Abstract
Background/Aims
We investigated the prevalence and relationship of peptic ulcer disease and Helicobacter pylori infection to liver cirrhosis.
Methods
We examined 288 patients with liver cirrhosis, 322 patients with non-ulcer dyspepsia, and 339 patients with peptic ulcer disease. Rapid urease test and Wright-Giemsa staining were used for diagnosis of H. pylori infection.
Results
The prevalence of peptic ulcer disease in patients with cirrhosis was 24.3%. The prevalence of peptic ulcer disease in patients with cirrhosis divided into Child-Pugh classes A, B, and C was 22.3%, 21.0%, and 31.3%, respectively (p>0.05). The prevalence of H. pylori infection in the patients with cirrhosis, non-ulcer dyspepsia, and peptic ulcer without chronic liver disease were 35.1%, 62.4%, and 73.7%, respectively (p<0.001). The prevalence of H. pylori infection did not differ depending on whether there was peptic ulcer (35.6%) or not (34.9%) in patients with liver cirrhosis (p>0.05). The prevalence of H. pylori infection in patients with hepatitis virus-related liver cirrhosis and in the patients with alcohol-related liver cirrhosis was 42.5% and 22.0%, respectively (p<0.001). The prevalence of H. pylori infection in patients with Child-Pugh classes A, B, and C liver cirrhosis was 51.5%, 30.5%, and 20.0%, respectively (p<0.001).
Conclusions
Factors other than H. pylori may be involved in the pathogenesis of peptic ulcer disease in the setting of liver cirrhosis.
Keywords: Helicobacter pylori, Liver cirrhosis, Peptic ulcer
INTRODUCTION
Chronic liver disease and its complications are major health problems. Peptic ulcer disease is one of the most frequently observed complications in patients with liver cirrhosis. Although the incidence and prevalence of peptic ulcer disease appear to be increased in cirrhosis, the underlying mechanism of peptic ulcer disease in cirrhosis is unclear1-3). In the general population, Helicobacter pylori infection is central to the pathogenesis of peptic ulcer disease4, 5). However, the role of H. pylori infection in the pathogenesis of peptic ulcer disease in cirrhotic patients still remains to be elucidated. Many studies have suggested a role for H. pylori infection in the pathogenesis of peptic ulcer disease in cirrhotic patients, but several studies have found no relationship6-24). Moreover, there is debate concerning the relationship between H. pylori infection and the etiology or severity of cirrhosis.
The aim of this study was to define the prevalence of peptic ulcer disease and H. pylori infection in patients with liver cirrhosis. We additionally evaluated the relationship between H. pylori infection and the etiology or severity of cirrhosis.
MATERIALS AND METHODS
The study population included 288 consecutive Korean patients (229 men and 59 women, 49.3±11.1 years of age) with newly diagnosed liver cirrhosis presenting at Chuncheon Sacred Heart Hospital, Hallym University Medical Center, Chuncheon, Korea, between January 2000 and December 2004. 322 age- and sex-matched non-ulcer dyspepsia patients (259 men and 63 women, 49.0±10.0 years of age) without chronic liver disease were drawn to serve as a control group. Additionally, 339 age- and sex-matched peptic ulcer patients (272 men and 67 women, 48.1±11.5 years of age) without chronic liver disease were selected as a reference group. Those patients who had taken antibiotics, proton pump inhibitors, or non-steroidal anti-inflammatory drugs within 2 weeks before entry, and those patients who had a history of gastric surgery, were excluded.
Liver cirrhosis was diagnosed using a combination of clinical, biochemical, radiologic, and histologic methods. The severity of cirrhosis was scored according to the Child-Pugh classification. Etiology of the cirrhosis was defined as alcoholic if viral markers (HBsAg, anti-HCV) were negative and there was a history of ethanol intake of over 60 g/day for 5 years or more.
All the enrolled patients underwent upper gastrointestinal endoscopy with 4 biopsies taken from the antrum (within 2 cm from the pylorus) and 4 biopsies taken from the gastric body (greater curvature side of the midbody). Endoscopy was performed using an Olympus videoscope GIF 240 (Olympus Optical Co., Ltd., Tokyo, Japan). Endoscopic diagnosis of peptic ulcer disease was made when a distinct ulcer crater with fibrin-coated base larger than 5 mm was observed. The diagnosis of gastric ulcer was always confirmed by multiple biopsies of the ulcer.
Four biopsy specimens 2 from the antrum and 2 from the gastric body were used to identify H. pylori, using the rapid urease test (CLOtest®, Ballard Medical Products, UT, USA or ProntoDry®, Medical Instruments Co., Herford, Germany). Four other specimens 2 from the antrum and 2 from the gastric body were used for determining H. pylori presence (hematoxylin-eosin & Wright-Giemsa staining). The presence of H. pylori infection was determined by positivity of rapid urease test and/or histology (Wright-Giemsa staining).
This study was approved by the Hospital's Ethics Committee, and informed consent for endoscopy was obtained from all the patients.
The demographic and clinical characteristics were analyzed statistically using the chi-square test and Spearman correlation test, as appropriate. A value of p<0.05 was considered to be statistically significant. Statistical calculation was made with the help of SPSS statistical package for Windows version 8.0.0. (SPSS Inc, Chicago, IL, USA).
RESULTS
The etiologies of the 288 cirrhosis patients were: Hepatitis B virus (HBV)-related in 145, Hepatitis C virus (HCV)-related in 24, HBV and HCV-related in 5, alcohol-related in 109, and cryptogenic in 5 patients.
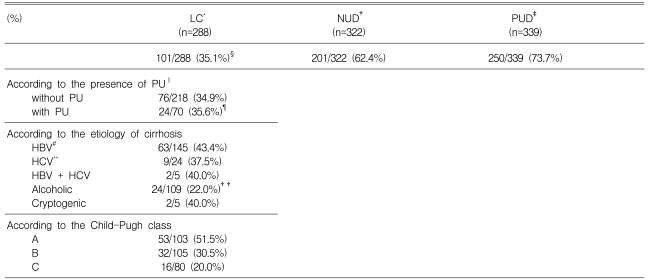
The prevalence of H. pylori infection in patients with cirrhosis, patients with non-ulcer dyspepsia without chronic liver disease, and peptic ulcer disease patients without chronic liver disease, were 35.1% (101/288), 62.4% (201/322), and 73.7% (250/339), respectively. The prevalence of H. pylori infection in patients with cirrhosis was significantly lower than in non-ulcer dyspepsia (p<0.001) or peptic ulcer disease patients without chronic liver disease (p<0.001) (Table 1).
Table 1.
The prevalence of H. pylori infection in patients with liver cirrhosis, non-ulcer dyspepsia, and peptic ulcer disease
*Liver Cirrhosis, †Non-Ulcer Dyspepsia, ‡Peptic Ulcer Disease, ‖Peptic Ulcer, #Hepatitis B Virus, **Hepatitis C Virus
§p<0.001 (LC vs. NUD, LC vs. PUD), ¶p<0.001 (PU with LC vs. PU without LC), ††p<0.001 (alcohol vs. viral etiology)
The point prevalence of peptic ulcer disease in patients with cirrhosis was 24.3% (36 gastric ulcer, 31 duodenal ulcer, 3 gastric and duodenal ulcer). The prevalence of H. pylori infection was 35.6% in peptic ulcer disease patients with liver cirrhosis and 73.7% in peptic ulcer disease patients without chronic liver diseases (p<0.001). The prevalence of H. pylori infection did not differ in patients with liver cirrhosis, whether peptic ulcer was present (35.6%) or no (34.9%) (p>0.05) (Table 1).
The prevalence of H. pylori infection in patients with HBV-related, HCV-related, HBV- and HCV-related, alcohol-related, and cryptogenic liver cirrhosis was 43.4%, 37.5%, 40.0%, 22.0%, and 40.0%, respectively. The prevalence of H. pylori infection in patients with hepatitis virus-related liver cirrhosis (42.5%) was significantly higher than that in patients with alcohol-related liver cirrhosis (22.0%, p<0.001) (Table 1).
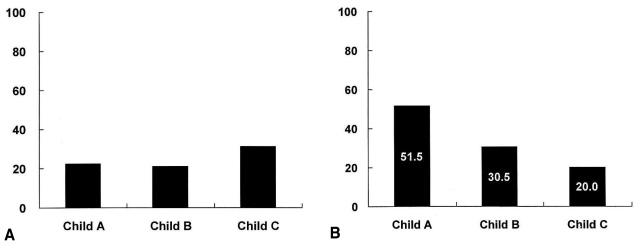
As shown in Table 1, the prevalence of H. pylori infection in patients with Child-Pugh class A, B, and C liver cirrhosis was 51.5%, 30.5%, and 20.0%, respectively. A negative correlation was noted between the prevalence of H. pylori infection and the severity of liver cirrhosis according to Child-Pugh class (Spearman's rho=-0.267, p<0.001). The prevalence of peptic ulcer disease in cirrhotic patients according to Child-Pugh class A, B, and C was 22.3%, 21.0%, and 31.3%, respectively (p>0.05) (Figure 1).
Figure 1.
The prevalence of peptic ulcer (A) and H. pylori infection (B) in patients with liver cirrhosis. (A) The prevalence of peptic ulcer disease in cirrhotic patients is not significantly different according to Child-Pugh class (p>0.05). (B) A negative correlation is demonstrated between the prevalence of H. pylori infection and the severity of liver cirrhosis according to Child-Pugh class (Spearman's rho=-0.267, p<0.001).
DISCUSSION
Although the point prevalence of peptic ulcer disease in patients with liver cirrhosis is high, reaching 24.3%, the exact mechanism remains to be determined. The high prevalence of peptic ulcer disease in patients in our study with liver cirrhosis is consistent with previous reports1-3, 6-8).
The role of H. pylori infection in the pathogenesis of peptic ulcer disease has been extensively evaluated in non-cirrhotic patients4, 5). The increased rate of peptic ulcer disease in patients with cirrhosis has been explained in some studies, by higher rates of gastric colonization with H. pylori6, 7, 14-18, 23, 24). However, other studies have found no relationship between H. pylori infection and peptic ulcer disease in cirrhosis9-12, 18-20). For a proper interpretation of the studies investigating the prevalence of H. pylori infection in patients with liver cirrhosis, several factors, such as diagnostic methods, etiology of liver cirrhosis, and geographic and racial differences in H. pylori prevalence should be considered.
In our study, the prevalence of H. pylori infection in patients with liver cirrhosis was 35.1%. Previous studies have reported a wide range (39~89%) in the prevalence of H. pylori infection in patients with liver cirrhosis, most of them somewhat higher than our study7, 8, 12, 14, 17, 18). Moreover, the prevalence of H. pylori infection in cirrhotic patients in our study was significantly lower than that in the control and reference groups, a finding that was different from that found by many groups6, 7, 13-18, 23, 24). It was consistent with the results of others19, 20). This relatively low prevalence of H. pylori infection in cirrhotic patients in our study can be explained by the enrollment of more than 50% of Child-Pugh B and C cirrhotic patients in our study. Although those who took antibiotics within 2 weeks before entry were excluded, those who had a history of extensive but remote use of antibiotics were included. Therefore, inclusion of decompensated cirrhotic patients with a history of extensive but remote use of antibiotics due to various reasons might affect the prevalence of H. pylori.
The relatively low prevalence of H. pylori infection in cirrhotic patients in our study can also be partially explained by the difference in the diagnostic methods used in the setting of H. pylori infection. In general, studies that use serologic tests (IgG to H. pylori) report higher prevalence than the studies that use the rapid urease test, histology, or urea breath test for detection of H. pylori infection (76.2~89% vs. 39~59.7%). If H. pylori infection and liver cirrhosis have the same risk factors, but H. pylori infection cannot easily persist in the stomach of cirrhotic patients, serologic determination of H. pylori infection may lead to confounding results. The 2007 Maastricht Consensus Report on H. pylori diagnosis and treatment does not recommend serological determination of H. pylori infection in routine clinical practice. It also recommends that the primary diagnosis of H. pylori infection should be established by rapid urease test and/or histology25-28).
The accuracy of gastric mucosal biopsy may be affected by various factors: the presence of bacteria other than H. pylori that produce urease, the patch distribution of H. pylori in gastric mucosa, and the histologic overestimation of H. pylori due to the presence of confounding bacteria. Thus, primary diagnosis by gastric mucosa biopsy urease testing and/or histology has some limitations19). So the accuracy of diagnostic methods use to detect H. pylori infection in our study may affect the result of low prevalence of H. pylori infection. The concordance of rapid urease test and histology for the diagnosis of H. pylori infection in our study was 255 out of 288 in cirrhotic patients and 293 out of 339 in peptic ulcer patients without chronic liver disease.
The prevalence of H. pylori infection in the control group of the present study (62.4%) was slightly lower than the seroprevalence (66.9%) in asymptomatic Korean adults29). This discrepancy may be due to the different study groups and diagnostic methods. H. pylori infection in the general population correlates with age, social class, education level, overcrowding, bed-sharing, and economic level during childhood13). Thus, a valid comparison of the prevalence of H. pylori infection in patients with cirrhosis and the general population, a control group matched by prognostic variables (age, socioeconomic status, ethanol intake, etc.) would be required. Although our study used age- and sex-matched control and reference groups, socioeconomic status and ethanol intake were not matched. Consequently, out study may not provide definitive data.
Besides H. pylori, other factors may contribute to the increased risk of peptic ulcer in cirrhotic patients30, 31). Reduced prostaglandins, decreased gastric acid secretion, elevated serum gastrin concentration, impaired mucus secretion, a reduction in potential difference of the gastric mucosa, and portal hypertensive gastropathy may all play a role in the pathogenesis of peptic ulcer disease in cirrhotic patients1-3).
Several studies have been previously performed regarding the relationship between the prevalence of H. pylori infection and peptic ulcer disease according to the etiology and severity of liver cirrhosis. The present study demonstrated an interesting finding in that the prevalence of H. pylori infection in patients with liver cirrhosis varied according to the etiology of cirrhosis (viral vs. alcoholic = 42.5 vs. 22.0%, p<0.001). In Korea, HBV infection is still the leading cause of liver cirrhosis (53%), followed by alcohol (31%) and HCV infection (10%)32). Since our study included a relatively small proportion of cirrhotic patients with HCV infection, the fact that the prevalence of H. pylori infection in virus-related liver cirrhosis was higher than that in alcohol-related cirrhosis patients may not be generalizable in other countries where the major cause of liver cirrhosis is HCV infection.
It is of interest to note that the prevalence of H. pylori infection was related inversely to Child-Pugh classification. Although this observation is consistent with a previous report, which suggested that the prevalence of H. pylori infection among cirrhotic patients might be inversely related to the severity of liver cirrhosis, many previous data have noted no relationship between H. pylori infection and severity of liver cirrhosis8, 10, 11, 33, 34). Several factors, such as diagnostic methods, etiology of liver cirrhosis, proportion of decompensated cirrhosis, and geographic and racial differences in H. pylori prevalence, may explain the discrepancy. Based on our study, we hypothesize that the environment of the stomach in cirrhotic patients may not be suitable for the growth of H. pylori, and the progression of liver disease may lead to a more hostile milieu to H. pylori.
Many studies have argued for the relationship between H. pylori and peptic ulcer in cirrhotic patients, but the 'statistically high' (which is at most 2 times higher than control) prevalence of H. pylori in cirrhotic patients in those studies cannot explain completely the 'vividly and absolutely high' (which is at least 10 times higher than control) prevalence of peptic ulcer in cirrhotic patients. Our study showed that even though H. pylori infection was decreased in patients with severe liver cirrhosis, peptic ulcer disease was increased. Based on our observations and those of others, we hypothesize that H. pylori may not be a main cause of peptic ulcer disease in cirrhotic patients6).
The followings are major findings of our study: (1) Even though the prevalence of peptic ulcer disease was increased in patients with liver cirrhosis, H. pylori infection rate in peptic ulcer disease patients with cirrhosis was significantly lower than that in patients without chronic liver disease. (2) The prevalence of H. pylori infection did not differ in patients with liver cirrhosis, whether there was peptic ulcer or no. (3) Although the prevalence of H. pylori infection decreased with Child's class progression, the prevalence of peptic ulcer disease did not change. Taking the above major findings into account, we can conclude that H. pylori infection may not be one of the main factors in the pathogenesis of peptic ulcer disease in patients with liver cirrhosis.
Footnotes
This study was supported by Hallym University Research Fund.
References
- 1.Cryer B, Spechler SJ. Peptic ulcer disease. In: Felman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran's gastrointestinal and liver disease. 8th ed. Philadelphia: Saunders; 2006. pp. 1089–1110. [Google Scholar]
- 2.Zullo A, Hassan C, Morini S. Helicobacter pylori infection in patients with liver cirrhosis: facts and fiction. Dig Liver Dis. 2003;35:197–205. doi: 10.1016/s1590-8658(03)00029-x. [DOI] [PubMed] [Google Scholar]
- 3.Fraser AG, Pounder RE, Burroughs AK. Gastric secretion and peptic ulceration in cirrhosis. J Hepatol. 1993;19:171–182. doi: 10.1016/s0168-8278(05)80191-6. [DOI] [PubMed] [Google Scholar]
- 4.Marshall BJ. Helicobacter pylori. Am J Gastroenterol. 1994;89(8 Suppl):S116–S128. [PubMed] [Google Scholar]
- 5.Graham DY, Sung JJ. Helicobacter pylori. In: Felman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran's gastrointestinal and liver disease. 8th ed. Philadelphia: Saunders; 2006. pp. 1049–1066. [Google Scholar]
- 6.Calvet X, Navarro M, Gil M, Lafont A, Sanfeliu I, Brullet E, Campo R, Dalmau B, Rivero E, Mas P. Epidemiology of peptic ulcer disease in cirrhotic patients: role of Helicobacter pylori infection. Am J Gastroenterol. 1998;93:2501–2507. doi: 10.1111/j.1572-0241.1998.00711.x. [DOI] [PubMed] [Google Scholar]
- 7.Zullo A, Rinaldi V, Meddi P, Folino S, Lauria V, Diana F, Winn S, Attili AF. Helicobacter pylori infection in dyspeptic cirrhotic patients. Hepatogastroenterology. 1999;46:395–400. [PubMed] [Google Scholar]
- 8.Tsai CJ. Helicobacter pylori infection and peptic ulcer disease in cirrhosis. Dig Dis Sci. 1998;43:1219–1225. doi: 10.1023/a:1018899506271. [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara Y, Arakawa T, Higuchi K, Kuroki T. Gastrointestinal lesions in liver cirrhosis. Nippon Rinsho. 1998;56:2387–2390. [PubMed] [Google Scholar]
- 10.Wu CS, Lin CY, Liaw YF. Helicobacter pylori in cirrhotic patients with peptic ulcer disease: a prospective, case controlled study. Gastrointest Endosc. 1995;42:424–427. doi: 10.1016/s0016-5107(95)70044-7. [DOI] [PubMed] [Google Scholar]
- 11.Calvet X, Navarro M, Gil M, Mas P, Rivero E, Sanfeliu I, Brullet E, Campo R, Dalmau B, Lafont A. Seroprevalence and epidemiology of Helicobacter pylori infection in patients with cirrhosis. J Hepatol. 1997;26:1249–1254. doi: 10.1016/s0168-8278(97)80459-x. [DOI] [PubMed] [Google Scholar]
- 12.Siringo S, Vaira D, Menegatti M, Piscaglia F, Sofia S, Gaetani M, Migliolo M, Corinaldesi R, Bolondi L. High prevalence of Helicobacter pylori in liver cirrhosis: relationship with clinical and endoscopic features and the risk of peptic ulcer. Dig Dis Sci. 1997;42:2024–2030. doi: 10.1023/a:1018849930107. [DOI] [PubMed] [Google Scholar]
- 13.Schmulson MJ, De Leon G, Kershenovich A, Vargas Vorackova F, Kershenobich D. Helicobacter pylori infection among patients with alcoholic and nonalcoholic cirrhosis. Helicobacter. 1997;2:149–151. doi: 10.1111/j.1523-5378.1997.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 14.Pellicano R, Leone N, Berrutti M, Cutufia MA, Fiorentino M, Rizzetto M, Ponzetto A. Helicobacter pylori seroprevalence in hepatitis C virus positive patients with cirrhosis. J Hepatol. 2000;33:648–650. doi: 10.1034/j.1600-0641.2000.033004648.x. [DOI] [PubMed] [Google Scholar]
- 15.Kamalaporn P, Sobhonslidsuk A, Jatchavala J, Atisook K, Rattanasiri S, Pramoolsinsap C. Factors predisposing to peptic ulcer disease in asymptomatic cirrhotic patients. Aliment Pharmacol Ther. 2005;21:1459–1465. doi: 10.1111/j.1365-2036.2005.02507.x. [DOI] [PubMed] [Google Scholar]
- 16.Ponzetto A, Pellicano R, Leone N, Berrutti M, Turrini F, Rizzetto M. Helicobacter pylori seroprevalence in cirrhotic patients with hepatitis B virus infection. Neth J Med. 2000;56:206–210. doi: 10.1016/s0300-2977(00)00033-4. [DOI] [PubMed] [Google Scholar]
- 17.Chen CT, Wang TF, Chan CC, Lee FY, Chang FY, Lin HC, Hou MC, Lu RH, Chu CJ, Wang SS, Lee SD. Role of chronic Helicobacter pylori infection in hyperdynamic circulation of cirrhotic patients. Hepatogastroenterology. 2002;49:208–212. [PubMed] [Google Scholar]
- 18.Chang CS, Kao CH, Yeh HZ, Lien HC, Chen GH, Wang SJ. Helicobacter pylori infection and gastric emptying in cirrhotic patients with symptoms of dyspepsia. Hepatogastroenterology. 1999;46:3166–3171. [PubMed] [Google Scholar]
- 19.Shahin WA, Abdel Baset EZ, Nassar AK, Atta MM, Kabil SM, Murray JA. Low incidence of Helicobacter pylori infection in patients with duodenal ulcer and chronic liver disease. Scand J Gastroenterol. 2001;36:479–484. doi: 10.1080/003655201750153250. [DOI] [PubMed] [Google Scholar]
- 20.Auroux J, Lamarque D, Roudot Thoraval F, Deforges L, Chaumette MT, Richardet JP, Delchier JC. Gastroduodenal ulcer and erosions are related to portal hypertensive gastropathy and recent alcohol intake in cirrhotic patients. Dig Dis Sci. 2003;48:1118–1123. doi: 10.1023/a:1023772930681. [DOI] [PubMed] [Google Scholar]
- 21.Vergara M, Calvet X, Roque M. Helicobacter pylori is a risk factor for peptic ulcer disease in cirrhotic patients: a meta analysis. Eur J Gastroenterol Hepatol. 2002;14:717–722. doi: 10.1097/00042737-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Queiroz DM, Rocha AM, Rocha GA, Cinque SM, Oliveira AG, Godoy A, Tanno H. Association between Helicobacter pylori infection and cirrhosis in patients with chronic hepatitis C virus. Dig Dis Sci. 2006;51:370–373. doi: 10.1007/s10620-006-3150-y. [DOI] [PubMed] [Google Scholar]
- 23.Dore MP, Mura D, Deledda S, Maragkoudakis E, Pironti A, Realdi G. Active peptic ulcer disease in patients with hepatitis C virusrelated cirrhosis: the role of Helicobacter pylori infection and portal hypertensive gastropathy. Can J Gastroenterol. 2004;18:521–524. doi: 10.1155/2004/150674. [DOI] [PubMed] [Google Scholar]
- 24.Konturek SJ, Gonciarz M, Gonciarz Z, Bielanski W, Mazur W, Mularczyk A, Konturek PC, Goetze JP, Rehfeld JF. Progastrin and its products from patients with chronic viral hepatitis and liver cirrhosis. Scand J Gastroenterol. 2003;38:643–647. doi: 10.1080/00365520310002472. [DOI] [PubMed] [Google Scholar]
- 25.Tsai CJ. Does quantitative serologic testing for Helicobacter pylori predict peptic ulcer disease in cirrhosis? Gastrointest Endosc. 1999;50:381–386. doi: 10.1053/ge.1999.v50.98595. [DOI] [PubMed] [Google Scholar]
- 26.Calvet X, Sanfeliu I, Musulen E, Mas P, Dalmau B, Gil M, Bella MR, Campo R, Brullet E, Valero C, Puig J. Evaluation of Helicobacter pylori diagnostic methods in patients with liver cirrhosis. Aliment Pharmacol Ther. 2002;16:1283–1289. doi: 10.1046/j.1365-2036.2002.01293.x. [DOI] [PubMed] [Google Scholar]
- 27.The report of the digestive health initiative international update conference on Helicobacter pylori. Gastroenterology. 1997;113(6 supple):S4–S8. doi: 10.1016/s0016-5085(97)80003-0. [DOI] [PubMed] [Google Scholar]
- 28.Malfertheiner P, Megraud F, O'morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JH, Kim HY, Kim NY, Kim SW, Kim JG, Kim JJ, Roe IH, Seo JK, Sim JG, Ahn H, Yoon BC, Lee SW, Lee YC, Chung IS, Jung HY, Hong WS, Choi KW. Seroepidemiological study of Helicobacter pylori infection in asymptomatic people in South Korea. J Gastroenterol Hepatol. 2001;16:969–975. doi: 10.1046/j.1440-1746.2001.02568.x. [DOI] [PubMed] [Google Scholar]
- 30.Kitano S, Dolgor B. Does portal hypertension contribute to the pathogenesis of gastric ulcer associated with liver cirrhosis? J Gastroenterol. 2000;35:79–86. doi: 10.1007/s005350050018. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki H, Ishii H. Peptic ulcer disease complicated with liver cirrhosis. Nippon Rinsho. 2004;62:532–540. [PubMed] [Google Scholar]
- 32.You K, Kim DJ, Choi SK, Sohn JH, Yoon JH, Choi MS, Yeon JE, Cho M, Kwon YO, Lee JH, Lee JS, Bail YH, Han CJ, Kim YS, Bae SH, Lim YS. The Korean Association of the Study of liver practice guideline for complications of liver cirrhosis. Korean J Hepatol. 2005;11(Suppl):S111–S163. [Google Scholar]
- 33.Chen NL, Bai L, Deng T, Zhang C, Kong QY, Chen H. Expression of hepatitis B virus antigen and Helicobacter pylori infection in gastric mucosa of patients with chronic liver disease. Hepatobiliary Pancreat Dis Int. 2004;3:223–225. [PubMed] [Google Scholar]
- 34.Pan WD, Xun RY, Chen YM. Correlations of portal hypertensive gastropathy of hepatitis B cirrhosis with other factors. Hepatobiliary Pancreat Dis Int. 2002;1:527–531. [PubMed] [Google Scholar]