Abstract
Background
Recent genetic association studies have investigated the possible genetic role of the dopaminergic system in migraine. Catechol-O-methyltransferase (COMT) is an enzyme that plays a crucial role in the metabolism of dopamine and its genetic polymorphism is associated with three- to fourfold variation of enzymatic activity.
Objectives
The objective of this study was to elucidate the role of the COMT polymorphism in the genetic susceptibility to migraine and its phenotypic expression in patients with migraine without aura (MWOA).
Methods
Ninety-seven patients with MWOA and 94 healthy volunteers were included in the study. After amplifying COMT genes by the polymerase chain reaction, we assessed their genotype frequencies and allele distributions by based on restriction fragment length polymorphisms. We classified all MWOA patients into two groups according to their COMT genotype: with the L allele (N = 43), and without this allele (N = 54).
Results
The genotype frequency and allele distribution of the COMT polymorphism did not differ between MWOA patients and the control group. During migraine attacks, MWOA patients with the L allele showed a higher pain intensity of headache (P = 0.001) and a higher incidence of the accompanying nausea/vomiting (94% vs 75%; P = 0.026) compared with MWOA patients without the L allele.
Conclusions
Although the COMT polymorphism does not appear to be involved in predisposition to the development of MWOA, this genetic factor could be involved in the phenotypic expression of MWOA.
Keywords: Catechol-O-methyltransferase, Migraine without aura, Polymorphism, Dopamine
INTRODUCTION
Many investigators have reported that dopaminergic neurotransmission is altered in migraineurs.1-4 The frequency and severity of attacks in migraineurs decreases after the development of Parkinson's disease, and serum levels of dopamine increase in patients with migraine without aura (MWOA).5,6 Clinical trials involving treatment with dopamine receptor antagonists for migraine provide strong evidence for the involvement of dopamine.7,8 The accompanying symptoms during the migraine attack (nausea, vomiting, dizziness, and orthostatic faintness) may be related to dopaminergic activation.9-11
Migraine shows strong familial aggregation and presumably has a genetic basis, but the type and number of genes involved are currently unclear.12-14 Recent genetic association studies have investigated the possible genetic role of the dopaminergic system in migraine. Catechol-O-methyltransferase (COMT) is an enzyme that inactivates catecholamines or catechol-containing drugs. The gene encoding for COMT has been mapped to chromosome 22q11.15 Several studies indicate that the genetic polymorphism due to a G→A substitution at codon 158 of the COMT gene, leading to a valine-to-methionine substitution, results in differences in COMT activity: a valine at codon 158 results in a heat-stable, high-activity COMT variant, whereas a methionine at this position results in heat-labile, low-activity variants.16,17 The potential role of this functional polymorphism of COMT in Parkinson's disease and other neuropsychiatric disorders, including schizophrenia and bipolar affective disorders, has already been investigated.17-20 However, few studies have examined the association between COMT polymorphisms and migraine.21 Moreover, no study has investigated the phenotypic expression of dopamine-related accompanying symptoms.
We therefore investigated the possible role of COMT enzyme polymorphism in the genetic susceptibility to migraine and its phenotypic expression.
SUBJECTS AND METHODS
Subjects
All subjects included in this study were identified from a larger pool of subjects involved in an ongoing prospective study of the genetic contribution to headache at the headache clinic of the Catholic university of Korea. Ninety-seven patients with MWOA consecutively recruited from the headache clinic, and 94 healthy normal subjects without MWOA agreed to participate in this study. None of the participants were from the same family. The migraine patients and healthy control group were closely age matched. Given the known effects of gender on this gene system, only women were included in this study. This study was approved by the institutional ethnical committee of our institute, and written informed consent was obtained from all participating subjects.
All participants were interviewed clinically and examined physically and neurologically by an experienced neurologist. Psychiatric interviews were performed by a psychiatrist, and patients with personality or major psychiatric disorders were excluded according to DSM-IV criteria. During the interview, detailed data were obtained regarding clinical symptoms and headache variables using a structured questionnaire. The diagnosis of MWOA was made based on the operational diagnostic criteria of the International Headache Society (ICHD-II [International Classification of Headache Disorders]).22 We did not include patients suffering migraine with aura, because there were too few of them in the pool of subjects from which they were selected.
Determination of the COMT genot
Blood samples were collected into heparinized tubes and stored at -80℃ before isolation of DNA. Genomic DNA was isolated and amplified by the polymerase chain reaction (PCR) using the specific oligonucleotide primers 5'-CTC ATC ACC ATC GAG ATCAA-3' and 5'-GAT GAC CCT GGT GAT GTGG-3'. PCR reaction mixtures contained 50 ng of genomic DNA, 50 pmol of each primer, 10 mM Tris-HCL, 50 mM KCl, 50 mM MgCl2, 0.01% Tween 20, each of dATP, dCTP, dGTP, and dTTP at 0.2 and 2.5 units of Taq polymerase in a total volume of 100 µL. The following cycling conditions were used: (1) an initial denaturation at 95℃ for 3 min; (2) three cycles of denaturation at 94℃ for 30 s, touch-down annealing at 65℃ for 1 min, and synthesis at 72℃ for 1 min; (3) 34 cycles of denaturation at 94℃ for 30 s, annealing at 63℃ for 1 min, and synthesis at 72℃ for 1 min; and (4) a final extension at 72℃ for 10 min. PCR was conducted in a thermal cycler (BioRad, Richmond, California).
The resulting PCR products were subjected to restriction digestion for 3 h at 37℃ using 5 U NlaIII (New England BioLabs, Beverly, Massachusetts). The digested products were separated at 150 V for 2 h on a 4% NuSieve 3:1 agarose gel (FMC Bio-Products, Rockland, Maine) containing 0.5 g/mL ethidium bromide. The gel was visualized under UV light using a gel electrophoresis visualization system (Vilber Lourmat, Marne La Vallée, France). The COMT-Met/Met genotype (L/L) was represented by 54-, 67-, and 71-bp fragments, COMT-Met/Val (L/H) by 54-, 71-, and 85-bp fragments, and COMT-Val/Val (H/H) by 54-, 67-, 71-, and 85-bp fragments. The 18-bp fragment was difficult to visualize because of both its small size and its comigration with the similarly sized primer residue; however, detection of this fragment was not critical to determining the genotypes. Genotyping was based upon independent scoring of the results by two reviewers who were unaware of the case/control status.
Associations of migraine phenotypes with COMT genotypes
We classified all patients with MWOA into two groups according to their COMT genotype: those with the L allele (N = 44, L/L and L/H genotype) and those without this allele (N = 53, H/H genotype). The physical variables of the patients' headaches were evaluated according to the pain index of headache intensity, as measured on a visual analog scale (scale anchored with 0 and 10, where 0 means no pain at all and 10 means pain as bad as it can be), the headache frequency (per month), and the duration of each headache attack (hours) during the previous 6 months. Clinical manifestations associated with MWOA (location, quality, aggravation by activity, nausea/vomiting, phonophobia/photophobia, and dizziness) were assessed during this evaluation. Photophobia and phonophobia (0, at least one present; 1, both present) as well as nausea and vomiting (0, not present; 1, at least one present) were combined into a single index variable for later analysis.
Data analysis
Comparisons of genotype distribution and allele frequency were performed on raw frequencies using a chi-squared test. A chi-squared analysis of the Hardy-Weinberg equilibrium for the genotypes was conducted to determine whether the allele frequencies were stable within the patients and controls. Comparisons of clinical symptoms and physical variables of migraine according to the COMT allele were performed using the t-test for parametric variables and the chi-squared test for non-parametric variables. SPSS statistical software (version 10.0; SPSS, Chicago, Illinois, USA) was used for all the analyses, with a probability value of P < 0.05 considered significant.
RESULTS
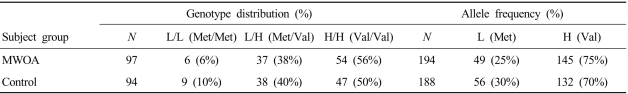
The genotype distributions in patients with MWOA and controls did not deviate from those expected based on the Hardy-Weinberg equilibrium. Table 1 indicates that the COMT genotype distribution and allele frequencies did not differ significantly between MWOA patients and controls.
Table 1.
Distributions of genotypes and allele frequencies of the catechol-O-methyltransferase (COMT) polymorphism in patients with migraine without aura (MWOA) and in controls
Genotype distribution: χ2 = 1.05 (two degrees of freedom), P = 0.059
Allele frequency: χ2 = 0.983 (one degree of freedom), P > 0.05
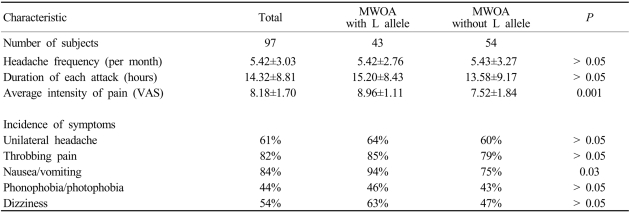
The pain intensity of headache was significantly higher in patients with the L allele than in those without this allele. Variables including the headache frequency and duration of headache attack did not differ significantly between the two groups (Table 2). Nausea or vomiting accompanying migraine attacks was more frequent in patients with L allele than in those without this allele (94% vs 75%, P = 0.026). Although the tendency to experience dizziness during a migraine attack was higher in patients with the L allele than in those without this allele (63% vs 47%), the difference was not statistically significant (Table 2).
Table 2.
Clinical symptoms of patients with MWOA according to their COMT polymorphism
VAS; visual analog scale
DISCUSSION
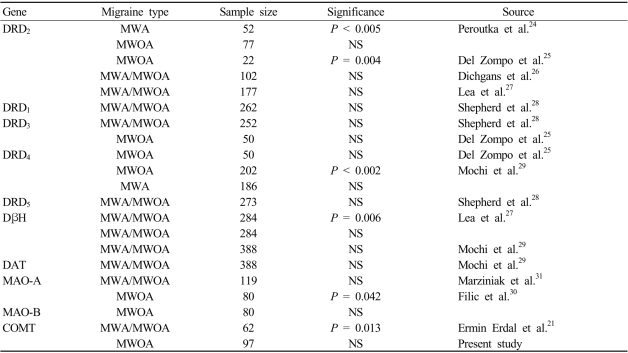
Interest in the contribution of dopaminergic pathways to migraine biogenesis stems from reports of the induction of migraine by dopaminergic agonist at doses that do not appear to affect nonmigraineurs.10,23 Migraine association studies investigating dopamine-related candidate genes have produced some interesting but as yet inconclusive results (Table 3).21,24-31 A recent analysis of the association between COMT polymorphism and migraine in a Caucasian population revealed that the frequency of the L/L genotype was higher in migraine patients.21 However, in the present study we found no differences in the genotype distribution and allele frequency of the COMT polymorphism between patients with MWOA and controls. The interactions of the dopaminergic system are complex, and the control of dopamine activity may be determined by various enzymes involved in dopamine metabolism. Therefore, this possibility could be considered in addition to ethnic differences.32-36
Table 3.
Summary of association studies investigating dopamine-related genes in migraine
DRD; dopamine receptor gene, DβH; dopamine beta hydroxylase gene, DAT; dopamine transporter gene, MAO; monoamine oxidase gene, MWA; migraine with aura
NS; P > 0.05
In this study, the pain intensity of headache was higher and the associated nausea and/or vomiting was more common in patients with the L allele. The higher dopaminergic activity could presumably result from the COMT activity being lower in individuals with the L allele than in those without this allele. With regard to migraine headache, basal ganglia and dopaminergic neurotransmission are well known to be involved in the regulation of nociception.37 Administration of L-dopa facilitates the perception of pain in rats, and L-dopa agonists can induce pain in Parkinson's patients.38,39 Dopamine antagonists are commonly and successfully used to reduce pain intensity of headache in acute migraine, which is additional to their antiemetic effect.7,8,40,41 Symptoms accompanying migraine, such as nausea, vomiting, and dizziness, may be mediated by dopaminergic activation.9-11 Different expression of the D2 family receptor in the brainstem has been frequently reported in patients with migraine, which may be due to these receptors regulating visceral function, including gastrointestinal motility and blood pressure.42,43 D2, D3, and D4 receptors have been detected in intermediate and medial subnuclei among the nucleus of the solitary tract and in the dorsal motor nucleus of the vagus. D3 receptors are also found in the area postrema. Peripheral D2 receptors are located presynaptically on sympathetic nerves and ganglia, and may be the mechanism by which dopaminergic stimulation induces dizziness or orthostatic hypotension.44 Del Zompo, et al. studied genetic aspects of dopamine receptor polymorphism in migraine patients, and considered that "dopaminergic migraineurs" - selected based on the presence of both nausea and vomiting during the pain phase of migraine - could be differentiated from "nondopaminergic migraineurs".25 In their study, although no association was detected between dopamine receptor polymorphism and migraine in the overall groups, the allelic distribution differed significantly according to the existence of dopaminergic symptoms.25 Considering those studies, our results suggest that genetic factors of dopaminergic system are involved in the associated clinical manifestation of MWOA. The COMT polymorphism may be of potential pharmacological importance regarding individual differences in the metabolism of dopaminergic drugs in patients with MWOA. Moreover, antidopaminergic drugs may be useful as an antimigraine treatment in patients who are refractory to primary treatment and have prominent accompanying dopaminergic symptoms.
In conclusion, our results do not confirm an association of MWOA with the COMT gene, but they do suggest that allelic variation in COMT is involved in the phenotypic expression of migraine in some individuals.
References
- 1.Fanciullacci M, Michelacci S, Curradi C, Sicuteri F. Hyperresponsiveness of migraine patients to the hypotensive action of bromocriptine. Headache. 1980;20:99–102. doi: 10.1111/j.1526-4610.1980.hed2002099.x. [DOI] [PubMed] [Google Scholar]
- 2.Geraud G, Guell A, Courtade M, Dupui P, Bes A. Clinical value of a dopaminergic agonist administration in migraine patients. Headache. 1983;23:191–192. doi: 10.1111/j.1526-4610.1983.hed2304191.x. [DOI] [PubMed] [Google Scholar]
- 3.Montastruc JL, David J, Rascol A. Does the piribedil test permit predicting the efficacy of domperidone, a dopaminergic antagonist, in the treatment of migraine? Headache. 1985;25:332–333. doi: 10.1111/j.1526-4610.1985.hed2506332.x. [DOI] [PubMed] [Google Scholar]
- 4.Bes A, Dupui P, Guell A, Bessoles G, Geraud G. Pharmacological exploration of dopamine hypersensitivity in migraine patients. Int J Clin Pharmacol Res. 1986;6:189–192. [PubMed] [Google Scholar]
- 5.van Hilten JJ. The migraine - dopamine link: do migraine and Parkinson's disease coexist? Clin Neurol Neurosurg. 1992;94(Suppl):S168–S170. doi: 10.1016/0303-8467(92)90060-g. [DOI] [PubMed] [Google Scholar]
- 6.Nagel-Leiby S, Welch KMA, D'Andrea G, Grunfeld S, Brown E. Event-related slow potentials and associated catecholamine function in migraine. Cephalalgia. 1990;10:317–329. doi: 10.1046/j.1468-2982.1990.1006317.x. [DOI] [PubMed] [Google Scholar]
- 7.Fisher H. A new approach to emergency department therapy of migraine headache with intravenous haloperidol: a case series. J Emerg Med. 1995;13:119–122. doi: 10.1016/0736-4679(94)00121-9. [DOI] [PubMed] [Google Scholar]
- 8.Emilien G, Maloteaux JM, Geurts M, Hoogenberg K, Cragg S. Dopamine receptors-physiological understanding to therapeutic intervention potential. Pharmacol Ther. 1999;84:133–156. doi: 10.1016/s0163-7258(99)00029-7. [DOI] [PubMed] [Google Scholar]
- 9.Mascia A, Afra J, Schoenen J. Dopamine and migraine: a review of pharmacological, biochemical, neurophysiological, and therapeutic data. Cephalalgia. 1998;18:174–182. doi: 10.1046/j.1468-2982.1998.1804174.x. [DOI] [PubMed] [Google Scholar]
- 10.Cerbo R, Barbanti P, Buzzi MG, Fabbrini G, Brusa L, Roberti C, et al. Dopamine hypersensitivity in migraine: role of the apomorphine test. Clin Neuropharmacol. 1997;20:36–41. doi: 10.1097/00002826-199702000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Sicuteri F. Dopamine, the second putative protagonist in headache. Headache. 1977;17:129–131. doi: 10.1111/j.1526-4610.1977.hed1703129.x. [DOI] [PubMed] [Google Scholar]
- 12.Honkasalo ML, Kaprio J, Winter T, Heikkila K, Sillanpaa M, Koskenvuo M. Migraine and concomitant symptoms among 8167 adult twin pairs. Headache. 1995;35:70–78. doi: 10.1111/j.1526-4610.1995.hed3502070.x. [DOI] [PubMed] [Google Scholar]
- 13.Larsson B, Bille B, Pedersen NL. Genetic influence in headaches: a Swedish twin study. Headache. 1995;35:513–519. doi: 10.1111/j.1526-4610.1995.hed3509513.x. [DOI] [PubMed] [Google Scholar]
- 14.Russell MB, Olesen J. The genetics of migraine without aura and migraine with aura. Cephalalgia. 1993;13:245–248. doi: 10.1046/j.1468-2982.1993.1304245.x. [DOI] [PubMed] [Google Scholar]
- 15.Lachman HM, Morrow B, Shprintzen R, Veit S, Parsia SS, Faedda G, Goldberg R, Kucherlapati R, Papolos DF. Association of codon 108/158 catechol-O-methyltransferase gene polymorphism with the psychiatric manifestations of velo-cardio-facial syndrome. Am J Med Genet. 1996;67:468–472. doi: 10.1002/(SICI)1096-8628(19960920)67:5<468::AID-AJMG5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 16.Strous RD, Bark N, Woerner M, Lachman HM. Lack of association of a functional catechol-O-methyltransferase gene polymorphism in schizophrenia. Biol Psychiatry. 1997;41:493–495. doi: 10.1016/s0006-3223(96)00474-x. [DOI] [PubMed] [Google Scholar]
- 17.Syvanen AC, Tilgmann C, Rinne J, Ulmanen I. Genetic polymorphism of catechol-O-methyltransferase (COMT): correlation of genotype with individual variation of S-COMT activity and comparison of the allele frequencies in the normal population and parkinsonian patients in Finland. Pharmacogenetics. 1997;7:65–71. doi: 10.1097/00008571-199702000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Daniels JK, Williams NM, Williams J, Jones LA, Cardno AG, Murphy KC, et al. No evidence for allelic association between schizophrenia and a polymorphism determining high or low catechol-O-methyltransferase activity. Am J Psychiatry. 1996;153:268–270. doi: 10.1176/ajp.153.2.268. [DOI] [PubMed] [Google Scholar]
- 19.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Papolos DF, Veit S, Faedda GL, Saito T, Lachman HM. Ultra-ultra rapid cycling bipolar disorder is associated with the low activity catechol-O-methyltransferase allele. Mol Psychiatry. 1998;3:346–349. doi: 10.1038/sj.mp.4000410. [DOI] [PubMed] [Google Scholar]
- 21.Ermin Erdal M, Herken H, Yilmaz M, Bayazit YA. Significance of the catechol-O-methyltransferase gene polymorphism in migraine. Brain Res Mol Brain Res. 2001;94:193–196. doi: 10.1016/s0169-328x(01)00219-4. [DOI] [PubMed] [Google Scholar]
- 22.Headache classification subcommittee of the international headache society. The international classification of headache disorders. 2nd Edn. Cephalalagia. 2004;24(Suppl):1–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 23.Peroutka SJ. Dopamine and migraine. Neurology. 1997;49:650–656. doi: 10.1212/wnl.49.3.650. [DOI] [PubMed] [Google Scholar]
- 24.Peroutka SJ, Wilhoit T, Jones K. Clinical susceptibility to migraine with aura is modified by dopamine D2 receptor (DRD2) NcoI alleles. Neurology. 1997;49:201–206. doi: 10.1212/wnl.49.1.201. [DOI] [PubMed] [Google Scholar]
- 25.Del Zompo M, Cherchi A, Palmas MA, Ponti M, Bocchetta A, Gessa GL, et al. Association between dopamine receptor genes and migraine without aura in a Sardinian sample. Neurology. 1998;51:781–786. doi: 10.1212/wnl.51.3.781. [DOI] [PubMed] [Google Scholar]
- 26.Dichgans M, Forderreuther S, Deiterich M, Pfaffenrath V, Gasser T. The D2 receptor NcoI allele: absence of allelic association with migraine with aura. Neurology. 1998;51:928. doi: 10.1212/wnl.51.3.928. [DOI] [PubMed] [Google Scholar]
- 27.Lea RA, Dohy A, Jordan K, Quinlan S, Brimage PJ, Griffiths LR. Evidence for allelic association of the dopamine beta-hydroxylase gene (DBH) with susceptibility to typical migraine. Neurogenetics. 2000;3:35–40. doi: 10.1007/pl00022977. [DOI] [PubMed] [Google Scholar]
- 28.Shepherd AG, Lea RA, Hutchins C, Jordan KL, Brimage PJ, Griffiths LR. Dopamine receptor genes and migraine with and without aura: an association study. Headache. 2002;42:346–351. doi: 10.1046/j.1526-4610.2002.02105.x. [DOI] [PubMed] [Google Scholar]
- 29.Mochi M, Cevoli S, Cortelli P, Pierangeli G, Soriani S, Scapoli C, et al. A genetic association study of migraine with dopamine receptor 4, dopamine transporter and dopamine-beta-hydroxylase genes. Neurol Sci. 2003;23:301–305. doi: 10.1007/s100720300005. [DOI] [PubMed] [Google Scholar]
- 30.Filic V, Vladic A, Stefulj J, Cicin-Sain L, Balija M, Sucic Z, Jernej B. Monoamine oxidases A and B gene polymorphisms in migraine patients. J Neurol Sci. 2005;15:149–153. doi: 10.1016/j.jns.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 31.Marziniak M, Mossner R, Benninghoff J, Syagailo YV, Lesch KP, Sommer C. Association analysis of the functional monoamine oxidase A gene promotor polymorphism in migraine. J Neural Transm. 2004;111:603–609. doi: 10.1007/s00702-004-0108-0. [DOI] [PubMed] [Google Scholar]
- 32.Tiihonen J, Hallikainen T, Lachman H, Saito T, Volavka J, Kauhanen J, et al. Association between the functional variant of the catechol-O-methyltransferase (COMT) gene and type 1 alcoholism. Mol Psychiatry. 1999;4:286–289. doi: 10.1038/sj.mp.4000509. [DOI] [PubMed] [Google Scholar]
- 33.Kauhanen J, Hallikainen T, Tuomainen TP, Koulu M, Karvonen MK, Salonen JT, et al. Association between the functional polymorphism of catechol-O-methyltransferase gene and alcohol consumption among social drinkers. Alcohol Clin Exp Res. 2000;24:135–159. [PubMed] [Google Scholar]
- 34.Hallikainen T, Lachman H, Saito T, Volavka J, Kauhanen J, Salonen JT, et al. Lack of association between thefunctional variant of the catechol-O-methyltransferase (COMT) gene and early-onset alcoholism associated with severe antisocial behavior. Am J Med Genet. 2000;96:348–352. doi: 10.1002/1096-8628(20000612)96:3<348::aid-ajmg22>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 35.Ishiguro H, Haruo Shibuya T, Toru M, Saito T, Arinami T. Association study between high and low activity polymorphism of catechol-O-methyltransferase gene and alcoholism. Psychiatr Genet. 1999;9:135–138. doi: 10.1097/00041444-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura A, Inada T, Kitao Y, Katayama Y. Association between catechol-O-methyltransferase (COMT) polymorphism and severe alcoholic withdrawal symptoms in male Japanese alcoholics. Addict Biol. 2001;6:233–238. doi: 10.1080/13556210120056562. [DOI] [PubMed] [Google Scholar]
- 37.Chudler EH, Dong WK. The role of the basal ganglia in nociception and pain. Pain. 1995;60:3–38. doi: 10.1016/0304-3959(94)00172-B. [DOI] [PubMed] [Google Scholar]
- 38.Paalzow GH. L-dopa induces opposing effects on pain in intact rats: (-)-sulpiride, SCH 23390 or alpha-methyl-DL-p-tyrosine methylester hydrochloride reveals profound hyperalgesia in large antinociceptive doses. J Pharmacol Exp Ther. 1992;263:470–479. [PubMed] [Google Scholar]
- 39.Quinn NP, Koller WC, Lang AE, Marsden CD. Painful Parkinson's disease. Lancet. 1986;1:1366–1369. doi: 10.1016/s0140-6736(86)91674-0. [DOI] [PubMed] [Google Scholar]
- 40.Silberstein SD, Young WB, Mendizabal JE, Rothrock JF, Alam AS. Acute migraine treatment with droperidol: a randomized, double-blind, placebo-controlled trial. Neurology. 2003;60:315–321. doi: 10.1212/01.wnl.0000042477.63516.b2. [DOI] [PubMed] [Google Scholar]
- 41.Wang SJ, Silberstein SD, Young WB. Droperidol treatment of status migrainosus and refractory migraine. Headache. 1997;37:377–382. doi: 10.1046/j.1526-4610.1997.3706377.x. [DOI] [PubMed] [Google Scholar]
- 42.Hyde TM, Knable MB, Murray AM. Distribution of dopamine D1-D4 receptor subtypes in human dorsal vagal complex. Synapse. 1996;24:224–232. doi: 10.1002/(SICI)1098-2396(199611)24:3<224::AID-SYN4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 43.Kohli JD, Glock D, Goldberg LI. Selective DA2 versus DA1 antagonist activity of domperidone in the periphery. Eur J Pharmacol. 1983;89:137–141. doi: 10.1016/0014-2999(83)90618-0. [DOI] [PubMed] [Google Scholar]
- 44.Sicuteri F, Bonucci M, Cangi F, Pietrini U, Fanciullacci M. Syncope and dopamine receptor hyperreactivity: myth and facts about basilar artery migraine. In: Carroll JD, Pfaffenrath V, Sjaastad O, editors. Migraine and Beta Blockade. Molndal: Hassle; 1985. pp. 147–160. [Google Scholar]