Abstract
AIM: To study the effect of nicotine on the migration and invasion of human esophageal squamous carcinoma cells and to investigate whether nimesulide can inhibit the effect of nicotine.
METHODS: The esophageal squamous carcinoma cell line (TE-13) was treated with different concentrations of nicotine (100 μg/mL and 200 μg/mL) or 200 μg/mL nicotine plus 100 μmol/L nimesulide. Cell migration and invasion were measured using migration and invasion chamber systems. COX-2 expression was determined by Western blotting. Matrix metalloproteinase-2 (MMP-2) was analyzed by zymography and ELISA.
RESULTS: Nicotine (100 μg/mL, 200 μg/mL) enhanced TE-13 cells migration and invasion, and increased the protein expression of COX-2 and the activity of MMP-2. Nicotine (200 μg/mL) stimulated TE-13 cells migration and invasion which were partly blocked by nimesulide. This was associated with decreased protein expression of COX-2 and decreased activity and protein expression of MMP-2.
CONCLUSION: Nicotine enhances the migration and invasion of the esophageal squamous carcinoma cell line, and nimesulide partly blocks the effect of nicotine-enhanced esophageal squamous carcinoma cell migration and invasion.
Keywords: Carcinoma, Cyclooxygenase 2 inhibitors, Esophagus, Nicotine, Squamous cell
INTRODUCTION
Esophageal carcinoma is relatively common in China, especially esophageal squamous cell carcinoma (ESCC), which has a high mortality rate. It is well established that cigarette smoking increases the risk and mortality of ESCC. Nicotine is a major component of cigarettes. Conventionally, nicotine is regarded as a relatively inert chemical in carcinogenesis. A recent finding suggested that nicotine may at least be partially involved in the initiation, promotion, and even progression of tumors[1,2]. However, the effect of nicotine on tumorigenesis of the esophagus is still not clear.
Many of the critical steps in malignant tumorigenesis, such as cell proliferation, evading apoptosis, stimulating angiogenesis, enhancing cell motility, cell invasiveness and mediating immune suppression, have been associated with cyclooxygenase-2 (COX-2) expression. It has been observed that the expression of COX-2 was increased in ESCC[3] and COX-2 may play a critical role in cancer progression. A number of epidemiological studies have suggested that the administration of COX inhibitors (NSAIDs, aspirin, indomethacin) reduce the incidence of breast, colon, prostate and esophagus cancers[4–12]. In our previous study, cigarette smoke extract dose-dependently stimulated esophageal squamous carcinoma cell proliferation through up-regulation of COX-2 expression, and a COX-2 inhibitor inhibited this effect. COX-2 inhibitors may decrease the incidence of ESCC, however, the mechanism of COX-2 inhibitors in the metastasis of ESCC is also not very clear.
In order for cancer cells to metastasize, the cells must digest and dissolve the extracellular matrix (ECM) and the basement membrane, which requires the secretion and activation of matrix metalloproteinases (MMPs). The activity of MMPs is associated with invasiveness and metastasis in tumor cells. MMP-2 is a major enzyme that can selectively degrade type IV collagen and facilitate tumor invasion and metastasis. The expression and activation of MMPs may be directly proportional to the overexpression and activity of COX-2 in tumor cells. A previous study showed that Hs578T breast cancer cells transfected with COX-2 resulted in the activation of MMP-2[13]. Administration of nicotine increased the vascular endothelial growth factor (VEGF)-induced suppression of MMP-2 activity in mice. However, the direct action of nicotine on migration and invasion of esophageal squamous carcinoma cells remains unknown.
In the present study, we evaluated the effect of nicotine on migration and invasion in the human esophageal squamous carcinoma cell line and we investigated whether nimesulide, a selective COX-2 inhibitor, could inhibit migration and invasion in the ESCC cell line treated with nicotine.
MATERIALS AND METHODS
Cell lines and cell culture
TE-13, a human esophageal squamous carcinoma cell line was purchased from Hebei Cancer Hospital of China. Cells were cultured in RPMI-1640 (Hyclone, USA) containing 10% fetal bovine serum (Hyclone, USA). Cells were maintained at 37°C, 95% humidity, and 5% CO2.
Drug treatment
To examine the effect of nicotine on esophageal squamous carcinoma cells, TE-13 cells were incubated directly with nicotine (Sigma, USA) (100 μg/mL or 200 μg/mL). Dimethyl sulfoxide (0.5%, v/v) was used as a control. TE-13 cells were incubated with nicotine (200 μg/mL) and nimesulide (100 μmol/L) in order to study the effect of the cyclooxygenase-2 (COX-2) inhibitor.
Western blotting for protein
For the detection of COX-2 protein in TE-13 cells, 50 μg protein from the cell extracts of each cell line which had been treated with different drugs for 48 h was electrophoresed through polyacrylamide gel. The separated protein was then transferred to a nitrocellulose membrane (Amersham, USA) and probed with diluted rabbit polyclonal anti-COX-2 (1:200) (Cayman, USA). The next day, after incubation with secondary antibody (Santa Cruz, USA), protein bands on the membranes were then developed by a chemiluminescence detection system (Pierce, USA) and exposed on an X-ray film.
Migration assay
Cell migration assays were performed using a modification of the protocol described by Larkins et al[14] and Shin et al[15]. The 6.5 mm Transwell® with an 8.0 μm pore polycarbonate membrane insert (Corning Company, USA) was utilized in this assay. The TE-13 cells were harvested and resuspended into serum-free medium containing nicotine (100 μg/mL or 200 μg/mL) or nicotine (200 μg/mL) and nimesulide (100 μmol/L). The upper chamber of the insert was filled with 200 μL of the cells and drug suspension (8 × 104 cells). The lower chamber was filled with culture medium supplemented with 10% FCS as the chemoattractant. The plate was incubated in a humidified environment at 37°C with 5% CO2 for 48 h. After incubation, the cells were removed from the upper surface of the membrane by wiping with a moist cotton swab. The migrated cells that passed through the membrane and adhered to the lower surface of the membrane were fixed with methanol, stained for 3 min with hematoxylin and eosin, rinsed with distilled water to remove excess stain not absorbed by cells and counted under a light microscope (× 400).
Invasion assay
Matrigel was purchased from BD Biosciences (USA) and stored at -20°C. After thawing at 4°C overnight, the matrigel was diluted in serum-free RPMI-1640 medium. 50 μL of the diluted matrigel were evenly inoculated into the upper chamber of the 6.5 mm Transwell® membrane and allowed to form a gel at 37°C. The remaining processes of the matrigel invasive assay were the same as those for the migration assay.
Matrix metalloproteinase-2 activity by Gelatin Zymography
Metalloproteinases are capable of degrading gelatin, therefore, by incorporating gelatin into the polyacrylamide gel a clear zone indicates the presence of a matrix degrading enzyme. Gelatin zymography was carried out as described by Tsujii et al[16]. In brief, cells were incubated with nicotine in the absence or presence of nimesulide for 48 h. The supernatants were collected after 48 h and centrifuged 12 000 r/m for 10 min at 4°C. The protein content of the supernatant was determined by dye-reagent protein assay. Twenty-one microgram protein from each supernatant was separated on 10% SDS/PAGE with 1 mg/mL gelatin incorporated into the gel mixture. Following electrophoresis at 4°C, the gel was washed with 2.5% Triton X-100 to remove the SDS, rinsed in H2O three times, and transferred to a bath containing 50 mmol/L Tris-HCl (pH 8.0), 50 mmol/L NaCl and 10 mmol/L CaCl2 at 37°C for 24 h. To visualize the presence of gelatinolytic bands, gels were stained with Coomassie blue (R-250) and destained with Coomassie blue destaining solution until all bands of lysis became clear. Quantification of bands on the gels was carried out by video densitometry.
ELISA
We examined the level of MMP-2 in conditioned media from TE-13 cells, using ELISA and commercially available antibodies.
Statistical analysis
Results were expressed as mean ± SE. Statistical analysis was performed using ANOVA. P-values less than 0.05 were considered statistically significant.
RESULTS
Effect of nicotine on cellular migration and invasion
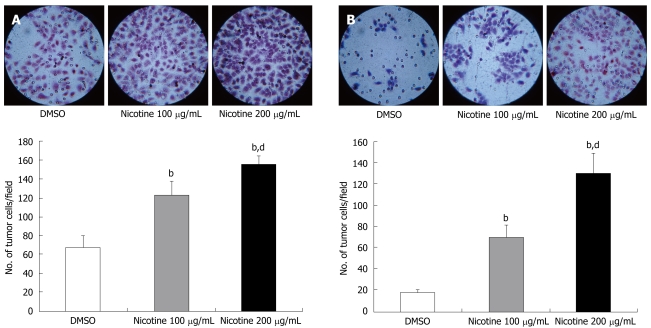
Firstly, we tested the effects of nicotine on cellular invasive and migratory potentials. Nicotine (100 μg/mL) induced a 1.8-fold increase and nicotine (200 μg/mL) induced a 2.3-fold increase in TE-13 cellular migration through the 8.0 μm pore polycarbonate membrane of Transwell®, relative to untreated cells (P < 0.01) (Figure 1A). Nicotine also increased invasive ability of the tumor cells, and the number of invading cells was increased by 3.6-fold and 6.8-fold in nicotine 100 μg/mL and 200 μg/mL, respectively, compared with the control (P < 0.01) (Figure 1B).
Figure 1.
Effect of nicotine on the motility (A) and invasiveness (B) of the esophageal squamous carcinoma cell line TE-13. Numbers of TE-13 cells which migrated through the polycarbonate (A) or matrigel (B) membrane of Transwell® to the lower surface of the membrane were counted under a light microscope (× 400). Columns, mean of three triplicate experiments; bars, standard error of mean. bP < 0.01 vs the control group; dP < 0.01 vs the nicotine 100 mg/mL group.
COX-2 expression
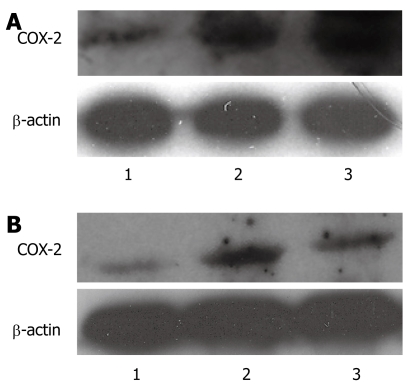
We examined COX-2 expression in the human esophageal squamous carcinoma cell line (TE-13) following treatment with nicotine or nicotine and nimesulide using Western blotting. Nicotine (100 μg/mL or 200 μg/mL) significantly increased the expression of COX-2 in TE-13 cells when compared with the control group, and the higher concentration of nicotine increased the expression of COX-2 more than the lower concentration of nicotine (Figure 2A). Nimesulide (100 μmol/L) suppressed the increased expression of COX-2 induced by nicotine (200 μg/mL) (Figure 2B).
Figure 2.
Western blotting analysis. A: Effect of nicotine on COX-2 protein expression in TE-13 cells, 1: DMSO; 2: Nicotine 100 μg/mL; 3: Nicotine 200 μg/mL; B: Effect of nimesulide on COX-2 protein expression in TE-13 cells, 1: DMSO; 2: Nicotine 200 μg/mL; 3: Nicotine 200 μg/mL + Nimesulide 100 μmol/mL.
Effect of COX-2 on tumor cell migration and invasiveness induced by nicotine
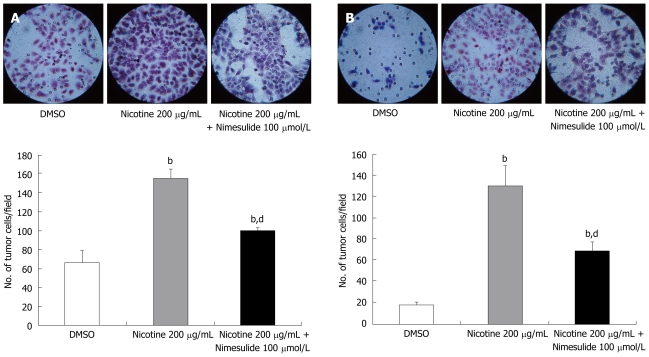
To determine if the effect of nicotine on cellular migration and invasion was associated with COX-2 in tumor cells, we examined the effect of the COX-2 inhibitor, nimesulide, on tumor cell migration and invasiveness induced by nicotine. Nimesulide partially, but significantly, inhibited the migration of TE-13 cells through the Transwell® membrane (Figure 3A) and significantly inhibited the invasion of TE-13 cells through the matrigel membrane (Figure 3B).
Figure 3.
Effect of nimesulide on the motility (A) and invasiveness (B) of the esophageal squamous carcinoma cell line TE-13 induced by nicotine treatment. Numbers of TE-13 cells which migrated through the polycarbonate (A) or matrigel (B) membrane of Transwell® to the lower surface of the membrane were counted under a light microscope (× 400). Data are expressed as mean ± SE. bP < 0.01 vs the control group; dP < 0.01 vs the nicotine 200 μg/mL group.
Effect of nicotine on MMP-2 in TE-13 cells
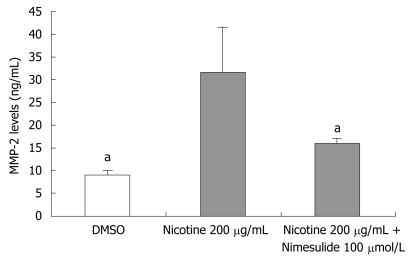
Because activation of MMP-2 can selectively degrade type IV collagen, which contributes to tumor invasion and metastasis, its activity is an important determinant of tumor cellular invasive potential. We measured MMP-2 activity and expression in TE-13 cells treated with nicotine or nicotine and nimesulide. Nicotine significantly increased MMP-2 (72 kDa) activity (Figure 4A). Nimesulide markedly reduced this increased activity and the protein level of MMP-2 induced by nicotine (Figures 4B and 5).
Figure 4.
A Gelatin zymography of MMP-2 activity of TE-13. A: Effect of nicotine on MMP-2 activity of TE-13 cells. Our study showed nicotine increased the activity of MMP-2. 1: DMSO; 2: Nicotine 100 μg/mL; 3: Nicotine 200 μg/mL; B: Effect of nimesulide on MMP-2 activity of TE-13 cells treated by nicotine. Our study showed nimesulide suppressed the effect of nicotine that increased the activity of MMP-2. 1: DMSO; 2: Nicotine 200 μg/mL; 3: Nicotine 200 μg/mL + Nimesulide 100 mmol/L.
Figure 5.
The protein levels of MMP-2 of TE-13 by treatment of nicotine or nicotine and nimesulide were analyzed by ELISA. The experiments were done as described in Materials and Methods. Data are expressed as mean ± SE. aP < 0.05 versus the nicotine 200 μg/mL group.
DISCUSSION
Cigarette smoking causes cancer of various types, including cancers of the lung, oropharynx, larynx, and esophagus. Nicotine, a major component of cigarettes, has been proposed to be responsible for many pharmacological effects of cigarette smoke. Smoking is a neuronal nicotinic acetylcholine (nACh) receptor-mediated addiction[17]. Conventionally, nicotine is regarded as a relatively inert chemical in carcinogenesis and is responsible for the addictive potential of tobacco smoke. However, recently many studies have reported the toxicity of nicotine[18–20] and have suggested that nicotine may at least be partially involved in the initiation, promotion, and even progression of tumors in the gastrointestinal tract[1,2]. However, reports on the effect of nicotine on esophageal squamous carcinoma are very few. There are three important steps in cancer metastasis: adhesion to the extracellular matrix (ECM), degradation of ECM, and ultimately migration[21]. During the whole process, the motility and invasiveness of tumor cells are the most important characteristics. Therefore, in order to study the effect of nicotine on esophageal squamous carcinoma metastasis, we examined the motility and invasiveness of TE-13 cells, an esophageal squamous carcinoma cell line treated with nicotine. In the present study, nicotine stimulated the motility and invasiveness of TE-13 cells through the reconstituted membrane.
Several studies have reported that COX-2 is involved in these complex steps of cancer metastasis. In Tsujii’s study[16], human colon cancer cells (Caco-2) were permanently transfected with a COX-2 expression vector or the identical vector lacking the COX-2 insert. The Caco-2 cells, which constitutively expressed COX-2, acquired increased invasiveness compared with the parental Caco-2 cells or the vector-transfected control cells. Chen et al[22] investigated the association between COX-2 expression and colorectal cancer cell invasiveness. Three different colon cancer cell lines, SW620, Lovo, HT-29 and a metastatic variant of HT-29, HT-29/Inv3, were employed to evaluate COX-2 expression and prostaglandin E2 (PGE2) production in relation to their invasive abilities in vitro. Among the 4 colon cancer cell lines, HT-29/Inv3 manifested the highest COX-2 expression, PGE2 production and in vitro invasive activity. These authors’ results implied that COX-2 expression might be associated with the invasive and metastatic properties of colorectal cancer cells. Up-regulation of COX-2 mRNA was observed after exposure to nicotine in human gingival fibroblasts and rat microglial cells[23,24]. Therefore, we studied whether nicotine affected the motility and invasiveness of TE-13 cells through COX-2 up-regulation. Our findings showed that nicotine increased COX-2 expression and nimesulide, a COX-2 inhibitor, partly inhibited the effect of nicotine on motility and invasiveness of TE-13 cells. This implied that the effect of nicotine is at least partly dependent on COX-2 expression. In the study by Shin et al[15], nicotine enhanced gastric cancer cell invasion through the matrigel membrane by 4-fold and the effect of nicotine was blocked by a COX-2 inhibitor, which is consistent with our finding.
In order for the cells to invade and migrate through the basement membrane, proteolysis of the extracellular matrix must occur. This is accomplished by the secretion and activation of MMPs, which will degrade all extracellular matrix components. Among MMPs, MMP-2 plays an important role in tumor metastasis. In our study, we also determined the mediation of MMP-2 expression and secretion by nicotine, and used an inhibitor approach to investigate the action of COX-2 on the effect of nicotine. Our findings showed that nicotine increased MMP-2 expression and activity, and nimesulide blocked the effects of nicotine. Several other studies have also implicated that the activity of COX-2 gene expression leads to higher MMP expression. Tsujii et al[16] indicated that activation of MMP-2 can be modulated by COX-2 and treatment with a COX inhibitor can reverse the increased invasiveness of Caco-2 cells (which constitutively expressed COX-2) and inhibit activation of MMP-2. Pan et al[25] showed that NS398, a COX-2 inhibitor, inhibited MMP-2 mRNA expression, reduced the amount of MMP-2 released into the medium and attenuated the degrading activity of MMP-2. Inhibition of the MMP-2 promoter activity by NS-398 was partially reversed by exogenous PGE2. From these studies and from our findings, we suggest that the effect of nicotine on the stimulation of invasiveness of tumor cells is associated with increased activity and expression of MMP-2 by nicotine, and increased MMP-2 was associated with increased expression of COX-2 by nicotine. Therefore, COX-2 inhibition inhibited the action of nicotine which enhanced the invasiveness of tumor cells by inhibiting the activity of COX-2 and decreasing the activity and expression of MMP-2.
In conclusion, nicotine can enhance the migration and invasion of the esophageal squamous carcinoma cell line (TE-13), and increase the expression of COX-2 and activity of MMP-2 in these cells. Nimesulide partly blocked the effect of nicotine.
COMMENTS
Background
Esophageal squamous cell carcinoma (ESCC) is relatively common in China, and has a high mortality rate. It is well established that cigarette smoking increases the risk and mortality of ESCC. Nicotine is a major component of cigarettes. A recent finding suggests that nicotine may at least be partially involved in the initiation, promotion, and even progression of tumors. However, the effect of nicotine on tumorigenesis in the esophagus is still not clear. It has been observed that an increased expression of cyclooxygenase-2 (COX-2) in ESCC, and COX-2 inhibitors can decrease the incidence of ESCC, however, the mechanism of COX-2 inhibitors in the metastasis of ESCC is not very clear.
Research frontiers
It has been observed that COX-2 may play a critical role in cancer progression. The activity of matrix metalloproteinases (MMPs) is associated with invasiveness and metastasis in tumor cells. The expression and activation of MMPs may be directly proportional to the overexpression and activity of COX-2 in tumor cells. A number of epidemiological studies have suggested that the administration of COX-2 inhibitors can reduce the incidence of breast, colon, prostate and esophagus cancers.
Innovations and breakthroughs
Nicotine can enhance the migration and invasion of esophageal squamous carcinoma cells and nimesulide partly blocked the effect of nicotine.
Applications
COX-2 inhibitors can inhibit the action of nicotine which enhanced the migration and invasiveness of esophageal squamous carcinoma cells. This indicated that COX-2 inhibitors may be an effective preventive and therapeutic strategy for esophagus cancer, however, further investigations are needed to prove this.
Terminology
COX is the rate limiting enzyme involved in the conversion of arachidonic acid to prostaglandin H2. Two isoforms of COX have been identified which share 60% homology, COX-1 and COX-2. Recently many studies have showed that COX-2 expression is up-regulated in several types of human cancers.
Peer review
In this paper the authors evaluated the effects of nicotine and nicotine and nimesulide on the migration and invasion in esophageal squamous carcinoma TE-13 cells. The manuscript is interesting and deals with an important subject.
Supported by Beijing Municipal Commission of Education, Science and Technology Program, No. KM200610025029; Beijing Municipal Natural Science Foundation, No. 7072022
Peer reviewers: Dr. Katerina Dvorak, Research Assistant Professor, Cell Biology and Anatomy, The University of Arizona, 1501 N. campbell Ave, Tucson 85724, United States; You-Yong Lu, Professor, Beijing Molecular Oncology Laboratory, Peking University School of Oncology and Beijing Institute for Cancer Reaearch, #52, Fucheng Road, Haidian District, Beijing 100036, China; Satoshi Osawa, MD, First Department of Medicine, Hamamatsu University School of Medicine, 1-20-1 Handayama, Hamamatsu, 431-3192, Japan
S- Editor Tian L L- Editor Webster JR E- Editor Yin DH
References
- 1.Ye YN, Liu ES, Shin VY, Wu WK, Luo JC, Cho CH. Nicotine promoted colon cancer growth via epidermal growth factor receptor, c-Src, and 5-lipoxygenase-mediated signal pathway. J Pharmacol Exp Ther. 2004;308:66–72. doi: 10.1124/jpet.103.058321. [DOI] [PubMed] [Google Scholar]
- 2.Heusch WL, Maneckjee R. Signalling pathways involved in nicotine regulation of apoptosis of human lung cancer cells. Carcinogenesis. 1998;19:551–556. doi: 10.1093/carcin/19.4.551. [DOI] [PubMed] [Google Scholar]
- 3.Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schrör K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198–204. [PubMed] [Google Scholar]
- 4.Attiga FA, Fernandez PM, Weeraratna AT, Manyak MJ, Patierno SR. Inhibitors of prostaglandin synthesis inhibit human prostate tumor cell invasiveness and reduce the release of matrix metalloproteinases. Cancer Res. 2000;60:4629–4637. [PubMed] [Google Scholar]
- 5.Schreinemachers DM, Everson RB. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology. 1994;5:138–146. doi: 10.1097/00001648-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Harris RE, Namboodiri KK, Farrar WB. Nonsteroidal antiinflammatory drugs and breast cancer. Epidemiology. 1996;7:203–205. doi: 10.1097/00001648-199603000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409–412. [PubMed] [Google Scholar]
- 8.Kundu N, Fulton AM. Selective cyclooxygenase (COX)-1 or COX-2 inhibitors control metastatic disease in a murine model of breast cancer. Cancer Res. 2002;62:2343–2346. [PubMed] [Google Scholar]
- 9.Subbaramaiah K, Zakim D, Weksler BB, Dannenberg AJ. Inhibition of cyclooxygenase: a novel approach to cancer prevention. Proc Soc Exp Biol Med. 1997;216:201–210. doi: 10.3181/00379727-216-44170. [DOI] [PubMed] [Google Scholar]
- 10.Norrish AE, Jackson RT, McRae CU. Non-steroidal anti-inflammatory drugs and prostate cancer progression. Int J Cancer. 1998;77:511–515. doi: 10.1002/(sici)1097-0215(19980812)77:4<511::aid-ijc6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 11.Thun MJ, Namboodiri MM, Calle EE, Flanders WD, Heath CW Jr. Aspirin use and risk of fatal cancer. Cancer Res. 1993;53:1322–1327. [PubMed] [Google Scholar]
- 12.Funkhouser EM, Sharp GB. Aspirin and reduced risk of esophageal carcinoma. Cancer. 1995;76:1116–1119. doi: 10.1002/1097-0142(19951001)76:7<1116::aid-cncr2820760703>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi Y, Kawahara F, Noguchi M, Miwa K, Sato H, Seiki M, Inoue H, Tanabe T, Yoshimoto T. Activation of matrix metalloproteinase-2 in human breast cancer cells overexpressing cyclooxygenase-1 or -2. FEBS Lett. 1999;460:145–148. doi: 10.1016/s0014-5793(99)01328-9. [DOI] [PubMed] [Google Scholar]
- 14.Larkins TL, Nowell M, Singh S, Sanford GL. Inhibition of cyclooxygenase-2 decreases breast cancer cell motility, invasion and matrix metalloproteinase expression. BMC Cancer. 2006;6:181. doi: 10.1186/1471-2407-6-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin VY, Wu WK, Chu KM, Wong HP, Lam EK, Tai EK, Koo MW, Cho CH. Nicotine induces cyclooxygenase-2 and vascular endothelial growth factor receptor-2 in association with tumor-associated invasion and angiogenesis in gastric cancer. Mol Cancer Res. 2005;3:607–615. doi: 10.1158/1541-7786.MCR-05-0106. [DOI] [PubMed] [Google Scholar]
- 16.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dajas-Bailador F, Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci. 2004;25:317–324. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Chang YC, Huang FM, Tai KW, Yang LC, Chou MY. Mechanisms of cytotoxicity of nicotine in human periodontal ligament fibroblast cultures in vitro. J Periodontal Res. 2002;37:279–285. doi: 10.1034/j.1600-0765.2002.01612.x. [DOI] [PubMed] [Google Scholar]
- 19.Chen YC, Shen SC, Lin HY, Tsai SH, Lee TJ. Nicotine enhancement of lipopolysaccharide/interferon-gamma-induced cytotoxicity with elevating nitric oxide production. Toxicol Lett. 2004;153:191–200. doi: 10.1016/j.toxlet.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Cooke JP, Bitterman H. Nicotine and angiogenesis: a new paradigm for tobacco-related diseases. Ann Med. 2004;36:33–40. doi: 10.1080/07853890310017576. [DOI] [PubMed] [Google Scholar]
- 21.Mignatti P, Rifkin DB. Biology and biochemistry of proteinases in tumor invasion. Physiol Rev. 1993;73:161–195. doi: 10.1152/physrev.1993.73.1.161. [DOI] [PubMed] [Google Scholar]
- 22.Chen WS, Wei SJ, Liu JM, Hsiao M, Kou-Lin J, Yang WK. Tumor invasiveness and liver metastasis of colon cancer cells correlated with cyclooxygenase-2 (COX-2) expression and inhibited by a COX-2-selective inhibitor, etodolac. Int J Cancer. 2001;91:894–899. doi: 10.1002/1097-0215(200102)9999:9999<894::aid-ijc1146>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Chang YC, Tsai CH, Yang SH, Liu CM, Chou MY. Induction of cyclooxygenase-2 mRNA and protein expression in human gingival fibroblasts stimulated with nicotine. J Periodontal Res. 2003;38:496–501. doi: 10.1034/j.1600-0765.2003.00681.x. [DOI] [PubMed] [Google Scholar]
- 24.De Simone R, Ajmone-Cat MA, Carnevale D, Minghetti L. Activation of alpha7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures. J Neuroinflammation. 2005;2:4. doi: 10.1186/1742-2094-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan MR, Chuang LY, Hung WC. Non-steroidal anti-inflammatory drugs inhibit matrix metalloproteinase-2 expression via repression of transcription in lung cancer cells. FEBS Lett. 2001;508:365–368. doi: 10.1016/s0014-5793(01)03118-0. [DOI] [PubMed] [Google Scholar]