Abstract
Background and Purpose
We investigated the relationship between the β-fibrinogen gene (FGB) -455 G/A polymorphism and plasma fibrinogen levels in Korean ischemic stroke patients. We also determined whether the frequency of the -455 G/A polymorphism differed between two subtypes of noncardioembolic stroke: large-artery atherosclerosis (LAA) and small-vessel occlusion (SVO).
Methods
A total of 267 patients with noncardioembolic stroke were enrolled. Plasma fibrinogen and other risk factors for stroke were evaluated. FGB -455 G/A genotypes were determined by polymerase chain reaction with restrictive enzyme Hae III and automatic DNA sequencing.
Results
The FGB -455 G/A polymorphism was significantly associated with an elevated plasma fibrinogen level (p<0.001). The frequency of the A allele in Korean stroke patients was 16.7%. However, the frequency of the -455 G/A polymorphism did not differ between LAA and SVO.
Conclusions
The plasma fibrinogen level might be affected by the -455 G/A polymorphism in noncardioembolic stroke patients. However, the LAA and SVO subtypes of ischemic stroke were not affected by the -455 G/A polymorphism.
Keywords: Fibrinogen, Polymorphism, Stroke
INTRODUCTION
Prospective studies with large samples have suggested that the plasma fibrinogen level is an independent risk factor for coronary artery disease or stroke.1-3 It is well known that elevated plasma fibrinogen levels can be affected by environmental and genetic factors. It has been reported that some of the 10 or more genetic polymorphisms of the fibrinogen gene that have been investigated to date may be involved in elevation of the plasma fibrinogen level.4-9 Polymorphisms of the β-fibrinogen gene (FGB) including the β-455 G/A polymorphism - which is especially involved in the rate-limiting steps of the formation of the β-chain - have been shown to be closely related to elevation of the plasma fibrinogen level. Several studies have suggested that the FGB -455 G/A polymorphism is associated with an elevated plasma fibrinogen concentration and ischemic stroke.10-12
In terms of subtypes of ischemic stroke, it has been suggested that the FGB -455 polymorphism increases the incidence of multiple lacunar infarction.11 The FGB -455 polymorphism may also be associated with large-artery atherosclerosis (LAA) rather than smallvessel occlusion (SVO),12 and hence there is controversy regarding the relationship between the subtypes of cerebral infarction and the FGB -455 polymorphism.
This study was designed to determine the relationship between the FGB -455 polymorphism and plasma fibrinogen levels in patients with noncardioembolic stroke (LAA and SVO), and to elucidate whether the frequency of the FGB -455 polymorphism differs with the subtype of ischemic stroke.
MATERIALS AND METHODS
1. Subjects
This study investigated acute-onset (< 7 days) cerebral infarction patients who visited our clinic and were diagnosed using brain magnetic resonance imaging. The patients were divided into subtypes according to Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification.13 Patients with cardiogenic embolism, at least two etiologies, or with unknown etiologies at the time of diagnosis were excluded. Patients with the following potential factors that could affect plasma fibrinogen levels were also excluded: (1) administration of antiplatelet agents prior to the onset of cerebral infarction, (2) apparent infections, and (3) cancers. Infection was regarded as present if there was a positive history and/or clinical signs of any current infection in combination with laboratory findings (e.g., elevated C-reactive protein, leukocytosis of >12,000 cells/µL, or pyuria in urinalysis). Of 344 patients who were initially recruited, 77 patients were excluded due to previous antiplatelet-agent medication (n=35), apparent infection (n=33), and cancer (n=9).
Demographic characteristics, medical history, and risk factors for cerebral infarction such as hypertension, diabetes mellitus, hyperlipidemia, and smoking were investigated in all subjects. Patients were diagnosed as hypertensive if they were currently receiving antihypertensive medication or had a systolic blood pressure of greater than 140 mmHg or a diastolic blood pressure of greater than 90 mmHg on two separate examinations. Patients were diagnosed as diabetes mellitus if they were currently undergoing treatment with insulin or oral hypoglycemic agents, or if their fasting blood glucose level was higher than 140 mg/dL on two separate examinations. Patients were diagnosed as hyperlipidemia if they were currently receiving lipid-lowering agents, or if their total cholesterol level and LDL-cholesterol level exceeded the normal limits (240 and 130 mg/dL, respectively). The smoking habit was defined as either smoking (current smokers and ex-smokers) or nonsmoking.
Blood samples were collected prior to any patient management. The plasma fibrinogen level was measured using a coagulation analyzer (CA-7000, Sysmex, IL, USA). Routine hematology, blood coagulation, and serum lipid tests were also performed. The study was approved by the ethics committee of our hospital, and all patients or their caregivers signed a consent form for the genetic analysis.
2. Genetic analysis
Genomic DNA was extracted from peripheral blood lymphocytes using a standard protocol. The polymerase chain reaction (PCR) primers for DNA -fragments in the promoter region of the fibrinogen gene -455 G/A polymorphism were 5'-TGT TTA GAA TTT GTT GAA CAT TTT ACC-3' (sense) and 5'-GAG GAG TGC CCT AAA CTT CCC-3' (antisense). PCR amplification was performed with a 50-µL reaction volume containing 1 µL of DNA template, 1 µL of each of the sense and antisense primers, 4 µL of deoxynucleotide triphosphate, 5 µL of MgCl2, and 0.5 µL of AmpliTaq Gold™ polymerase (Perkin Elmer, CT, USA). The amplification conditions were as follows: an initial denaturing step at 96℃ for 7 min, followed by 35 amplification cycles of denaturation at 94℃ for 30 sec, annealing at 58℃ for 30 sec, and extension at 72℃ for 30 sec, and a final extension step of 72℃ for 10 min. PCR products were electrophoresed on a 2% agarose gel, and the amplified genomic DNA fragments were extracted from the gel and purified using a QIAquick® gel extraction kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. After 45 µL of amplified PCR products was mixed with 5 U of restrictive enzyme Hae III and buffer, they were allowed to react at 37℃ for 4 hours. The final product was electrophoresed on a 4% gel containing EtBr, and its genotypes were analyzed using a UV transilluminator. Direct sequencing was performed using BigDye terminator kits (PE Biosystems, CA, USA) to determine individual genotypes.
3. Data analysis
The frequency of analyzed genotypes, including a minor A allele, were determined. The study subjects were divided into wild (G/G) and mutant (G/A or A/A) groups according to genotypes, and demographic characteristics such as age and gender, risk factors for cerebral infarction such as hypertension, diabetes mellitus, hyperlipidemia, and smoking, and fibrinogen level were compared between the two groups. Additionally, variations in the frequencies of A allele with subtypes of cerebral infarction were investigated. Fibrinogen levels were compared between the wild and mutant groups in patients with LAA and those with SVO.
Statistical analyses were performed using SPSS software, version 12.0 (SPSS, IL, USA). Student's t test and the chi-square test were used to compare not only fibrinogen levels and risk factors for cerebral infarction with respect to fibrinogen polymorphisms, but also clinical features and fibrinogen levels with respect to pathogenic mechanisms of cerebral infarction between the two groups.
RESULTS
1. FGB -455 G/A polymorphism in all patients
The 267 patients with cerebral infarction were aged 64.6±11.4 (mean±SD) years, and comprised 156 (58.4%) males and 111 (41.6%) females. The risk factors for cerebral infarction were hypertension (178 patients, 66.7%), diabetes mellitus (75 patients, 28.1%), and hyperlipidemia (56 patients, 21.1%).
The FGB -455 G/A polymorphism was present in 79 (29.6%) patients. Overall, the wild (G/G), G/A, and A/A types were present in 188 (70.4%), 69 (25.8%), and 10 (3.7%) patients, respectively. The overall frequency of the minor A allele was 16.7%.
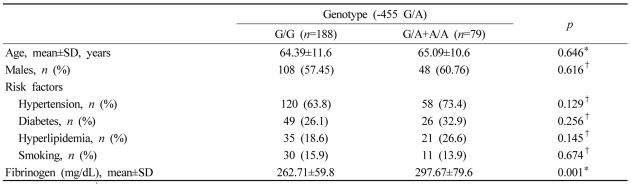
Demographic characteristics such as age and gender, and risk factors for cerebral infarction such as hypertension, diabetes mellitus, and smoking did not differ significantly between the wild and mutant groups. However, plasma fibrinogen levels were significantly higher in the mutant group than in the wild group (p=0.001, Table 1).
Table 1.
Demographic features, risk factors, and fibrinogen levels in noncardioembolic stroke patients according to genotypes of the β-fibrinogen gene (FGB) -455 G/A polymorphism
*Student's t test, †chi-square test
2. Plasma fibrinogen and the -455G/A polymorphism in LAA and SVO
1) Frequency of the -455 G/A polymorphism in LAA and SVO
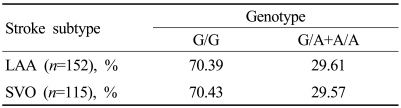
One hundred and fifty-two patients had LAA and the others had SVO according to the TOAST classification. The mutant group contained 45 (29.61%) of the 152 patients with LAA and 34 (29.57%) of the 115 patients with SVO. The frequency of the FGB -455 G/A polymorphism did not differ between the wild and mutant groups (Table 2).
Table 2.
Distribution of genotypes of the FGB .455 G/A polymorphism according to stroke subtype
LAA; large-artery atherosclerosis, SVO; small-vessel occlusion.
2) Plasma fibrinogen levels according to the -455 G/A polymorphism in LAA and SVO
Patients with LAA and those with SVO were divided into wild and mutant groups according to the -455G/A polymorphism. Plasma fibrinogen levels were higher in patients with the A allele in each of the LAA and SVO groups (p=0.003 and 0.011, respectively). However, the other factors did not differ significantly between the wild and mutant groups.
DISCUSSION
An elevated plasma fibrinogen level is known to be an independent risk factor for cerebral infarction, ischemic heart disease, venous thrombosis, and peripheral artery disease.1,14-17 Fibrinogen is an important protein involved in blood coagulation, and is synthesized in the liver. On activation in vivo, it is converted into fibrin and forms thrombi with subsequent hemostasis.17-19 The fibrinogen polymer consists of single α-, β-, and γ-chains, the gene clusters for each of which are located within a 50 kb region on the long arm of chromosome 4 (q28).20 Although mRNA for each of the three chains is independently synthesized in the fibrinogen genes, the regulation of transcription by the three genes is known to be closely related. Several studies have suggested that synthesis of β-fibrinogen is a rate-limiting step in the assembly of experimentally induced mature fibrinogen, suggesting that it is the most important factor affecting plasma fibrinogen levels.20,21
This study investigated the relationship between the FGB -455 G/A polymorphism and plasma fibrinogen levels in patients with two subtypes of ischemic stroke: LAA and SVO. Although elevated plasma fibrinogen can contribute to the development of cardioembolism,22 this condition was excluded in the present study because the focus was on clarifying the debate about differences in the genetic influence of the -455 G/A polymorphism between LAA and SVO.11,12
The G→A substitution in the promoter region of FGB was associated with an increase in plasma fibrinogen levels in 267 patients with cerebral infarction. In a meta-analysis, Iacoviello et al. found that the fibrinogen levels were higher in patients with the A allele in 19 of 23 articles, which corresponds well with the results of this study.21
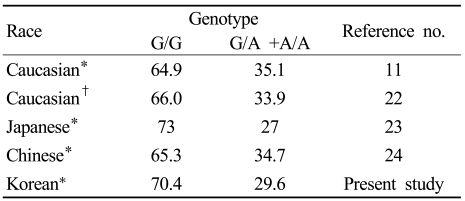
We initially suspected that genotypes of the FGB -455 G/A polymorphism would differ with race. However, the genotypes of patients with ischemic stroke or myocardial infarction were quite similar across different races (Table 3),11,23-25 which suggests that the genotype of the FGB -455 G/A polymorphism does not vary with race. However, since our results and those of the studies referenced in Table 3 applied to patients with ischemic stroke or myocardial infarction, these findings might not be representative of all Koreans and other races.
Table 3.
Distribution of genotypes of the FGB .455 G/A polymorphism according to race
Data in columns 2 and 3 are percentages relative to all the cases studied.
*Patients with cerebral infarction, †patients with myocardial infarction
This study found that the frequency of the FGB -455G/A polymorphism did not differ significantly between patients with LAA and those with SVO. Previous studies have found that this polymorphism is a risk factor for multiple lacunar cerebral infarction, namely SVO, with others suggesting that it is also a risk factor for LAA.11,12 With regards to SVO, the A allele elevates the fibrinogen concentration in the circulation, which might contribute to the progression of arteriosclerosis primarily in smaller cerebral arteries with a slower blood flow. This would predispose to the development of occlusions in small cerebral arteries and finally to multiple lacunar infarcts.11 However, in patients with LAA, elevated fibrinogen concentrations eventually promote atherosclerosis. It has also been reported that fibrinogen is involved in the formation of thrombi and the development of early atherosclerotic plaques, and is closely associated with the progression of atherosclerotic plaques.12 Possible mechanisms underlying these observations are the affinity of fibrin to lipoprotein a and the proliferation of vascular smooth-muscle cells by FDP, a fibrinogen metabolite.26
To the best of our knowledge, this is the first comparison of the FGB -455 G/A polymorphism between patients with LAA and those with SVO. Whilst we cannot confirm the relationship between FGB -455 G/A polymorphism and subtypes of cerebral infarction (LAA and SVO) from the results of this study, the absence of a significant difference in the frequency of this polymorphism between LAA and SVO suggests that it has little effect on different subgroups of ischemic stroke. However, the pathogenic mechanisms underlying LAA and SVO might be similar,27,28 and the FGB polymorphism might commonly contribute to the polygenic potential for the development of both types of ischemic stroke. LAA can be distinguished from SVO according to the TOAST classification, which is based on clinical determinations of ischemic stroke including neuroimaging findings (e.g., size of infarction and degree of arterial stenosis). However, the possibility of subgroups from the TOAST classification overlapping might make it difficult to elucidate the exact underlying pathogenic mechanisms.
Our study was subject to some limitations. The relationship between the baseline fibrinogen level before stroke and the -455 G/A polymorphism could not be determined since only ischemic stroke patients were enrolled (i.e., no healthy control subjects were included). Also, acute stroke patients were recruited, and their fibrinogen levels could have been elevated as an acute-phase reaction. Further examinations of the plasma fibrinogen level in the chronic state would provide more information about the relationship between fibrinogen and the -455 G/A polymorphism.
In summary, the level of plasma fibrinogen was higher in ischemic stroke patients with the FGB -455 A allele. The genotypes of the FGB -455 G/A polymorphism in Korean stroke patients were similar to those of other races. Also, the frequency of the polymorphism did not differ between patients with LAA and those with SVO. Further studies on the FGB -455 G/A polymorphism with larger series of patients and including healthy control subjects are needed to elucidate the underlying mechanisms.
Footnotes
This paper was supported by grant no. CUHRICM-U-2004006 from the Chonnam University Hospital Research Institute of Clinical Medicine.
References
- 1.Wilhelmsen L, Svärdsudd K, Korsan-Bengtsen K, Larsson B, Welin L, Tibblin G. Fibrinogen as a risk factor for stroke and myocardial infarction. N Engl J Med. 1984;311:501–505. doi: 10.1056/NEJM198408233110804. [DOI] [PubMed] [Google Scholar]
- 2.Maresca G, Di Blasio A, Marchioli R, Di Minno G. Measuring plasma fibrinogen to predict stroke and myocardial infarction: an update. Arterioscler Thromb Vasc Biol. 1999;19:1368–1377. doi: 10.1161/01.atv.19.6.1368. [DOI] [PubMed] [Google Scholar]
- 3.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 4.Tybjaerg-Hansen A, Agerholm-Larsen B, Humphries SE, Abildgaard S, Schnohr P, Nordestgaard BG. A common mutation (G-455→A) in the β-fibrinogen promoter is an independent predictor of plasma fibrinogen, but not of ischemic heart disease. A study of 9,127 individuals based on the Copenhagen City Heart Study. J Clin Invest. 1997;99:3034–3039. doi: 10.1172/JCI119499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leander K, Wiman B, Hallqvist J, Falk G, De Faire U. The G-455A polymorphism of the fibrinogen beta-gene relates to plasma fibrinogen in male cases, but does not interact with environmental factors in causing myocardial infarction in either men or women. J Intern Med. 2002;252:332–341. doi: 10.1046/j.1365-2796.2002.01041.x. [DOI] [PubMed] [Google Scholar]
- 6.Komitopoulou A, Platokouki H, Kapsimali Z, Pergantou H, Adamtziki E, Aronis S. Mutations and polymorphisms in genes affecting hemostasis proteins and homocysteine metabolism in children with arterial ischemic stroke. Cerebrovasc Dis. 2006;22:13–20. doi: 10.1159/000092332. [DOI] [PubMed] [Google Scholar]
- 7.Doggen CJ, Bertina RM, Cats VM, Rosendaal FR. Fibrinogen polymorphisms are not associated with the risk of myocardial infarction. Br J Haematol. 2000;110:935–938. doi: 10.1046/j.1365-2141.2000.02266.x. [DOI] [PubMed] [Google Scholar]
- 8.Yang Z, Li F, Liu G, Cai W, Ling G. The study of β-fibrinogen gene -455G/A, -148 C/T, 448 G/A polymorphisms and their association with plasma fibrinogen levels. Zhonghua Xue Ye Xue Za Zhi. 2000;21:463–465. [PubMed] [Google Scholar]
- 9.van't Hooft FM, von Bahr SJF, Silveira A, Iliadou A, Eriksson P, Hamsten A. Two common, functional polymorphisms in the promoter region of the β-fibrinogen gene contribute to regulation of plasma fibrinogen concentration. Arterioscler Thromb Vasc Biol. 1999;19:3063–3070. doi: 10.1161/01.atv.19.12.3063. [DOI] [PubMed] [Google Scholar]
- 10.Maumus S, Marie B, Vincent-Viry M, Siest G, Visvikis-Siest S. Analysis of the effect of multiple genetic variants of cardiovascular disease risk on insulin concentration variability in healthy adults of the STANISLAS cohort. The role of FGB -455 G/A polymorphism. Atherosclerosis. 2007;191:369–376. doi: 10.1016/j.atherosclerosis.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Martiskainen M, Pohjasvaara T, Mikkelsson J, Mäntylä R, Kunnas T, Laippala P, et al. Fibrinogen gene promoter -455 A allele as a risk factor for lacunar stroke. Stroke. 2003;34:886–891. doi: 10.1161/01.STR.0000060029.23872.55. [DOI] [PubMed] [Google Scholar]
- 12.Kessler C, Spitzer C, Stauske D, Mende S, Stadlmüller J, Walther R, et al. The apolipoprotein E and betafibrinogen G/A-455 gene polymorphisms are associated with ischemic stroke involving large-vessel disease. Arterioscler Thromb Vasc Biol. 1997;17:2880–2884. doi: 10.1161/01.atv.17.11.2880. [DOI] [PubMed] [Google Scholar]
- 13.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 14.Lee AJ, Lowe GD, Woodward M, Tunstall-Pedoe H. Fibrinogen in relation to personal history of prevalent hypertension, diabetes, stroke, intermittent claudication, coronary heart disease, and family history: the Scottish Heart Health Study. Br Heart J. 1993;69:338–342. doi: 10.1136/hrt.69.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maresca G, Di Blasio A, Marchioli R, Di Minno G. Measuring plasma fibrinogen to predict stroke and myocardial infarction: an update. Arterioscler Thromb Vasc Biol. 1999;19:1368–1377. doi: 10.1161/01.atv.19.6.1368. [DOI] [PubMed] [Google Scholar]
- 16.Kofoed SC, Wittrup HH, Sillesen H, Nordestgaard BG. Fibrinogen predicts ischaemic stroke and advanced atherosclerosis but not echolucent, rupture-prone carotid plaques: the Copenhagen City Heart Study. Eur Heart J. 2003;24:567–576. doi: 10.1016/s0195-668x(02)00467-0. [DOI] [PubMed] [Google Scholar]
- 17.Fuss C, Palmaz JC, Sprague EA. Fibrinogen: Structure, function, and surface interactions. J Vasc Interv Radiol. 2001;12:677–682. doi: 10.1016/s1051-0443(07)61437-7. [DOI] [PubMed] [Google Scholar]
- 18.Lane DA, Grant PJ. Role of hemostatic polymorphisms in venous and arterial thrombotic disease. Blood. 2000;95:1517–1532. [PubMed] [Google Scholar]
- 19.Di Napoli M, Papa F, Bocola V. Prognostic influence of increased C-reactive protein and fibrinogen levels in ischemic stroke. Stroke. 2001;32:133–138. doi: 10.1161/01.str.32.1.133. [DOI] [PubMed] [Google Scholar]
- 20.Resch KL, Ernst E, Matrai A, Paulsen HF. Fibrinogen and viscosity as risk factors for subsequent cardiovascular events in stroke survivors. Ann Intern Med. 1992;117:371–375. doi: 10.7326/0003-4819-117-5-371. [DOI] [PubMed] [Google Scholar]
- 21.Iacoviello L, Vischetti M, Zito F, Benedetta Donati M. Genes encoding fibrinogen and cardiovascular risk. Hypertension. 2001;38:1199–1203. doi: 10.1161/hy1101.099478. [DOI] [PubMed] [Google Scholar]
- 22.Ohira T, Shahar E, Chambless LE, Rosamond WD, Mosley TH, Jr, Folsom AR. Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities study. Stroke. 2006;37:2493–2498. doi: 10.1161/01.STR.0000239694.19359.88. [DOI] [PubMed] [Google Scholar]
- 23.Scarabin PY, Bara L, Ricard S, Poirier O, Cambou JP, Arveiler D, et al. Genetic variation at the β-fibrinogen locus in relation to fibrinogen concentrations and risk of myocardial infarction: the ECTIM study. Arterioscler Thromb. 1993;13:886–891. doi: 10.1161/01.atv.13.6.886. [DOI] [PubMed] [Google Scholar]
- 24.Nishiuma S, Kario K, Yakushijin K, Maeda M, Murai R, Matsuo T, et al. Genetic variation in the promoter region of the beta-fibrinogen gene is associated with ischemic stroke in a Japanese population. Blood Coagul Fibrinolysis. 1998;9:373–379. doi: 10.1097/00001721-199806000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Pan J, Wang S, Li X, Huang Y. Beta-fibrinogen gene -455A/G polymorphism and plasma fibrinogen level in Chinese stroke patients. Chin Med J (Engl) 2002;115:214–216. [PubMed] [Google Scholar]
- 26.Anderson GM, Shaw AR, Schafer JA. Functional characterization of promoter elements involved in regulation of human B beta-fibrinogen expression. Evidence for binding of novel activator and repressor proteins. J Biol Chem. 1993;268:22650–22655. [PubMed] [Google Scholar]
- 27.Hollander M, Bots ML, Del Sol AI, Koudstaal PJ, Witteman JC, Grobbee DE, et al. Carotid plaques increase the risk of stroke and subtypes of cerebral infarction in asymptomatic elderly: the Rotterdam study. Circulation. 2002;105:2872–2877. doi: 10.1161/01.cir.0000018650.58984.75. [DOI] [PubMed] [Google Scholar]
- 28.Tejada J, Díez-Tejedor E, Hernnádez-Echebarría L, Balboa O. Does a relationship exist between carotid stenosis and lacunar infarction? Stroke. 2003;34:1404–1409. doi: 10.1161/01.STR.0000072520.53106.8C. [DOI] [PubMed] [Google Scholar]