Abstract
Background and purpose
N-methyl-D-aspartate (NMDA)-mediated neurotoxicity and oxidative stress have been implicated in the etiology of amyotrophic lateral sclerosis (ALS). Memantine is a low-affinity, noncompetitive NMDA receptor antagonist that may protect against motor neuron degeneration.
Methods
Thirty transgenic mice expressing the G93A SOD1 mutation were randomly divided into control, low-dose memantine (30 mg/kg/day), and high-dose memantine (90 mg/kg/day) groups, with memantine supplied daily with drinking water beginning at 75 days of age. Body weight, survival, and behavioral performances including a rotarod test, paw grip endurance, and hindlimb extension reflex were assessed in the control and memantine-diet groups.
Results
Clinical symptoms were evident in the G93A transgenic mice by 11 weeks of age. Memantine was tolerated well. Compared to control, mice treated with memantine performed better in the rotarod test and hindlimb extension reflex. Moreover, low-dose memantine treatment significantly prolonged the survival of the transgenic mice relative to control mice (141 vs 134 days, p<0.05).
Conclusions
These findings suggest that memantine, even when administered at the time of symptom onset, has beneficial effects on patients with ALS.
Keywords: Amyotrophic lateral sclerosis, Memantine, N-methyl-D-aspartate receptor antagonist
INTRODUCTION
Glutamate is a major excitatory neurotransmitter in the central nervous system, and excessive activation of glutamate receptors can damage neurons via excitotoxicity. There are several lines of evidence that excitotoxicity plays a role in selective motor neuron death in amyotrophic lateral sclerosis (ALS).1-3 Elevation of the extracellular glutamate level causes degeneration of motor neurons through excessive stimulation of glutamate receptors. Both N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors are responsible for excitotoxicity in motor neurons.4,5 This has led to several antiglutamate drugs being tested on patients with ALS,6 of which riluzole has a modest effect on survival and is the only such drug approved by the US Food and Drug Adminstration for ALS.7,8
Riluzole inhibits glutamate release by inactivating voltage-dependent sodium channels and a G-protein-dependent signal transduction process at glutamatergic nerve terminals.9 Because NMDA receptors play crucial roles in various forms of synaptic plasticity, which is required in several normal processes such as learning and memory,10,11 drugs such as riluzole that completely block NMDA receptors would inevitably impair normal synaptic transmission and thereby cause numerous unwanted side effects. Such side effects could be avoided by producing a partial rather than a complete blockade of NMDA receptors.
Memantine is a low-affinity, noncompetitive open-channel receptor blocker with a rapid off-rate from the channel, permitting the blockade of excessive NMDA receptor activity without disrupting normal synaptic transmission.12,13 Neuroprotective properties of memantine have been shown in various in vitro and in vivo models of excitotoxicity,14,15 and it has been used clinically with excellent safety in various neurodegenerative disorders including Alzheimer's disease.16,17 In a recent study, memantine significantly delayed disease progress and increased the life span by about 1 week in a transgenic ALS mouse model carrying the G93A mutant of the human Cu-Zn superoxide dismutase (SOD) gene,18 with immunohistochemistry revealing the expression of NMDA receptor subunits within neurons of varying sizes in both dorsal and ventral areas of the spinal cord, including motor neurons.18 However, the starting point and delivery method of memantine treatment employed in that study are unfortunately not readily applicable in clinical treatments of patients with ALS.
In the present study, we attempted to determine the effect of oral administration of memantine given at the time of symptom onset on behaviors and survival of transgenic SOD1 mutant ALS mice.
MATERIALS AND METHODS
1. Animals and memantine treatment
Transgenic mice overexpressing human SOD1 with a Gly-to-Ala mutation at position 93 were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and maintained as hemizygotes by breeding transgenic males with wild-type B6SJL females. The transgenic progeny were genotyped for expression of the transgene by the polymerase chain reaction using primers specific for human SOD1. Throughout the experiments, animals were housed in a controlled environment (temperature of 20-22℃, humidity of 40-60%, and lights on from 0700 to 1900 hours) with free access to food and water. The animals were randomly divided into control, low-dose memantine (30 mg/kg/day), and high-dose memantine (90 mg/kg/day) groups, with 10 animals in each group. The transgenic mice were treated daily with memantine in the drinking water, beginning at 75 days of age. The daily dosage was calculated based on a daily water intake of 17.7 ml/100g/day.19 Fresh solutions were prepared weekly.
2. Behavioral analysis
Motor performance was evaluated twice weekly by a rotarod test (Columbus Instruments, OH, USA), paw grip endurance (PaGE), and an extension reflex of hindlimbs, beginning at 10 weeks of age after a 2-week learning period. The time period that mice remained on a rotarod rotating at a constant speed of 16 rpm was measured, with 300 s chosen as the arbitrarycut-off time. Animals performed three trials for each evaluation, and the longest duration achieved was recorded. PaGE was used to quantify the grip strength of the mouse. The wire lid was gently shaken to prompt the mouse to hold onto the grid before the lid was flipped upside down. The time before the mouse let go of the grid with at least both hindlimbs was measured. Each mouse was allowed three attempts to hold onto the inverted lid for an arbitrary maximum of 200 s, and the longest duration was recorded. The extension reflex was quantified using the following 4-point scoring system: 0 for loss of reflex, with hindlimbs and paws held close to the body; 1 for loss of reflex with marked flexion of the hindlimbs; 2 for hindlimbs extending to <90 degrees with a decreased extension reflex in bilateral hindlimbs; 3 for hindlimbs extending to <90 degrees with decreased extension reflex in unilateral hindlimbs; and 4 for hindlimbs reflexly extending to form an angle of approximately 120 degrees. The body weight of the mice was measured twice weekly.
The day of death was defined as when a mouse could no longer roll over within 30 s after being placed on its side on a flat surface.
3. Statistical analysis
All statistical analyses were performed using SPSS 12.0 for Windows software. Analysis of variance was used to analyze the significance of differences in behavioral performance and body weight. The Kaplan-Meier method was used to assess survival data. Statistical significance was accepted at the p<0.05 level.
RESULTS
The onset of disease in G93A transgenic mice was defined as the first day on which hindlimb weakness appeared, including tremor or altered splay reflex. Memantine was administered orally at the end of 11 weeks of age (75 days of age) when the mice showed the initial clinical signs.
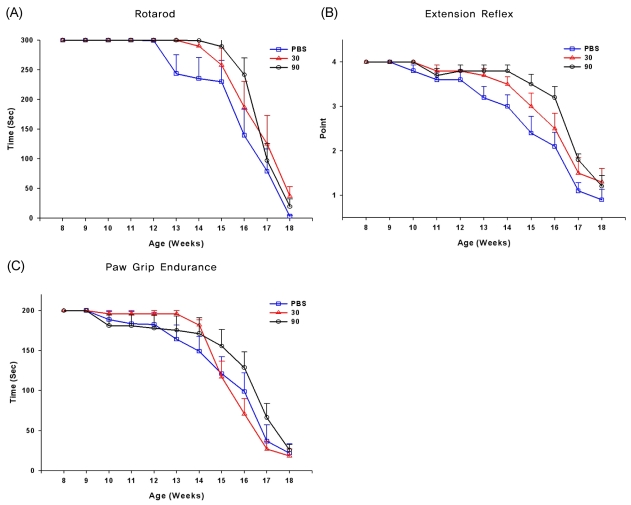
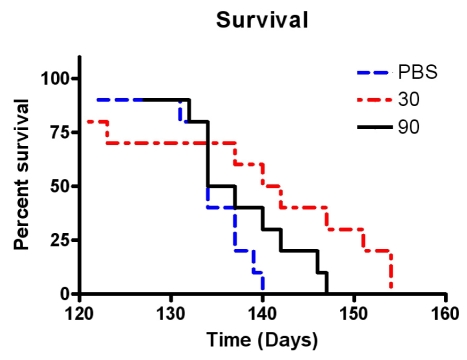
Low- and high-dose memantine treatments tended to delay disease progression as assessed by behavioral performances, but the protective effect of memantine was not statistically significant compared with control (values in the control, low-dose memantine, and high-dose memantine groups: 112, 116, and 119 days on the rotarod, and 91, 102, and 112 days on the extension reflex, respectively) (Fig. 1). In addition, no statistically significant difference was observed between the two memantine-treated groups, although further motor deterioration in the rotarod test and extension reflex tended to be delayed in the high-dose memantine group relative to the low-dose memantine group. Memantine treatment did not show any protective effect on the body weight or PaGE. However, low-dose memantine significantly prolonged the survival of the animals relative to control and high-dose memantine treatment: the median survival was 141 days in the low-dose memantine group but 135.5 and 134 days in the high-dose memantine and control groups, respectively (p<0.05, see Fig. 2).
Figure 1.
Effects of memantine treatment on motor performance in G93A transgenic mice. Cumulative probability of the onset of deficit in rotarod (A), paw grip endurance (PaGE) (B), and extension reflex (C) tests. There was a trend toward an improved rotarod performance and delayed loss of extension reflex, although the effect was not statistically significant. Memantine had no effect on PaGE.
Figure 2.
Cumulative probabilities for survival in animals treated with low-dose memantine (30 mg/kg/day), high-dose memantine (90 mg/kg/day), or water alone, starting at symptom onset at 75 days of age. Mortality was significantly delayed in G93A transgenic mice receiving low-dose memantine (p<0.05).
No significant adverse effects associated with memantine treatment were observed.
DISCUSSION
NMDA-receptor-mediated neurotoxicity has been implicated in a broad spectrum of neurological disorders, including ALS. The current data show that memantine (an NMDA receptor antagonist) prolongs the survival of G93A transgenic ALS mice by about 1 week (~5% of the life span). Although we observed some tendencies, we found that memantine had no significant protective effect on motor deterioration. These results are similar to the effects of riluzole found in two human clinical trials,7,8 as well as in an animal study.20 Riluzole exhibits a variety of pharmacologic actions, including inhibition of glutamate release from presynaptic nerve terminals,21 modulation of both NMDA22 and AMPA receptors,23 and inhibition of high-affinity uptake of γ-aminobutyric acid.24 Such diverse and nonspecific actions of riluzole would inevitably cause a variety of intolerable adverse effects. For this reason, an antiexcitotoxic therapy should act specifically and must block excessive activation of the relevant receptors, leaving normal function relatively intact in order to avoid side effects.
Memantine is a specific, noncompetitive open-channel NMDA receptor blocker with a rapid off-rate from the channel, permitting the preferential blockade of excessive NMDA receptor activity without disrupting normal activity.12,13 Many neurodegenerative diseases - including Alzheimer's disease, Parkinson's disease, Huntington's disease, and ALS . have a final common pathway of neuronal injury as a result of glutamate receptor overstimulation, especially of the NMDA subtype.25 Therefore, memantine might be a good candidate treatment for alleviating excitotoxicity associated with several neurodegenerative diseases. In fact, memantine has been used clinically for over 20 years to treat Parkinson's disease, vascular dementia, and Alzheimer's disease. Moreover, the long-term safety and efficacy of memantine has been confirmed in the treatment of patients with moderate-to-severe Alzheimer disease.26
Many in vitro and in vivo studies have demonstrated the antiexcitotoxic effects of memantine in several neurodegenerative diseases, but there have been surprisingly few attempts to determine its effects on ALS. To our knowledge only one recent study18 has investigated this, and concluded that the neuroprotective effect of memantine in a transgenic ALS mouse model carrying a high copy number of SOD1 (G93A) was probably due to the inhibition of spinal cord NMDA receptors, and suggested that memantine could be used in ALS patients to prolong survival. Despite these promising results, that study had limitations: (1) the memantine treatment began before the onset of symptoms due to there being no reliable diagnostic biomarkers for human ALS,27 and (2) the delivery method was a subcutaneous injection, which is not readily applicable to patients with ALS.
In the present study, we demonstrated that the administration of memantine even at symptom onset (75 days of age) had therapeutic effects in ALS. Although the earliest deficits in motor power and coordination in SOD1 (G93A) transgenic mice are observed as early as 8 weeks of age, the designated onset time may differ with the test used for motor assessment.28 The first clinical signs in the mice used in this study appeared at about 11 weeks of age, with the mice showing tremor, impairment of the hindlimb extension reflex, and reduced running time on the rotarod apparatus. While memantine significantly prolonged survival, no significant beneficial effects on motor performance, hindlimb extension reflex, or weight were detected. Such partial effects might be attributable to the delay in memantine treatment, or to motor neuron degeneration in ALS mice not being mediated via activation of NMDA receptors alone, since there is evidence that AMPA/kainate receptors play a major role in neurotoxicity.29 In this regard, a previous experimental study has demonstrated that an AMPA/kainate receptor antagonist significantly protected against the calcium influx and neurotoxicity, while an NMDA receptor antagonist had only a mild effect.30 Moreover, Tortarolo et al. demonstrated that a new noncompetitive AMPA antagonist partially protected motor neurons, improved motor function, and prolonged the survival of SOD1 (G93A) transgenic ALS mice.31
Memantine exerted a dose-dependent protective effect on motor deterioration but not on survival. This discrepancy might be due to erratic ingestion of the water containing memantine. We did not estimate the plasma concentration of memantine. The therapeutic plasma concentration of memantine for Alzheimer's disease is approximately 1 µM.32 The steady-state plasma levels following oral administration of 10, 30, and 100 mg/kg/day memantine were reportedly 0.49±0.06, 1.14±0.07, and 5.54±0.4 µM (mean±SD), respectively, in male C56BL/6J mice.33 Based on these data, a dose of 30 mg/kg/day should produce the therapeutic plasma level of 1 µM in Alzheimer's disease. Additional work is needed to explore whether a serum level of 1 µM is also effective in ALS.
In conclusion, we have confirmed that memantine prolongs the survival of and delays motor deterioration in SOD1 (G93A) transgenic ALS mice. These results combined with those from a previous memantine study for ALS18 suggest that memantine could be used as a new and effective NMDA receptor antagonist to manage human ALS patients with acceptable long-term safety and tolerability. The next steps are to determine whether memantine is also effective in human ALS and whether a combination therapy with other agents exerts better therapeutic effects than memantine treatment alone.
Footnotes
The authors thank Lundbeck, Inc., for providing the memantine used in this study. This study was supported by Korea Science and Engineering Foundation (KOSEF) grant no. R11-1998-052-06007-0.
References
- 1.Shaw PJ, Ince PG. Glutamate, excitotoxicity and amyotrophic lateral sclerosis. J Neurol. 1997;244:S3–S14. doi: 10.1007/BF03160574. [DOI] [PubMed] [Google Scholar]
- 2.Rothstein JD. Excitotoxic mechanisms in the pathogenesis of amyotrophic lateral sclerosis. Adv Neurol. 1995;68:7–20. [PubMed] [Google Scholar]
- 3.Van Den Bosch L, Van Damme P, Bogaert E, Robberecht W. The role of excitotoxicity in the pathogenesis of amyotrophic lateral sclerosis. Biochim Biophys Acta. 2006;1762:1068–1082. doi: 10.1016/j.bbadis.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 5.Prehn JH, Lippert K, Krieglstein J. Are NMDA or AMPA/kainate receptor antagonists more efficacious in the delayed treatment of excitotoxic neuronal injury? Eur J Pharmacol. 1995;292:179–189. doi: 10.1016/0926-6917(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 6.Traynor BJ, Bruijn L, Conwit R, Beal F, O'Neill G, Fagan SC, et al. Neuroprotective agents for clinical trials in ALS: a systemic assessment. Neurology. 2006;67:20–27. doi: 10.1212/01.wnl.0000223353.34006.54. [DOI] [PubMed] [Google Scholar]
- 7.Bensimon G, Lacomblez L, Meininger V. (ALS/Riluzole Study Group). A controlled trial of riluzole in amyotrophic lateral sclerosis. N Engl J Med. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 8.Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V, Amyotrophic Lateral Sclerosis/Riluzole Study Group II Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Lancet. 1996;347:1425–1431. doi: 10.1016/s0140-6736(96)91680-3. [DOI] [PubMed] [Google Scholar]
- 9.Doble A. The pharmacology and mechanism of action of riluzole. Neurology. 1996;47:S233–S241. doi: 10.1212/wnl.47.6_suppl_4.233s. [DOI] [PubMed] [Google Scholar]
- 10.Collingridge GL, Singer W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol Sci. 1990;11:290–296. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- 11.Danysz W, Zajaczkowski W, Parsons CG. Modulation of learning processes by ionotropic glutamate receptor ligands. Behav Pharmacol. 1995;6:455–474. [PubMed] [Google Scholar]
- 12.Chen HS, Pellegrini JW, Aggarwal SK, Lei SZ, Warach S, Jensen FE, et al. Open-channel block of N-methyl-Daspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992;12:4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97:1611–1626. doi: 10.1111/j.1471-4159.2006.03991.x. [DOI] [PubMed] [Google Scholar]
- 14.Volbracht C, van Beek J, Zhu C, Blomgren K, Leist M. Neuroprotective properties of memantine in different in vitro and in vivo models of excitotoxicity. Eur J Neurosci. 2006;23:2611–2622. doi: 10.1111/j.1460-9568.2006.04787.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee ST, Chu K, Park JE, Kang L, Ko SY, Jung KW, et al. Memantine reduces striatal cell death with decreasing calpain level in 3-nitropropionic model of Huntington's disease. Brain Res. 2006;1118:199–207. doi: 10.1016/j.brainres.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 16.Fleischhacker WW, Buchgeher A, Schubert H. Memantine in the treatment of senile dementia of the Alzheimer type. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:87–93. doi: 10.1016/0278-5846(86)90047-3. [DOI] [PubMed] [Google Scholar]
- 17.Reisberg G, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. (Memantine Study Group). Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003;348:1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 18.Wang R, Zhang D. Memantine prolongs survival in an amyotrophic lateral sclerosis mouse model. Eur J Neurosci. 2005;22:2376–2380. doi: 10.1111/j.1460-9568.2005.04431.x. [DOI] [PubMed] [Google Scholar]
- 19.Chu CP, Kunitake T, Kato K, Watanabe S, Qiu DL, Tanoue A, et al. The alpha 1D-adrenergic receptor modulates cardiovascular and drinking responses to central salt loading in mice. Neurosci Lett. 2004;356:33–36. doi: 10.1016/j.neulet.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Gurney ME, Cutting FB, Zhai P, Doble A, Taylor CP, Andrus PK, et al. Benefit of vitamin E, riluzole, and gabapentin in a transgenic model of familial amyotrophic lateral sclerosis. Ann Neurol. 1996;39:147–157. doi: 10.1002/ana.410390203. [DOI] [PubMed] [Google Scholar]
- 21.Martin D, Thompson MA, Nadler JV. The neuroprotective agent riluzole inhibits release of glutamate and aspartate from slices of hippocampal area of CA1. Eur J Pharmacol. 1993;250:473–476. doi: 10.1016/0014-2999(93)90037-i. [DOI] [PubMed] [Google Scholar]
- 22.Debono MW, Le Guern J, Canton T, Doble A, Pradier L. Inhibition by riluzole of electrophysiological responses mediated by rat kainateand NMDA receptors in Xenopus oocytes. Eur J Pharmacol. 1993;235:283–289. doi: 10.1016/0014-2999(93)90147-a. [DOI] [PubMed] [Google Scholar]
- 23.Albo F, Pieri M, Zona C. Modulation of AMPA receptors in spinal motor neurons by the neuroprotective agent riluzole. J Neurosci Res. 2004;78:200–207. doi: 10.1002/jnr.20244. [DOI] [PubMed] [Google Scholar]
- 24.Mantz J, Laudenbach V, Lecharny J-B, Henzel D, Desmonts J-M. Riluzole, a novel antiglutamate, blocks GABA uptake by striatal synaptosomes. Eur J Pharmacol. 1994;257:R7–R8. doi: 10.1016/0014-2999(94)90716-1. [DOI] [PubMed] [Google Scholar]
- 25.Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov. 2006;5:160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- 26.Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. A 24-week open-label extension of memantine in moderate to severe Alzheimer disease. Arch Neurol. 2006;63:49–54. doi: 10.1001/archneur.63.1.49. [DOI] [PubMed] [Google Scholar]
- 27.Bowser R, Cudkowicz M, Kaddurah-Daouk R. Biomarkers for amyotrophic lateral sclerosis. Expert Rev Mol Diagn. 2006;6:387–398. doi: 10.1586/14737159.6.3.387. [DOI] [PubMed] [Google Scholar]
- 28.Barneoud P, Lolivier J, Sanger DJ, Scatton B, Moser P. Quantitative motor assessment in FALS mice: a longitudinal study. Neuroreport. 1997;8:2861–2865. doi: 10.1097/00001756-199709080-00012. [DOI] [PubMed] [Google Scholar]
- 29.Van Den Bosch L, Vandenberghe W, Klassen H, Van Houtte E, Robberecht W. Ca2+-permeable AMPA receptors and selective vulnerability of motor neurons. J Neurol Sci. 2000;180:29–34. doi: 10.1016/s0022-510x(00)00414-7. [DOI] [PubMed] [Google Scholar]
- 30.Sen I, Nalini A, Joshi NB, Joshi PG. Cerebrospinal fluid from amyotrophic lateral sclerosis patients preferentially elevates intracellular calcium and toxicity in motor neurons via AMPA/kainate receptor. J Neurol Sci. 2005;235:45–54. doi: 10.1016/j.jns.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 31.Tortarolo M, Grignaschi G, Calvaresi N, Zennaro E, Spaltro G, Colovic M, et al. Glutamate AMPA receptors change in motor neurons of SOD1G93A transgenic mice and their inhibition by a noncompetitive antagonist ameliorates the progression of amytrophic lateral sclerosis-like disease. J Neurosci Res. 2006;83:134–146. doi: 10.1002/jnr.20715. [DOI] [PubMed] [Google Scholar]
- 32.Kornhuber J, Quack G. Cerebrospinal fluid and serum concentrations of the N-methyl-D-aspartate (NMDA) receptor antagonist memantine in man. Neurosci Lett. 1995;195:137–139. doi: 10.1016/0304-3940(95)11785-u. [DOI] [PubMed] [Google Scholar]
- 33.Minkeviciene R, Banerjee P, Tanila H. Memantine improves spatial learning in a transgenic mouse model of Alzheimer's disease. J Pharmacol Exp Ther. 2004;311:677–682. doi: 10.1124/jpet.104.071027. [DOI] [PubMed] [Google Scholar]