Abstract
Combined en bloc liver/pancreas transplantation (CLPT) was used primarily in the treatment of otherwise non-resectable upper abdominal malignancy. In fact, a more appropriate indication is in patients with liver disease and insulin-dependent diabetes mellitus (IDDM). Here, we report on two successful cases of CLPT at our hospital. One was a patient with non-resectable advanced liver cancer. The recipient survived for 23 mo and finally died of recurrent tumor. The other was a patient with severe biliary complication after orthotopic liver transplantation and preoperative IDDM. We performed CLPT with a modified surgical technique of preserving the native pancreas. He is currently liver-disease- and insulin-free more than 27 mo post-transplant. Based on our experience in two cases of abdominal cluster transplantation, we describe the technical details of CLPT and a modification of the surgical procedure.
Keywords: Transplantation, Liver, Pancreas, Diabetes mellitus, Liver cancer
INTRODUCTION
Although originally described several decades ago, combined liver/pancreas transplantation (CLPT) is still a relatively uncommon procedure. There are relatively few indications for CLPT. Previously, it was used mostly as a lifesaving method in the treatment of otherwise non-resectable upper abdominal malignancies[1,2]. However, with poor results, mainly because of tumor recurrence, this procedure fell out of favor. In fact, a more ideal indication for this so-called abdominal organ cluster transplantation is in patients with liver disease and insulin-dependent diabetes mellitus (IDDM). However, there have been only six case reports of successful CLPT in this group of patients[3,4], including three children[5].
Starzl originally designed the operation with removal and replacement of the entire grape cluster. During the procedure, upper abdominal exenteration (the liver, pancreas, spleen, duodenum, part of the stomach) is carried out. Exenteration is necessary for treatment of abdominal malignancies. However, in patients with liver disease and IDDM, such massive abdominal evisceration is unnecessary.
With the increased practicality of multivisceral transplantation, different innovative techniques have been introduced to further improve survival and reduce morbidity. Fishbein and Abu-Elmagd recently presented their experiences on preservation of the native organs in patients with hepatic-intestinal, and isolated intestinal transplantation[6,7]. According to Starzl, the main subtypes of multivisceral transplantation are full multivisceral, upper abdominal (cluster), hepatic-intestinal, and isolated intestinal transplantation[8]. In this paper, we present our experience of preservation of the native organs in a patient who underwent upper abdominal (cluster) transplantation. We compare this patient to another one with advanced liver cancer who underwent well-described standard CLPT.
CASE REPORT
Case 1
Patient 1 is a 44-year-old man (blood group B+; 68 kg; 174 cm) who presented with a 12-mo history of discomfort and vague pain in the right upper abdomen. Ultrasonic examination and follow-up computed tomography (CT) showed a lesion measuring 5 cm × 5 cm in the right lobe of the liver, portal vein embolus, and enlarged pancreatic head. The level of alpha-fetoprotein (AFP), serum alanine transaminase (ALT), and total serum bilirubin (T-Bil) was 1096 μg/L, 104 IU/L and 22.7 μmol/L respectively. The patient had a 20-year history of hepatitis B. He was diagnosed with advanced liver cancer. Multivisceral transplantation was an alternative that might have offered the only chance of radical tumor excision.
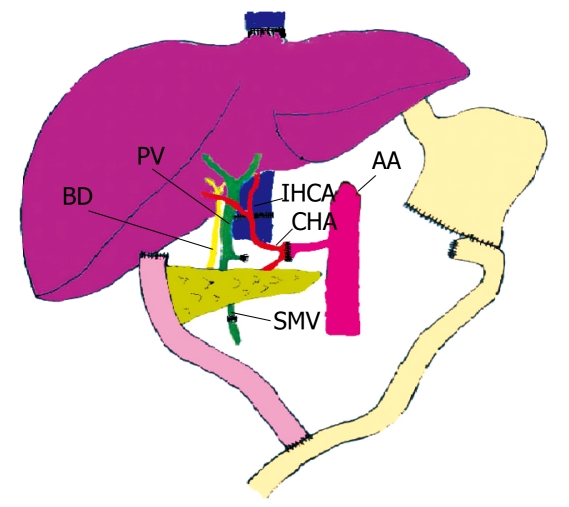
In September 2004, ABO compatible size matched organs from a 48-year-old 62-kg male donor were allocated. At laparotomy, pancreatic infiltration and embolus invading the portal vein and superior mesenteric vein were confirmed. After complete removal of the recipient liver, duodenum, part of the stomach and small bowel, CLPT was performed en bloc with the conventional technique. The grafts were sewn in to the native inferior vena cava in a standard fashion. Then, the superior mesenteric vein of the graft was anastomosed to the native one. Finally, a donor aortic patch with the celiac trunk and the superior mesenteric artery was anastomosed end-to-end to the receptor common hepatic artery. After reperfusion of the graft, the gastrointestinal tract was reestablished with a Roux-en-Y duodenojejunostomy (Figure 1). The operative time, amount of blood loss and blood transfusion during the operation were 600 min, 7 L and 32 units, respectively.
Figure 1.
Standard en bloc CLPT. The grafts were sewn into the native inferior vena cava. The superior mesenteric vein (SMV) of the graft was anastomosed to the native one. A donor aortic patch with the celiac trunk and the superior mesenteric artery was anastomosed end-to-end to the receptor common hepatic artery (CHA). The gastrointestinal tract was reestablished with a Roux-en-Y duodenojejunostomy. IHCA: Infrahepatic cava anastomosis; BD: Donor bile duct; PV: Donor portal vein; AA: Native abdominal aorta.
Immunosuppression included induction with daclizumab and maintenance treatment with the triple immunosuppressive therapy (tacrolimus, mycophenolate mofetil and prednisolone). His liver function recovered fast. On the seventh day after operation, the grafts test showed AFP, ALT, T-Bil were < 10 μg/L, 84 IU/L and 12.7 μmol/L, respectively. Blood sugars remained normal post-transplant. His postoperative course was complicated by severe mixed pulmonary infections on postoperative day 7, which lasted for 3 wk. Furthermore, intra-abdominal hemorrhage occurred on postoperative day 16, which required re-operation. He undertook six courses of postoperative chemotherapy with a pirarubicin-containing regimen. The recipient was doing well until ultrasound and CT showed hypodense areas in his new liver one and a half years post-operation. He finally died of cancer recurrence 23 mo post-operation (Table 1).
Table 1.
Patient characteristics
| Characteristics | Patient 1 | Patient 2 |
| Age (yr) | 44 | 49 |
| Gender | Male | Male |
| Indication | Malignancy | Liver disease and DM |
| During operation | ||
| Operative time | 600 min | 570 min |
| Amount of blood loss | 7000 mL | 3000 mL |
| Blood transfusion | 32 units | 13 units |
| Postoperative complications | ||
| Pulmonary infections | + | - |
| Intra-abdominal hemorrhage | + | - |
| Outcome | Dead (23 mo postoperation) | Alive (27 mo posttransplant) |
Case 2
In 2006, a 49-year-old man (blood group A+; 63 kg; 173 cm) presented with severe jaundice and high fever (39°C). He had a history of liver transplantation for terminal hepatitis-B cirrhosis in 2002. Unfortunately, severe biliary complication developed postoperatively. Since then, he suffered from very severe jaundice and repeated high fever with biliary infection. Meanwhile, he had a medical history of IDDM since about age 10 years. His blood sugar became difficult to control after liver transplantation. Furthermore, blurred vision occurred, which was finally diagnosed as diabetic ophthalmopathy.
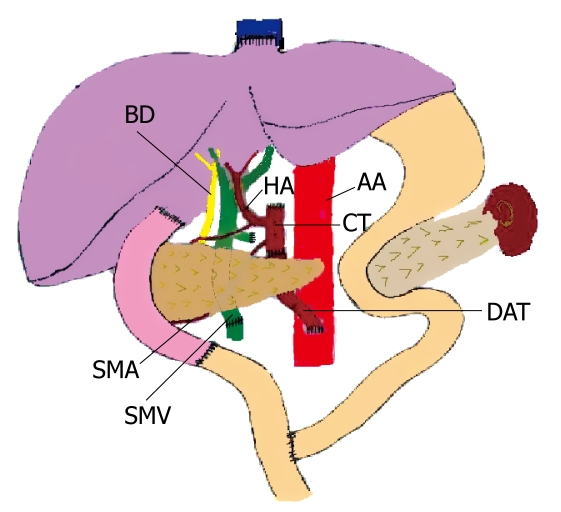
In September 2006, the patient underwent CLPT with organs from a 25-year-old male donor (blood group A+; 60 kg; 171 cm). Removal of the native organs was relatively simple. We only needed to resect the pathological liver, and then the organ cluster was transplanted orthotopically. A piggy-back anastomosis of the grafted suprahepatic vena cava onto the native one was performed. Then, the grafted superior mesenteric vein was anastomosed to the native portal vein. Next, a circular donor aortic patch including the celiac trunk and superior mesenteric artery was anastomosed end-to-end to a donor aortic tube that had been previously implanted on the receptor infrarenal aorta. The pancreas graft was draped directly over the native pancreas. The time of the anhepatic phase was 56 min. Finally, the digestive tract was reconstructed. A Roux-en-Y anastomosis of the grafted distal duodenum and the native proximal jejunum was performed (Figure 2). The operative time, amount of blood loss and blood transfusion during the operation were 570 min, 3 L and 13 units, respectively.
Figure 2.
Modified en bloc CLPT while retaining the native pancreas. A piggy-back anastomosis of the grafted suprahepatic vena cava onto the native one was performed. A circular donor aortic patch including both the celiac trunk (CT) and the superior mesenteric artery (SMA) was anastomosed end-to-end to a donor aortic tube (DAT) that had been implanted previously on the native abdominal aorta (AA). The donor superior mesenteric vein (SMV) was anastomosed end-to-end to the native portal vein. Roux-Y anastomosis of the grafted distal duodenum and the native proximal jejunum was performed. HA: Donor hepatic artery; BD: Donor bile duct.
The patient experienced an uneventful postoperative recovery. Since the second day post-operation, he no longer needed exogenous insulin. Liver function also recovered rapidly. On the first day after the operation, the grafts test showed that ALT and T-Bil was 531 U/L and 169.8 μmol/L, respectively. On postoperative day 7, they declined to 44 U/L and 63 μmol/L. Two weeks after transplantation, his liver function became normal. He is currently alive, liver-disease- and insulin-free more than 27 mo post-transplant (Table 1).
DISCUSSION
The operation of en bloc CLPT stemmed from the pioneer work of Thomas E. Starzl more than four decades ago[9]. Starzl and Williams published their first successful clinical experience in 1989[10,11]. Their initial attempts proved that the technique was feasible. However, because of postoperative complications and technical problems, the complex procedure is now still performed in small numbers in a few transplant centers.
There are relatively few indications for CLPT. Previously, it has been mostly indicated in patients with otherwise non-resectable upper abdominal malignancies[1,2]. Although some of the patients with advanced malignancies could benefit from this radical operative approach, it is not a wise choice to allocate scarce donor organs to such patients. There are several barriers to improving patient survival.
First, postoperative hemorrhage is one of the common life-threatening complications after such massive abdominal evisceration. Two of the first four recipients reported by Starzl and Williams succumbed from uncontrollable bleeding shortly after the complex surgery. Secondly, infection is a barrier to the improvement of graft survival[12], and represents the leading cause of mortality[13]. Thirdly, tumor recurrence remains the most difficult barrier to improving patient and graft survival. All three of these complications occurred in our patient 1.
In fact, a more appropriate indication for this so-called abdominal organ cluster transplant is in liver transplant candidates who coincidentally suffer from IDDM. For them, we only need to perform hepatectomy before transplanting the en bloc liver-duodeno-pancreatic graft. Case 2 showed the success of the surgical modification, while retaining the native pancreas. We believe that there are several potential advantages of such a modified technique.
First, the pancreas is located deep in the abdomen with an abundant blood supply. Avoiding native pancreas removal means avoiding surgical damage to the surrounding tissues. This procedure can dramatically decrease oozing of blood into the surgical field, particularly in patients with severe adhesions in the upper abdomen. Secondly, retaining the native pancreas endocrine and exocrine secretion can relieve the burden on the new pancreas. Thirdly, the gastrointestinal tract remains as a complete reunification of the whole, with retention of the native normal duodenum. Thus, the recipient can start feeding early postoperatively. Early feeding can prevent bacterial translocation and villus atrophy[14]. Fourthly, the recipient spleen is not removed during this procedure. Previous studies have revealed that the asplenic state is associated with increased incidence of sepsis[15]. Finally, avoiding removal of the native greater omentum may help to reduce postoperative complications, especially in cases of anastomotic leakage and duodenal or pancreatic fistula.
In conclusion, our experience demonstrates that en bloc CLPT can be modified according to the patient’s need. Although it plays a role as rescue therapy, the procedure for advanced abdominal malignancy needs careful consideration. The experience so far supports further cautious trials with this drastic cancer operation. A more appropriate indication is in patients with terminal benign liver disease and IDDM. We consider that the modified technique that preserves the native pancreas has the potential to become the standard procedure for this group of patients.
Acknowledgments
The authors thank Dr. Thomas Ritter (National University of Ireland, Galway, Ireland) for critical reading of this manuscript.
Supported by The Major Scientific and Technological Project of Hubei Province, No. 2006AA301A06
Peer reviewer: Salvatore Gruttadauria, MD, Assistant Professor, Abdominal Transplant Surgery, ISMETT, Via E. Tricomi, 190127 Palermo, Italy
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH
References
- 1.Mieles L, Todo S, Tzakis A, Starzl TE. Treatment of upper abdominal malignancies with organ cluster procedures. Clin Transplant. 1990;4:63–67. [PMC free article] [PubMed] [Google Scholar]
- 2.Abu-Elmagd K, Bond G, Reyes J, Fung J. Intestinal transplantation: a coming of age. Adv Surg. 2002;36:65–101. [PubMed] [Google Scholar]
- 3.Young AL, Peters CJ, Toogood GJ, Davies MH, Millson CE, Lodge JP, Pollard SG, Prasad KR. A combined liver-pancreas en-bloc transplant in a patient with cystic fibrosis. Transplantation. 2005;80:605–607. doi: 10.1097/01.tp.0000167007.58199.9b. [DOI] [PubMed] [Google Scholar]
- 4.Pirenne J, Deloose K, Coosemans W, Aerts R, Van Gelder F, Kuypers D, Maes B, Verslype C, Yap P, Van Steenbergen W, et al. Combined ‘en bloc’ liver and pancreas transplantation in patients with liver disease and type 1 diabetes mellitus. Am J Transplant. 2004;4:1921–1927. doi: 10.1111/j.1600-6143.2004.00588.x. [DOI] [PubMed] [Google Scholar]
- 5.Mekeel KL, Langham MR Jr, Gonzalez-Perralta R, Reed A, Hemming AW. Combined en bloc liver pancreas transplantation for children with CF. Liver Transpl. 2007;13:406–409. doi: 10.1002/lt.21070. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto CS, Fishbein TM. Modified multivisceral transplantation with splenopancreatic preservation. Transplantation. 2007;83:234–236. doi: 10.1097/01.tp.0000248885.76183.1b. [DOI] [PubMed] [Google Scholar]
- 7.Abu-Elmagd KM. Preservation of the native spleen, duodenum, and pancreas in patients with multivisceral transplantation: nomenclature, dispute of origin, and proof of premise. Transplantation. 2007;84:1208–1209; author reply 1209. doi: 10.1097/01.tp.0000287242.61220.4a. [DOI] [PubMed] [Google Scholar]
- 8.Starzl TE, Todo S, Tzakis A, Alessiani M, Casavilla A, Abu-Elmagd K, Fung JJ. The many faces of multivisceral transplantation. Surg Gynecol Obstet. 1991;172:335–344. [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE, Kaupp HA Jr, Brock DR, Butz GW Jr, Linman JW. Homotransplantation of multiple visceral organs. Am J Surg. 1962;103:219–229. doi: 10.1016/0002-9610(62)90491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alessiani M, Tzakis A, Todo S, Demetris AJ, Fung JJ, Starzl TE. Assessment of five-year experience with abdominal organ cluster transplantation. J Am Coll Surg. 1995;180:1–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Starzl TE, Todo S, Tzakis A, Podesta L, Mieles L, Demetris A, Teperman L, Selby R, Stevenson W, Stieber A. Abdominal organ cluster transplantation for the treatment of upper abdominal malignancies. Ann Surg. 1989;210:374–385; discussion 385-386. doi: 10.1097/00000658-198909000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loinaz C, Kato T, Nishida S, Weppler D, Levi D, Dowdy L, Madariaga J, Nery JR, Vianna R, Mittal N, et al. Bacterial infections after intestine and multivisceral transplantation. Transplant Proc. 2003;35:1929–1930. doi: 10.1016/s0041-1345(03)00728-0. [DOI] [PubMed] [Google Scholar]
- 13.Oltean M, Herlenius G, Gabel M, Friman V, Olausson M. Infectious complications after multivisceral transplantation in adults. Transplant Proc. 2006;38:2683–2685. doi: 10.1016/j.transproceed.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 14.Rayes N, Seehofer D, Theruvath T, Schiller RA, Langrehr JM, Jonas S, Bengmark S, Neuhaus P. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation--a randomized, double-blind trial. Am J Transplant. 2005;5:125–130. doi: 10.1111/j.1600-6143.2004.00649.x. [DOI] [PubMed] [Google Scholar]
- 15.Kato T, Kleiner G, David A, Selvaggi G, Nishida S, Madariaga J, Thompson J, Ruiz P, Tzakis A. Inclusion of spleen in pediatric multivisceral transplantation. Transplant Proc. 2006;38:1709–1710. doi: 10.1016/j.transproceed.2006.05.059. [DOI] [PubMed] [Google Scholar]