. A microanatomical study. J Neurosurg. 1985;62:261–268. doi: 10.3171/jns.1985.62.2.0261. [DOI] [PubMed] [Google Scholar]
- 15.Aydin IH, Taçki E, Kadioğlu HH, Kayaoğlu CR, Tüzün Y. The variations of lenticulostriate arteries in the middle cerebral artery aneurysms. Acta Neurochir (Wien) 1996;138:555–559. doi: 10.1007/BF01411176. [DOI] [PubMed] [Google Scholar]
- 16.Lodder J, Gorsselink EL. Progressive stroke caused by CT-verified small deep infarcts; relation with the size of the infarct and clinical outcome. Acta Neurol Scand. 1985;71:328–330. doi: 10.1111/j.1600-0404.1985.tb03208.x. [DOI] [PubMed] [Google Scholar]
- 17.Bang OY, Heo JH, Kim JY, Park JH, Huh K. Middle cerebral artery stenosis is a major clinical determinant in striatocapsular small, deep infarction. Arch Neurol. 2002;59:259–263. doi: 10.1001/archneur.59.2.259. [DOI] [PubMed] [Google Scholar]
- 18.Yamada K, Ito H, Nakamura H, Kizu O, Akada W, Kubota T, et al. Stroke patients' evolving symptoms assessed by tractography. J Magn Reson Imaging. 2004;20:923–929. doi: 10.1002/jmri.20215. [DOI] [PubMed] [Google Scholar]
- 19.Lansberg MG, O'Brien MW, Tong DC, Moseley ME, Albers GW. Evolution of cerebral infarct volume assessed by diffusion-weighted magnetic resonance imaging. Arch Neurol. 2001;58:613–617. doi: 10.1001/archneur.58.4.613. [DOI] [PubMed] [Google Scholar]
- 20.Park KY, Youn YC, Chung CS, Lee KH, Kim GM, Chung PW, et al. Large-artery stenosis predicts subsequent vascular events in patients with transient ischemic attack. J Clin Neurol. 2007;3:169–174. doi: 10.3988/jcn.2007.3.4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]