Abstract
We report a case of recurrent Streptococcus Pneumoniae meningoencephalitis with a transethmoidal meningoencephalocele (TEME) but without cerebrospinal fluid (CSF) leakage.
A 35-year-old man was admitted with S. pneumoniae meningitis. He had suffered from four episodes of recurrent pneumococcal meningitis during the previous 4 years. A computed tomography scan of the paranasal sinus showed the TEME protruding through a bony defect of the right frontal base. However, the patient did not have symptoms that could be attributable to CSF leakage, and radioisotope cisternography did not identify a leak. Brain magnetic resonance imaging revealed cortical lesions overlying the TEME, and electroencephalography revealed epileptiform discharges in frontal regions. Appropriate antibiotics therapy without steroids was given to improve his condition.
The presented case suggests that even in the absence of clinically demonstrable CSF leakage, an occult skullbase defect and its associated meningoencephalocele should be considered in patients with recurrent bacterial meningitis.
Keywords: Recurrent meningitis, Streptococcus Pneumoniae, Transethmoidal meningoencephalocele
Recurrent bacterial meningitis is indicative of an underlying pathological condition such as immunologic derangements or anatomic defects. Immunologic derangements that can cause recurrent bacterial meningitis include asplenia,1,2 human immunodeficiency virus infection,3 and complement system deficiency.4 The anatomic defects usually appear at the skull base, and are caused by a trauma5 or congenital anormalies.6,7 The defects allow persisting communication between the subarachnoid space and the air-filled cells of the temporal bone or the paranasal sinuses, and may offer a route of direct invasion of commensals such as Streptococcus Pneumoniae.8
Recurrent bacterial meningitis is usually caused by anatomic defects associated with the cerebrospinal fluid (CSF) leakage, which can manifest as rhinorrhea or otorrhea.5,6 CSF leakage has prompted an extensive search for an occult anatomic defect, such as a transethmoidal meningoencephalocele (TEME).7 CSF leakage is not only a subjective symptom but also an important objective sign of recurrent meningitis or anatomic defects.9
However, in the clinical setting it is necessary to interpret recurrent meningitis and the associated anatomic skull defects if there is no evidence of CSF leakage. Here we describe a case of recurrent S. pneumoniae meningoencephalitis with a TEME but without CSF leakage.
CASE REPORT
A 35-year-old man was admitted because of headache, fever, nausea, vomiting, and a generalized seizure attack that had appeared 1 day previously. He had suffered from three other episodes of meningitis within the previous 4 years, each of which was preceded by a mild infection of the upper respiratory tract. He had no history of trauma or surgery.
A physical examination showed a fever of 39.5℃, signs of meningeal irritation, and a stuporous mental state. A laboratory investigation revealed 14,600 leukocytes/mm3, an erythrocyte sedimentation rate of 12 mm/hour, and C-reactive protein at 249 mg/L. CSF was turbid and contained 149 leukocytes/mm3 (70% polymorphonuclear leukocytes and 30% lymphocytes), protein at 169.4 mg/dL, and glucose at 60 mg/dL (serum glucose was 143). Serologic testing for human immunodeficiency virus, immunoglobulin (Ig) G, IgA, IgM, and complement levels including terminal components (C5-C8) produced normal findings. Gram-positive diplococci were identified on CSF Gram staining, and CSF culturing yielded S. pneumoniae. Antibiotic therapy consisting of ceftriaxone (twice at 2 g/day) and vancomycin (once at 2 g/d) was initiated.
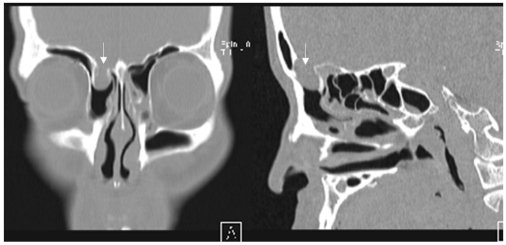
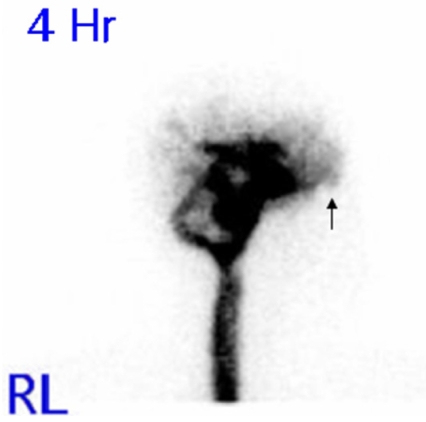
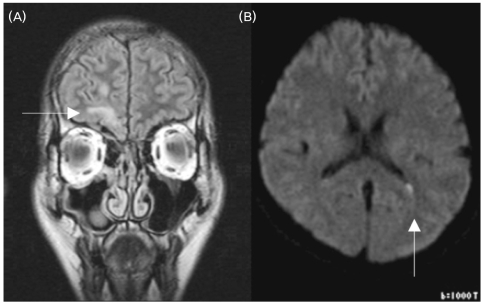
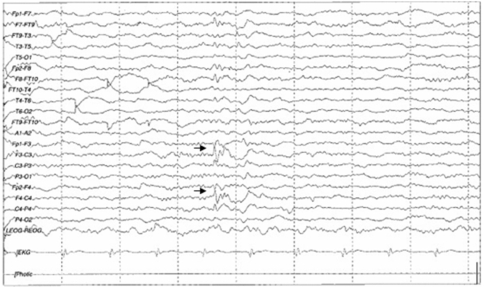
An otorhinolaryngological examination showed neither abnormal structures nor signs of rhinorrhea or otorrhea. However, a computed tomography scan of the paranasal sinus showed a bony defect in the right frontal base and a TEME (Fig. 1), and radioisotope (RI) cisternography showed radioactive foci in the right frontal base (Fig. 2). However, RI cisternography did not identify a CSF leak. Brain magnetic resonance imaging revealed right-sided cortical lesions overlying the TEME (Fig. 3-A), and that the left lateral ventricle was filled with small amounts of irregular debris on a diffusion-weighted image, indicating pyogenic ventriculitis (Fig. 3-B). Electroencephalography revealed spike-and-slow wave complexes with phase reversal in the right frontal regions (Fig. 4).
Figure 1.
Coronal (A) and sagittal (B) computed tomography views of the paranasal sinus, showing a bony defect of the right frontal base and a cystic mass lesion protruding into the ethmoid sinus (arrow).
Figure 2.
Radioisotope cisternography, indicating CSF activity with no leakage at the right frontal base in the 4-hour-delayed image (arrow). RL: Right lateral view.
Figure 3.
Brain magnetic resonance images. (A) Coronal FLAIR image, showing cerebral tissue herniating into the right ethmoid sinus and a focal encephalomalatic change in the anteroinferior aspect of the right frontal lobe (arrow). (B) Diffusion-weighted image showing the left lateral ventricle filled with small amounts of irregular debris, indicating pyogenic ventriculitis (arrow).
Figure 4.
Electroencephalogram showing spike-and-slow wave complexes with phase reversal in frontal regions (arrow).
No bacterium was seen or cultured with Gram staining and culturing of blood or urine. The fever resolved on the second day, and nuchal rigidity and Brudzinski signs disappeared on the third day. The patient refused immediate neurosurgical repair of the anterior cranial fossa, and was discharged on the 16th day of hospitalization. He had completely recovered 2 weeks after discharge, and was free of any disease at a 2-year follow-up.
DISCUSSION
In the present case, recurrent pneumococcal meningoencephalitis was associated with a TEME without CSF leakage evident either symptomatically or objectively. To our knowledge, this is the first report of a TEME related to recurrent meningitis with no CSF leakage.
History taking, an otorhinolaryngological examination, and RI cisternography did not reveal a CSF leak. The finding of the inward entry of S. pneumoniae into the CSF without evident outward CSF leakage suggests that the meningoencephalocele worked as a unidirectional gate, although the reason for the absence of CSF leakage with the TEME remained unclear since this was not confirmed histologically.
The high-signal-intensity lesions in the overlying right frontal cortex and the electroencephalographic findings in Fig. 3 and 4 confirmed a definitive diagnosis of meningoencephalitis rather than of simple meningitis. The prognosis of pneumococcal meningoencephalitis varies from a good recovery10 to mortality.2 Steroids were added in a previous study,10 but they were not used in our patient.
In a nontraumatic TEME, an osseous defect might result from a failure of the germ layer to close.11 In addition to the present case of pneumococcal meningitis, there have been two reports of recurrent pyogenic meningitis with a nontraumatic TEME: (1) a 12-year-old boy who presented with CSF rhinorrhea and recurrent meningitis for 3 years,12 and (2) a 52-year-old man with CSF rhinorrhea,13 in which Staphylococcus aureus was cultured.
Here we present a case of recurrent pneumococcal meningoencephalitis with a nontraumatic TEME but without an evident CSF leak. This case suggests that recurrent bacterial meningoencephalitis can be associated with a TEME even in the absence of clinically demonstrable CSF leakage.
Footnotes
This study was supported in part by the Research Institute of Clinical Medicine, Chonbuk National University Hospital.
References
- 1.Gilbert B, Menetrey C, Belin V, Brosset P, de Lumley L, Fisher A. Familial isolated congenital asplenia: a rare, frequently hereditary dominant condition, often detected too late as a cause of overwhelming pneumococcal sepsis. Report of a new case and review of 31 others. Eur J Pediatr. 2002;161:368–372. doi: 10.1007/s00431-002-0965-1. [DOI] [PubMed] [Google Scholar]
- 2.Heckmann JG, Schüttler M, Dörfler A. Pneumococcal meningoencephalitis. Wien Klin Wochenschr. 2005;117:739. doi: 10.1007/s00508-005-0465-1. [DOI] [PubMed] [Google Scholar]
- 3.Morand PC, Veuillez V, Poyart C, Abachin E, Quesne G, Dupont B, et al. Recurrent pneumococcal meningitis in a splenectomised HIV-infected patient. Ann Clin Microbiol Antimicrob. 2003;2:9. doi: 10.1186/1476-0711-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drummond DS, de Jong AL, Giannoni C, Sulek M, Friedman EM. Recurrent meningitis in the pediatric patient - the otolaryngologist's role. Int J Pediatr Otorhinolaryngol. 1999;48:199–208. doi: 10.1016/s0165-5876(99)00022-1. [DOI] [PubMed] [Google Scholar]
- 5.Kendirli T, Ünay B, Tosun F, Hacihamdioğlu B, Akin R, Özkaptan Y, et al. Recurrent Streptococcus pneumoniae meningitis in a child with traumatic anterior cranial base defect. Pediatr Int. 2006;48:91–93. doi: 10.1111/j.1442-200X.2006.02165.x. [DOI] [PubMed] [Google Scholar]
- 6.Rupa V, Rajshekhar V, Weider DJ. Syndrome of recurrent meningitis due to congenital perilymph fistula with two different clinical presentations. Int J Pediatr Otorhinolaryngol. 2000;54:173–177. doi: 10.1016/s0165-5876(00)00356-6. [DOI] [PubMed] [Google Scholar]
- 7.Dempsey PK, Harbaugh RE. Encephalomeningocele presenting with spontaneous cerebrospinal fluid rhinorrhea in an elderly man: case report. Neurosurgery. 1988;23:637–640. doi: 10.1227/00006123-198811000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Davachi F, Bregu H, Lito G. Recurrent Streptococcus pneumoniae meningitis. J Trop Pediatr. 2002;48:249–251. doi: 10.1093/tropej/48.4.249. [DOI] [PubMed] [Google Scholar]
- 9.Hur HY, Ahn GH, Chae KY. A case of spontaneous transsphenoidal encephalocele with recurrent bacterial meningitis. J Korean Child Neurol Soc. 2007;15:116–120. [Google Scholar]
- 10.Jorens PG, Parizel PM, Demey HE, Smets K, Jadoul K, Verbeek MM, et al. Meningoencephalitis caused by Streptococcus pneumoniae: a diagnostic and therapeutic challenge. Diagnosis with diffusion-weighted MRI leading to treatment with corticosteroids. Neuroradiology. 2005;47:758–764. doi: 10.1007/s00234-005-1423-3. [DOI] [PubMed] [Google Scholar]
- 11.Pollock JA, Newton TH, Hoyt WF. Transsphenoidal and transethmoidal encephaloceles. A review of clinical and roentgen features in 8 cases. Radiology. 1968;90:442–453. doi: 10.1148/90.3.442. [DOI] [PubMed] [Google Scholar]
- 12.Garg P, Rathi V, Bhargava SK, Aggarwal A. CSF rhinorrhea and recurrent meningitis caused by transethmoidal meningoencephaloceles. Indian Pediatr. 2005;42:1033–1036. [PubMed] [Google Scholar]
- 13.Hasegawa T, Sugeno N, Shiga Y, Takeda A, Karibe H, Tominaga T, et al. Transethmoidal intranasal meningoencephalocele in an adult with recurrent meningitis. J Clin Neurosci. 2005;12:702–704. doi: 10.1016/j.jocn.2004.08.027. [DOI] [PubMed] [Google Scholar]