Abstract
Backgraound/Aims
The direct toxic effects of antibiotics on the intestine can alter digestive functions and cause pathogenic bacterial overgrowth leading to antibiotic-associated diarrhea (AAD). Clostridium Difficile (C. Difficile) is widely known to be responsible for 10~20% of AAD cases. However, Klebsiella oxytoca, Clostridium perfringens, Staphylococcus aureus, and Candida species might also contribute to AAD.
Methods
We prospectively analyzed the organisms in stool and colon tissue cultures with a C. Difficile toxin A assay in patients with AAD between May and December 2005. In addition, we performed the C. Difficile toxin A assays using an enzyme-linked fluorescent assay technique. Patients were enrolled who had diarrhea with more than three stools per day for at least 2 days after the initiation of antibiotic treatment for up to 6~8 weeks after antibiotic discontinuation.
Results
Among 38 patients (mean age 59±18 years, M:F=18:20), the organism isolation rates were 28.9% (11/38) for stool culture, 18.4% (7/38) for colon tissue cultures and 13.2% (5/38) for the C. Difficile toxin A assay. The overall rate of identification of organisms was 50.0% (19/38). Of the five patients that had a positive result by the C. Difficile toxin A assay, two had no organism isolated by the stool or colon tissue culture. The organisms isolated from the stool cultures were C. Difficile (4), Klebsiella pneumoniae (K. pneumoniae) (3), Candida species (3), and Staphylococcus aureus (1). C. Difficile (4) and K. pneumoniae (3) were isolated from the colon tissue culture.
Conclusions
For C. Difficile negative AAD patients, K. pneumoniae, Candida species, and Staphylococcus aureus were found to be potential causative organisms.
Keywords: Antibiotic-associated, Diarrhea, Organism
INTRODUCTION
Antibiotic-associated diarrhea (AAD) is defined as diarrhea that occurs in association with the administration of antibiotics1). The direct toxic effects of antibiotics on the intestine can alter digestive functions secondary to reduced concentrations of the normal gut bacteria, or cause pathogenic bacterial overgrowth2, 3). Understanding the different mechanisms that cause AAD may help to prevent this condition, improve medical care and reduce medical cost. Clostridium Difficile (C. Difficile) is widely known to be responsible for approximately 10~20% of cases of AAD and almost all cases of pseudomembranous colitis4-7). However, Klebsiella oxytoca8), enterotoxin-producing Clostridium perfringens 9-11), Staphylococcus aureus12), Candida species13, 14), Salmonella species, and Pseudomonas aeruginosa15) might also contribute to the development of AAD2, 5).
However, little is known about other candidate organisms in this context and the clinical correlation between endoscopic findings and the organisms isolated in patients with AAD. Thus, a better understanding of the causes of AAD is needed. In this study, we prospectively analyzed the organisms associated with AAD using stool and colon tissue cultures in combination with the C. Difficile toxin A assay in patients with AAD. In addition, we evaluated the clinical correlation between endoscopic findings and the organisms isolated from stool and colon tissue cultures.
MATERIALS AND METHODS
Patient enrollment
Data on the stool and colon tissue cultures from 38 patients with AAD who were treated at the Ewha Womans Mokdong Hospital from May to December in 2005 were collected prospectively. In addition, we tested for the presence of C. Difficile toxin A using the enzyme-linked fluorescent assay (ELFA) technique (VIDAS® C. Difficile Toxin A II, bioMérieux®Sa, Marcy I'Etoile, France), an automated test for the qualitative detection of C. Difficile toxin A in stool specimens. The relative sensitivity and specificity of VIDAS® for C. Difficile toxin A assay compared with the commercial enzyme immunoassay are 87.6%, and 98.7%, respectively. We enrolled patients who had three or more stools a day for at least two days from the start of antibiotic administration to 6~8 weeks after antibiotic discontinuation.
We excluded patients without a history of antibiotic use, those who discontinued using antibiotics 2 months before the diarrhea started, and those with a diagnosis of acute gastroenteritis, radiation colitis, inflammatory bowel disease, ischemic colitis, or diarrhea due to a carcinoid tumor. The clinical characteristics of the patients with AAD were evaluated. Written informed consent was obtained from each patient for the protocol, which was approved by the ethics committee of the Ewha Womans University Mokdong Hospital.
Endoscopic and microbiologic investigations
For all patients who satisfied the above criteria, stool specimens and colon tissue specimens were obtained and cultured. The stool specimens were placed in transport medium (Amines®, w/o Charcol, Asan Pharmaceuticals Co. Ltd., Hwasung, Korea). Four tissue specimens from severely inflamed colon mucosa or normal mucosa were obtained during endoscopy using sterile biopsy forceps. The tissue specimens were collected into sterile eppendorf tubes (Safe lock test tube, Handok Pharmaceuticals Co. Hwasung, Korea).
All stool and tissue specimens were cultured in selective medium containing cycloserine, cefoxitin and fructose in egg-yolk agar (CCFA) for C. Difficile, Brucella medium for Clostridium perfringens, blood agar plate for Staphlyococcus aureus and Candida, MacConkey media for Klebsiella oxytoca and Klebsiella pneumoniae, and Salmonella-Shigella agar for Salmonella. Insult kits (VITEK II® GN, bioMerieux® Sa, Marcy I'Etoile, France) were used to identify gram-negative species. The endoscopic findings associated with AAD were classified into three groups as follows: 1) normal, no inflammation, 2) nonspecific colitis, which showed erythema, edema and shallow aphthous ulcers, and 3) pseudomembranous colitis (PMC) with whitish exudates, hemorrhage or deep ulcers (Figure 1).
Figure 1.
Three groups of antibiotic-associated diarrhea detected by endoscopy. Normal (A), non-specific colitis (B, C, D), and pseudomembranous colitis (E, F).
Statistical analysis
Statistical analysis was performed using the Fisher's exact test and the linear-by-linear association in SPSS for Windows (Ver. 11.0.1. 2001. Chicago, USA, SPSS Inc.). Throughout the study, p values less than 0.05 were considered statistically significant.
RESULTS
Demographic characteristics of the AAD patients
Thirty-eight patients who were treated between May and December 2005 were enrolled; there were 18 men and 20 women. The mean age was 59 (range, 22-86) years. Eight (21.1%) patients were evaluated by colonoscopy and 30 (78.9%) by sigmoidoscopy. All but one patient had co-morbid disease such as diabetes (14 cases), cerebrovascular disease (9), cancer (9), chronic renal failure (8), trauma (6), chronic liver disease (4), chronic obstructive pulmonary disease (3), and congestive heart failure (3). The reason for the antibiotic use was in descending order: surgical prophylaxis (9 cases), pneumonia (8), infection prophylaxis (6), and other (Table 1). The most commonly used antibiotics were cephalosporins (37.3%), aminoglycosides (11.8%), fluoroquinolone (11.8%), penicillin derivatives (6.4%), clindamycin (2.7%), and others (30.0%). Two (5.3%) of the 38 patients had recurrent AAD. The mean duration of antibiotic use, in patients with AAD, was 14 days (range, 5-29). When used for surgical prophylaxis, the mean duration of antibiotic use was 15 days (range, 8-21). In such cases, the antibiotics were usually used before surgery, then continued throughout the post-surgical period during the hospital stay.
Table 1.
Demographic characteristics of the AAD patients
AAD, antibiotic.associated diarrhea; CAPD, continous ambulatory peritoneal dialysis; SD, standard deviation
Isolated organisms
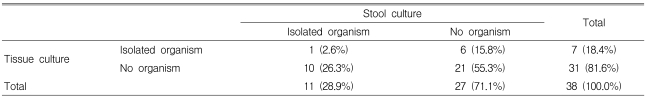
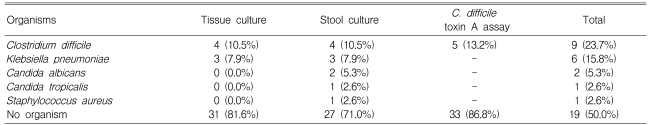
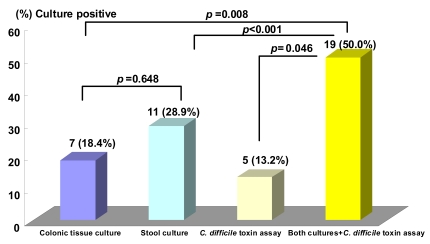
The rate of isolation of organisms with culture was 28.9% (11/38) from the stool and 18.4% (7/38) from colon tissue (p=0.648) (Table 2). Organisms were identified in 50% (19/38) of the patients including 13.2% (5/38) positive results for the C. Difficile toxin A assay (Table 3). However, no organism was isolated by stool or colon tissue culture in two patients out of five with a positive C. Difficile toxin A assay result (Table 4). The overall positive identification rate for organisms by both cultures in combination with the C. Difficile toxin A assay was significantly higher than when any of the techniques were used alone, colon tissue culture (p=0.008), stool culture (p<0.001) or the C. Difficile toxin A assay (p=0.046) (Figure 2). From the colon tissues, the organisms identified were C. Difficile (4 cases) and Klebsiella pneumoniae (K. pneumoniae) (3). From the stool cultures, the isolated organisms were C. Difficile, K. pneumoniae, Candida albicans, Candida tropicalis, and Staphylococcus aureus (Table 3). Overall, C. Difficile accounted for 23.7% (9/38) of the AAD patients and K. pneumoniae for 15.8% (6/38).
Table 2.
Comparison of isolated organism between stool and colon tissue cultures
p=0.648, tested by Fisher's exact test
Table 3.
Isolated organisms according to tissue and stool cultures with Clostridium difficile toxin A assays
C. Difficile, Clostridium difficile
Table 4.
Summary of Clostridium difficile-positive cases by tissue cultures and stool cultures with Clostridium difficile toxin A assays
C. Difficile, Clostridium difficile
Figure 2.
A comparison of positive colon tissue cultures and stool cultures for candidate organisms in patients with antibiotic-associated diarrhea The overall positive organism detection rate for both cultures in combination with C. Difficile toxin A assay were significantly higher than colon tissue culture (p=0.008), stool culture (p<0.001) or C. Difficile toxin A assay (p=0.046).
Clinical correlation between endoscopic findings and isolated organisms
The endoscopic findings associated with AAD were classified into three groups: 1) normal in six patients (15.8%), 2) nonspecific colitis in 17 patients (44.7%), which showed erythema, edema and shallow aphthous ulcers, and 3) PMC in 15 patients (39.5%). The isolated organisms can be categorized into four groups: 1) C. Difficile, 2) K. pneumoniae, 3) other organisms such as Candidia albicans, Candida tropicalis, and Staphylococcus aureus, and 4) no organism. There was no clinical correlation between the endoscopic findings and the organisms isolated (p=0.492) (Table 5).
Table 5.
Correlation between endoscopic findings and isolated organisms
p=0.492, *;Staphylococcus aureus (1), Candida albicans (1), †;Candidia albicans (1), Candida tropicalis (1), C. Difficile, Clostridium difficile; K. pneumoniae, Klebsiella pneumoniae; PMC, pseudomembranous colitis
DISCUSSION
The majority of cases with AAD are caused directly or indirectly by alterations of the gut microflora due to the antibiotics ingested. Changes in the gut flora ecosystem allow pathogens to proliferate. Using conventional anaerobic culture techniques, Bacteroides, Eubacterium, Clostridium, Ruminococcus, Peptococcus, Peptostreptococcus, Bifidobacterium, and Fusobacterium were found to represent the predominant genera of the stool flora2). However, culture-based methods provide an incomplete picture of the various microbial populations of the gut because many bacteria are difficult to culture. Multiple laboratories have reported that only 10~20% of stool specimens are positive with C. Difficile toxin testing4-7). This cytotoxin assay, for toxin B based on tissue culture, is the gold standard for the diagnosis of C. Difficile and still considered as the most sensitive test available for clinical testing16). In our study, however, the C. Difficile toxin A assay was neither superior to the C. Difficile anaerobic stool culture nor the tissue culture. This result is attributed to the fact that the methods used were based on the ELFA technique. C. Difficile was found to be the most common pathogen in our study, and accounted for 23.7% (9/38) of all AAD cases.
Overall, organisms were identified in 50% (19/38) of the patients by stool and tissue cultures including the C. Difficile toxin A assay. The organisms were isolated at a rate of 28.9% (11/38) from the stool culture and 18.4% (7/38) from the colon tissue culture (p=0.648). Only one patient had a positive result in both the stool and colon tissue cultures. There was no significant difference between the stool specimens and the colon tissue specimens with respect to the rate of positive cultures. However, there was a significant difference in the culture results in the comparisons between stool specimens and colon tissue specimens. This may be associated with the difference in the time interval between the onset of diarrhea and the microbiology examination of the two types of specimens. In addition, our results suggest that stool specimens are more likely to contain these organisms than are colon tissue specimens, although our results did not reach statistical significance (p=0.648). Recently, some reports have suggested that cultures of colon biopsy tissue, obtained during a colonoscopy, are more frequently positive than are stool cultures for some patients with presumed acute infectious colitis;17, 18) therefore, this finding is controversial19, 20).
C. Difficile isolates can be either toxigenic or nontoxigenic. Toxigenic strains of C. Difficile produce an enterotoxin (toxin A) as well as cytotoxin (toxin B). The toxin A may be a better marker for the presence of C. Difficile, because it is more stable than toxin B. Non-toxigenic strains have not been linked with C. Difficile-related disease21). Since many healthy infants, children and adults may be carriers of C. Difficile, stool cultures may yield misleading or "false-positive" results in patients with simple AAD because of coincidental intestinal carriage of the organism22). Although the culture methods have a high sensitivity, the potential problem with the culture is the delay of 48-72 hours for the results, and more importantly, the lack of specificity23). In this context (Table 4), four patients with culture positive C. Difficile and a negative toxin A assay might have had a non C. Difficile-associated diarrhea or a C. Difficile toxin B-associated disease. Therefore, stool and colon tissue cultures and the C. Difficile toxin A assay based on ELFA are considered useful complementary tools for identifying the causative pathogens in patients with AAD.
Other enteric organisms that can cause AAD include Klebsiella oxytoca8), enterotoxin-producing Clostridium perfringens9-11), Staphylococcus aureus12), Candida species13, 14), Salmonella species, and Pseudomonas aeruginosa15). In our study, K. pneumoniae (15.8%), Candida albicans (5.2%), Candida tropicalis (2.6%), and Staphylococcus aureus (2.6%) were isolated. We did not stratify patients by the treatment of K. pneumoniae, Candida species and S. aureus. We simply discontinued the antibiotics that were thought to cause the AAD and started treatment with metronidazole or vancomycin, well known treatment for AAD, regardless of the candidate organisms. In cases with K. pneumoniae, all six patients were completely improved whether they were treated with oral metronidazole (4 cases) or had no antibiotic treatment (2 cases).
Although Klebsiella oxytoca was isolated from stools and colon biopsy specimens of patients with C. Difficile negative antibiotic-associated hemorrhagic colitis8), in our study, K. pneumoniae was the second most common organism. Klebsiella is a ubiquitous gram-negative enterobacterium that causes community-acquired pneumonia and nosocomial infections24). Previous antibiotic therapy is significantly associated with the acquisition of Klebsiella25). In addition, K. pneumoniae, a commensal organism of the human gastrointestinal tract, has been reported occasionally to be a cause of diarrhea in humans26). Moreover, the enterotoxigenicity of K. pneumoniae has been reported to be a cause of childhood gastroenteritis27, 28). Although the exact mechanism by which K. pneumoniae causes diarrhea is not known, K. pneumoniae may be a potential cause of AAD.
In the present study, the endoscopic findings were not specific for C. Difficile infection and no diagnosis was made by endoscopic findings alone. The role of endoscopy for the diagnosis of C. Difficile colitis has been reported to be secondary in the workup of AAD, since endoscopy is costly and the findings not sensitive; whereas noninvasive stool tests are inexpensive and accurate29). However, endoscopic studies can eliminate other causes of diarrhea such as inflammatory bowel disease and ischemic colitis, and they allow biopsy of suspicious lesions as well as demonstrate the severity of the AAD.
This study is the first to investigate the clinical correlations between endoscopic findings and isolated organisms associated with AAD. The use of a selective medium for the stool and biopsy specimen cultures is necessary to demonstrate accurately the presence or absence of organisms. However, the cost-effectiveness of this method requires confirmation. The present study had several limitations. First, the sample size was small; a larger study is required to confirm our results. Second, a causal relationship between the presence of organisms in the stool and diarrhea is difficult to establish. In the context of AAD, the question as to whether such 'opportunistic' agents are innocent bystanders or true pathogens responsible for diarrhea is crucial. With the exception of C. Difficile, evidence for other heterogeneous organisms such as Clostridium perfringens, Staphylococcus aureus and Candida species was low to intermediate2). When a diagnosis of AAD is made in a patient with negative tests for C. Difficile and/or its toxins, these isolated organisms might be considered as possible pathogenic causes22). However, it is uncertain whether these species were colonized organisms or pathogens, because this study did not have a matched control group for comparison.
In conclusion, C. Difficile was found to be the most common pathogen in patients with AAD in the present study. In C. Difficile negative AAD patients, K. pneumoniae, Candida albicans, Candida tropicalis, Staphylococcus aureus, or other conventional enteric pathogens might be considered as possible causative organisms. Interestingly, K. pneumoniae was found to be the second most common organism found in patients with AAD. Further investigations are needed to evaluate the possible etiologic role of K. pneumoniae in AAD.
References
- 1.Bartlett JG. Antibiotic-associated diarrhea. N Engl J Med. 2002;346:334–339. doi: 10.1056/NEJMcp011603. [DOI] [PubMed] [Google Scholar]
- 2.Beaugerie L, Petit JC. Microbial-gut interactions in health and disease: antibiotic-associated diarrhoea. Best Pract Res Clin Gastroenterol. 2004;18:337–352. doi: 10.1016/j.bpg.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Hogenauer C, Hammer HF, Krejs GJ, Reisinger EC. Mechanisms and management of antibiotic-associated diarrhea. Clin Infect Dis. 1998;27:702–710. doi: 10.1086/514958. [DOI] [PubMed] [Google Scholar]
- 4.Kelly CP, Pothoulakis C, LaMont JT. Clostridium Difficile colitis. N Engl J Med. 1994;330:257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 5.Gorenek L, Dizer U, Besirbellioglu B, Eyigun CP, Hacibektasoglu A, van Thiel DH. The diagnosis and treatment of Clostridium difficile in antibiotic-associated diarrhea. Hepatogastroenterology. 1999;46:343–348. [PubMed] [Google Scholar]
- 6.Hull MW, Beck PL. Clostridium difficile-associated colitis. Can Fam Physician. 2004;50:1536–1545. [PMC free article] [PubMed] [Google Scholar]
- 7.Wilcox MH. Gastrointestinal disorders and the critically ill: clostridium difficile infection and pseudomembranous colitis. Best Pract Res Clin Gastroenterol. 2003;17:475–493. doi: 10.1016/s1521-6918(03)00017-9. [DOI] [PubMed] [Google Scholar]
- 8.Beaugerie L, Metz M, Barbut F, Bellaiche G, Bouhnik Y, Raskine L, Nicolas JC, Chatelet FP, Lehn N, Petit JC. Klebsiella oxytoca as an agent of antibiotic-associated hemorrhagic colitis. Clin Gastroenterol Hepatol. 2003;1:370–376. doi: 10.1053/s1542-3565(03)00183-6. [DOI] [PubMed] [Google Scholar]
- 9.Modi N, Wilcox MH. Evidence of antibiotic induced Clostridium perfringens diarrhoea. J Clin Pathol. 2001;54:748–751. doi: 10.1136/jcp.54.10.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asha NJ, Wilcox MH. Laboratory diagnosis of Clostridium perfringens antibiotic-associated diarrhoea. J Med Microbiol. 2002;51:891–894. doi: 10.1099/0022-1317-51-10-891. [DOI] [PubMed] [Google Scholar]
- 11.Vaishnavi C, Kaur S, Singh K. Clostridium perfringens type A & antibiotic associated diarrhoea. Indian J Med Res. 2005;122:52–56. [PubMed] [Google Scholar]
- 12.Gravet A, Rondeau M, Harf-Monteil C, Grunenberger F, Monteil H, Scheftel JM, Prevost G. Predominant Staphylococcus aureus isolated from antibiotic-associated diarrhea is clinically relevant and produces enterotoxin A and the bicomponent toxin LukE-LukD. J Clin Microbiol. 1999;37:4012–4019. doi: 10.1128/jcm.37.12.4012-4019.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine J, Dykoski RK, Janoff EN. Candida-associated diarrhea: a syndrome in search of credibility. Clin Infect Dis. 1995;21:881–886. doi: 10.1093/clinids/21.4.881. [DOI] [PubMed] [Google Scholar]
- 14.Crandall M. The pathogenetic significance of intestinal Candida colonization. Int J Hyg Environ Health. 2004;207:79–81. doi: 10.1078/1438-4639-00267. [DOI] [PubMed] [Google Scholar]
- 15.Kim SW, Peck KR, Jung SI, Kim YS, Kim SM, Lee NY, Song JH. Pseudomonas aeruginosa as a potential cause of antibiotic-associated diarrhea. J Korean Med Sci. 2001;16:742–744. doi: 10.3346/jkms.2001.16.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mylonakis E, Ryan ET, Calderwood SB. Clostridium difficile-associated diarrhea: a review. Arch Intern Med. 2001;161:525–533. doi: 10.1001/archinte.161.4.525. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto T, Iida M, Kimura Y, Fujishima M. Culture of colonoscopically obtained biopsy specimens in acute infectious colitis. Gastrointest Endosc. 1994;40:184–187. doi: 10.1016/s0016-5107(94)70164-4. [DOI] [PubMed] [Google Scholar]
- 18.Bellaiche G, Le Pennec MP, Choudat L, Ley G, Slama JL. Value of sigmoidoscopy with bacteriological biopsy cultures for the diagnosis of antibiotic-associated and haemorrhagic colitis due to Klebsiella oxytoca. Gastroenterol Clin Biol. 1997;21:764–767. [PubMed] [Google Scholar]
- 19.Schumacher G, Kollberg B, Sandstedt B, Jorup C, Grillner L, Ljungh A, Mollby R. A prospective study of first attacks of inflammatory bowel disease and non-relapsing colitis: microbiologic findings. Scand J Gastroenterol. 1993;28:1077–1085. doi: 10.3109/00365529309098313. [DOI] [PubMed] [Google Scholar]
- 20.Barbut F, Beaugerie L, Delas N, Fossati-Marchal S, Aygalenq P, Petit JC. Comparative value of colonic biopsy and intraluminal fluid culture for diagnosis of bacterial acute colitis in immunocompetent patients. Clin Infect Dis. 1999;29:356–360. doi: 10.1086/520215. [DOI] [PubMed] [Google Scholar]
- 21.Lyerly DM, Krivan HC, Wilkins TD. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988;1:1–18. doi: 10.1128/cmr.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fekety R. Guidelines for the diagnosis and management of Clostridium difficile-associated diarrhea and colitis. Am J Gastroenterol. 1997;92:739–750. [PubMed] [Google Scholar]
- 23.Bartlett JG. Management of Clostridium difficile infection and other antibiotic-associated diarrheas. Eur J Gastroenterol Hepatol. 1996;8:1054–1061. doi: 10.1097/00042737-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11:589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Struve C, Krogfelt KA. Pathogenic potential of environmental Klebsiella pneumoniae isolates. Environ Microbiol. 2004;6:584–590. doi: 10.1111/j.1462-2920.2004.00590.x. [DOI] [PubMed] [Google Scholar]
- 26.Guerin F, Le Bouguenec C, Gilquin J, Haddad F, Goldstein FW. Bloody diarrhea caused by Klebsiella pnemoniae: a new mechanism of bacterial virulence? Clin Infect Dis. 1998;27:648–649. doi: 10.1086/517141. [DOI] [PubMed] [Google Scholar]
- 27.Ananthan S, Raju S, Alavandi S. Enterotoxigenicity of Klebsiella pneumoniae associated with childhood gastroenteritis in Madras, India. Jpn J Infect Dis. 1999;52:16–17. [PubMed] [Google Scholar]
- 28.Niyogi SK, Pal A, Mitra U, Dutta P. Enteroaggregative Klebsiella pneumoniae in association with childhood diarrhoea. Indian J Med Res. 2000;112:133–134. [PubMed] [Google Scholar]
- 29.Bergstein JM, Kramer A, Wittman DH, Aprahamian C, Quebbeman EJ. Pseudomembranous colitis: how useful is endoscopy? Surg Endosc. 1990;4:217–219. doi: 10.1007/BF00316796. [DOI] [PubMed] [Google Scholar]